Omega 3 fatty acids can be obtained from several sources, and should be added to the daily diet to enjoy a good health and to prevent many diseases. The European Food Safety Agency (EFSA) proposed a recommended daily intake of 250 mg/d eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) for adults, because this intake is negatively related to cardiovascular diseases (CVD) risk in a dose-dependent way up to 250 mg/d (1–2 servings/week of oily fish) in healthy subjects(1). The American Heart Association (AHA) recommended for the general population a consumption of fish, at least twice a week(Reference Kris-Etherton, Harris and Appel2), estimating a consumption of one portion (125 g) of oily fish (2 g/100 g EPA and DHA) and one portion of lean fish (0·2 g/100 g), which results in an mean intake of 3 g/week or 430 mg/d of DHA and EPA. AHA also established intakes of 1 g of EPA and DHA from fish or fish oils for subjects with clinical history of CVD and a 2–4 g supplement for subjects with high blood triacylglycerides (TAG)(Reference Kris-Etherton, Harris and Appel3). The World Health Organization (WHO) recommended a regular fish consumption (1–2 servings/week; providing 200–500 mg/serving of EPA and DHA) for the general population, as being protective against coronary heart disease and ischemic stroke(4). WHO also indicates that vegetarians and not fish-eaters are recommended to ensure adequate intake of plant sources of alpha-linolenic acid (ALA), as some of it (0·5–20 % depending on various factors) is metabolized to EPA(Reference Pawlosky, Hibbeln and Novotny5, Reference Arterburn, Hall and Oken6). Worldwide, general population use omega-3 fatty acids supplements and enriched foods to get and maintain adequate amounts of these fatty acids, i.e.: milk and dairy products are every day consumed foods and constitute a good and popular source of omega-3 fatty acids, to produce ‘healthier’ milks and dairy products(Reference Lopez-Huertas7). The aim of this paper was to review main scientific evidence regarding the public health risks and benefits of the dietary sources of omega-3 fatty acids
Methods
A systematic literature search was performed up to April 2011. The literature search was conducted in Medlars Online International Literature (MEDLINE), via PubMed©; Scopus; OvidSP (Food Science and Technology Abstracts); EMBASE©, and Latin American and Caribbean Heath Sciences Literature (LILACS), using the following terms: ‘Fatty acids, omega-3’[Major] OR ‘alpha-linolenic acid’[Mesh] OR ‘docosahexaenoic acids’[Mesh] OR ‘eicosapentaenoic acid’[Mesh] AND (‘adverse effects’[Mesh] OR ‘contraindications’[Mesh] OR ‘standards’[Mesh] OR ‘supply and distribution’[Mesh] OR ‘therapeutic use’[Mesh] OR ‘toxicity’[Mesh] AND (‘humans’[MeSH Terms] AND (‘Clinical Trial’[ptyp] OR ‘Randomized Controlled Trial’[ptyp])).
Using the above mentioned data bases, 2476 articles were selected. Duplicates, review articles and non-relevant articles were excluded (n 2310). After reading the literature list of the remaining articles, and suggestions from other experts about relevant papers, 35 were included in the results. Fifty-four of the remaining 201 articles were rejected for the reasons shown in Fig. 1. Finally, just one hundred and forty-seven articles were included in the results. The articles were reviewed by at least two reviewers and were taken into account for the selection criteria listed on the JADAD scale or Oxford Quality Scoring System, a procedure to independently assess the methodological quality of a clinical trial. Reviewers extracted data from the published reports. Table 1 summarises the design of and the results provided by the 147 articles finally selected.
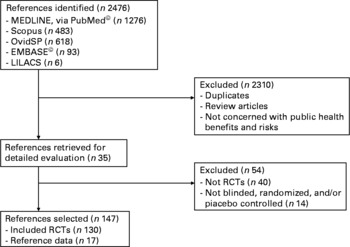
Fig. 1 Literature search flow chart.
Table 1 Description of the studies included in this review

Abbreviations: ALA: α-linolenic acid; ARA: arachidonic acid; CLA: conjugated linoleic acid; BMI: body mass index; CD69: cluster of differentiation 69; CRP: C-reactive protein; CVD: cardiovascular diseases; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; FFQ: food frequency questionnaire; HOMA-IR index: homeostasis model assessment-insulin resistence; hs-CRP: high-sensitivity C-reactive protein; HOSO: high-oleic sunflower oil; IL-6: interleukine-6; LA: linoleic acid; LTB4: leukotriene B4: LTB5: leukotriene B5; MDA: malondialdehyhe; MeHg: methylmercury; MI: myocardial infaction; MUFA: monounsaturated fatty acids; n-3 LC-PUFAs: omega-3 long chain polyunsaturated fatty acids; NO: nitric oxide; RBCs: red blood cells; SFA: saturated fatty acids; sICAM-1: soluble cell adhesion molecules-1; sVCAM-1: vascular cell adhesion molecules-1; TAG: triacylglycerides; TBARS: thiobarbituric acid reactive substances; T2DM: type II diabetes mellitus; TNFα: tumor necrosis factor-alpha; nd: no data.
Results
Algal omega-3 fatty acids
Clinical trials with algal DHA-rich oil supplementation resulted in potentially beneficial changes in some markers of cardiometabolic risk: decrease in VLDL and increase in LDL and HDL particle sizes, and reduction in VLDL, and total TAG concentrations, blood pressure and heart rate, and oxidative stress, indicating comparable efficacies to fish oil. Algal-DHA was safe and well tolerated(Reference Neff, Culiner and Cunningham-Rundles8). Unlike fish oil, algal-DHA seldom caused gastrointestinal complaints such as fishy taste and eructation, attributes of importance for patient compliance in high-dose therapy.
The consumption of Ulkenia sp. microalgae oil (0·94 g DHA/d) for 8 weeks decreased plasma TAG, and increased plasma total cholesterol, LDL- and HDL-cholesterol in normolipidaemic vegetarians. DHA-rich oil from Ulkenia sp. is well tolerated and a suitable vegetarian source of n-3 LC-PUFAs(Reference Geppert, Kraft and Demmelmair9).
Schizochytrium sp. microalgae provided substantial quantities of the docosapentaenoic acid (22 : 5n-6; DPA). Subjects received 4 g/d of this microalgae oil for 4 wk (providing 1·5 g/d DHA and 0·6 g/d DPA) and showed increased plasma concentrations of arachidonic acid (ARA), adrenic acid, DPA and DHA, increased DPA and DHA in erythrocyte phospholipids, increased total, LDL- and HDL-cholesterol, and increased Factor VII coagulant activity. This oil was well tolerated, without adverse effects(Reference Sanders, Gleason and Griffin10).
Fish oil omega-3 fatty acids
The consumption of equal amounts of EPA and DHA from oily fish on a weekly basis or from fish-oil capsules on a daily basis was equally effective at enriching blood lipids with n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs)(Reference Harris, Pottala and Sands11). Fish oil n-3 LC-PUFAs are readily incorporated into the healthy heart and skeletal muscle membranes, and may reduce both whole-body and myocardial O2 demand during exercise, without a decrement in performance. Fish oil also increased n-3 LC-PUFA contents of erythrocytes(Reference Milte, Coates and Buckley12), lowered heart rate during incremental workloads to exhaustion, and lowered steady-state submaximal exercise heart rate and whole-body O2 consumption, but time to voluntary fatigue was not altered(Reference Peoples, McLennan and Howe13).
Adding DHA (fish-oils) to staple foods reduced CVD morbidity and mortality(Reference Harrison, Sagara and Rajpura14), and has been recommended after myocardial infarction. Increased fish or fish-oil consumption has been associated with reduced risk of cardiac mortality, especially sudden death, by means of membrane stabilization in the cardiac myocite, inhibition of platelet aggregation, favourable modifications of the lipid profile, and decrease in systolic and diastolic blood pressure, probably due to the shift of balance between vasoconstrictive and vasodilator eicosanoids toward vasodilatation and reduction of the inflammatory response of the endothelium. Fish oil showed a propranolol-like blood pressure-lowering effect. Plasma norepinephrine and thromboxane B2 formation were likewise reduced, whereas plasma renin activity increased(Reference Singer, Melzer and Goschel15). Dietary intervention with fish oil n-3 LC-PUFAs reduced platelet-monocyte aggregation, and suggested that reduced platelet activation provides a potential mechanism through which fish oils confer their cardiovascular preventative benefits(Reference Din, Harding and Valerio16), and reduces atherothrombotic risk in patients with hyperlipoproteinemia(Reference Pirich, Gaszo and Granegger17).
Daily intake of fish oil n-3 LC-PUFAs for 37 months decreased 16 % all causes of mortality and 24 % the incidence of death due to myocardial infarction. This benefit putatively arises from the incorporation of EPA and DHA into cardiomyocyte phospholipids at the expense of ARA during high-dose fish-oil supplementation(Reference Metcalf, James and Gibson18). Fish oil consumption decreased tumour necrosis factor-alpha (TNFα) production in healthy subjects and improves body weight in severe heart failure(Reference Mehra, Lavie and Ventura19). However, restenosis after coronary angioplasty was not reduced by supplemental fish oil(Reference Kaul, Sanghvi and Bahl20).
Consumption of fish has been associated with a significantly reduced progression of coronary atherosclerosis in women with coronary artery disease(Reference Erkkilä, Lichtenstein and Mozaffarian21). Atlantic salmon fillets very high in n-3 LC-PUFAs of marine origin seemed to impose favourable biochemical changes (reductions of serum triglycerides, vascular cell adhesion molecule-1 and interleuki n-6) in patients with coronary heart disease(Reference Seierstad, Seljeflot and Johansen22). Findings from short- and long-term randomized trials pointed out that fish n-3 LC-PUFAs intake are inversely related to blood pressure, either on hypertensive or nonhypertensive persons, with small estimated effect size(Reference Ueshima, Stamler and Elliott23).
After 6-year follow up, the age-adjusted models showed no evidence of an association between fish consumption or omega-3 fatty acid intake and incident of atrial fibrillation (AF) in a large sample of older, postmenopausal women (44 720 participants from the Women's Health Initiative clinical trials) who were not enrolled in a dietary modification intervention arm and without AF at baseline(Reference Berry, Prineas and van Horn24). Fish oil n-3 LC-PUFAs have not a protective effect on cardiac arrhythmia. Current data neither proved nor disproved a beneficial or a detrimental effect for subgroups of patients with specific underlying pathologies(Reference Brouwer, Zock and Camm25).
DHA and EPA rich fish-oil supplements taken with a high-fat meal preserved impairments in endothelial function(Reference Fahs, Yan and Ranadive26). There was no effect on cardiovascular biomarkers or mood in patients with ischemic stroke submitted to 12 wk of treatment with moderate-dose fish oil supplements (3 g/d fish oil containing 1·2 g total omega-3: 0·7 g DHA; 0·3 g EPA). It is possible that insufficient dose, short duration of treatment, and/or oxidation of the fish oils may have influenced these outcomes(Reference Poppitt, Howe and Lithander27).
Beneficial effects of fish oil n-3 LC-PUFAs on cardiac risk factors and heart rate variability have been also found in people with epilepsy(Reference DeGiorgio, Miller and Meymandi28). However, the administration of five fish oil capsules with every meal (1260 mg/d EPA and 540 mg/d DHA) in healthy middle-aged Japanese men with a high level of fish consumption for 4 weeks did not demonstrate a decrease in plasma TAG, cholesterol, LDL-cholesterol, and whole-blood viscosity. Further, no changes in the fatty acid composition of plasma and erythrocyte phospholipids were noted(Reference Watanabe, Watanabe and Kumagai29). A progressive and significant increase in total hyperhomocysteinemia was observed after 8 weeks of dietary supplementation with 6 g/d of fish oil. This increase was not associated with changes in plasma folate or vitamin B12 concentrations(Reference Piolot, Blache and Boulet30).
In comparison with corn oil, fish oil tended to increase HDL and decreased LDL concentration, and to decrease insulin sensitivity, but it has no effect on oxidized LDL(Reference Mostad, Bjerve and Lydersen31). Once-a-day intakes of plant sterol-enriched yoghurt drink (2 g plant sterols/d) and fish oil capsules (2 g/d fish oil n-3 LC-PUFAs) reduced 15 % TAG and increased 5·4 % HDL-cholesterol in mildly hypercholesterolaemic 35–55 y-o adults(Reference Khandelwal, Demonty and Jeemon32).
A 30-year follow-up survey of the Dutch and Finnish cohorts of the Seven Countries Study showed that an increase in the fish consumption was inversely related to glycaemia(Reference Feskens, Virtanen and Rasanen33). To take ≥ 1 versus < 1 portion/week of fish was associated with a lower risk of T2DM(Reference Patel, Sharp and Luben34). Moreover, the risk of T2DM in an elderly population was lowered by increased fish and n-3 LC-PUFAs consumption(Reference Feskens, Bowles and Kromhout35). However, a large epidemiological study of healthy adults showed that the relative risk of T2DM was slightly higher in women who consumed ≥ 5 servings fish/wk than those who consumed fish ≤ 1/mo, after adjustment by other dietary and lifestyle risk factors. The authors explained the results by the fact that toxins such as dioxins and methylmercury may interrupt insulin signalling pathways. The authors also hypothesized that n-3 LC-PUFAs may contribute to higher glucose concentrations through other mechanisms, i.e.: n-3 LC-PUFAs can decrease glucose utilization and increase glucagon-stimulated C-peptide, or increase hepatic gluconeogenesis(Reference Kaushik, Mozaffarian and Spiegelman36). Several clinical studies also reported that n-3 LC-PUFAs may worsen glucose tolerance and insulin resistance in T2DM patients who consumed large amounts of fish oil(Reference Friday, Childs and Tsunehara37–Reference Borkman, Chisholm and Furler40). It has been pointed out that these negative effects were due to the high doses of n-3 LC-PUFAs used, such as ≥ 10 g/d fish oil.
A prospective study of 36 328 women (mean age 54·6 y) who participated in the Women's Health Study (1992–2008) suggested an increased risk of T2DM with the intake of marine n-3 LC-PUFAs, especially with high intakes ( ≥ 0·2 g omega-3/d or ≥ 2 servings of fish/d)(Reference Djoussé, Gaziano and Buring41). However, unfavourable associations between marine n-3 LC-PUFAs intake and glucose control was not found(Reference Belalcazar, Reboussin and Haffner42). In healthy individuals a moderate supplementation of fish oil did not affect insulin sensitivity, insulin secretion, beta-cell function or glucose tolerance(Reference Giacco, Cuomo and Vessby43). Further, in a crossover study of subjects with T2DM, enrichment with fish oil n-3 LC-PUFAs failed to affect insulin sensitivity and secretion(Reference Mostad, Bjerve and Basu44), but another randomized crossover dietary intervention study with two 8-week periods reported that an increase in oily fish consumption increased insulin sensitivity in young iro n-deficient women(Reference Navas-Carretero, Pérez-Granados and Schoppen45).
Current evidence indicates that fish oil EPA and DHA can prevent the development of inflammatory diseases by affecting different steps of the immune response. DHA, but not EPA, suppresses T lymphocyte activation(Reference Kew, Mesa and Tricon46). The capacity of n-3 LC-PUFAs to modulate the synthesis of eicosanoids, activity of nuclear receptor and transcription factors, and production of resolvins, may also mitigate inflammatory processes already present. In a 8-wk intervention trial, 324 subjects (aged 20–40 years, and BMI 27·5–32·5 kg/m2) that took salmon (3 × 150 g/wk, 2·1 g/d LC-PUFA) or cod (3 × 150 g/wk, 0·3 g/d n-3 LC-PUFAs) or fish oil capsules (1·3 g/d n-3 LC-PUFAs) showed significant decreases in inflammation parameters (high-sensitivity C-reactive protein, interleuki n-6, glutathione reductase, and prostaglandin F2α), a mechanism by which PUFAs reduce CVD, but also they experienced weight loss ( − 5·2 ± 3·2 kg)(Reference Ramel, Martinez and Kiely47), and decreased diastolic and systolic blood pressure(Reference Ramel, Martinez and Kiely48). Similar results were obtained in subjects (35–70 years) after an 8-wk food-based intervention trial taking salmon, an oily fish(Reference Zhang, Wang and Li49). Dietary fish oil n-3 LC-PUFAs supplementation had a markedly protective effect in suppressing exercise-induced bronchoconstriction in elite athletes, which may be attributed to their antiinflammatory properties. Fish oil n-3 LC-PUFAs supplementation decreased leukotriene (LT)E4, 9α, 11β-prostaglandin F2, LTB4, TNFα, and interleukin-1β(Reference Mickleborough, Murray and Ionescu50).
n-3 LC-PUFAs are potentially useful anti-inflammatory agents. To intake fish oil 960 mg/d of EPA and 600 mg/d of DHA can decrease C-reactive protein levels(Reference Bowden, Wilson and Deike51). An 8-wk consumption of fatty fish decreased lipids which are potential mediators of lipid-induced insulin resistance and inflammation(Reference Lankinen, Schwab and Erkkilä52). Dietary n-3 fatty acids have been associated with lower levels of inflammation and endothelial activation, which may partially explain the effect of n-3 LC-PUFAs in preventing cardiovascular disease(Reference Lopez-Garcia, Schulze and Manson53).
Parenteral supplementation with fish oil n-3 LC-PUFAs emulsion decreased the magnitude and persistence time of the systemic inflammatory response syndrome (SIRS), markedly retrieve the unbalance of the pro-/anti-inflammatory cytokines, improve severe condition of illness and may provide a new way to regulate the SIRS(Reference Xiong, Zhu and Zhou54).
Fish oil n-3 LC-PUFAs reduced the requirement for nonsteroidal antiinflammatory drugs (NSAID) in patients with rheumatoid arthritis(Reference Lau, Morley and Belch55), and are a safer alternative to NSAID for treatment of nonsurgical neck or back pain(Reference Maroon and Bost56). Cod liver oil supplements containing n-3 LC-PUFAs may be used as NSAID-sparing agents in rheumatoid arthritis patients(Reference Galarraga, Ho and Youssef57). The combination of fish oil and paracetamol suppressed PGE2 synthesis by an amount equivalent to that from maximum therapeutic doses of NSAID, and enhanced suppression of nociceptive PGE2 synthesis and thereby provided additive symptomatic benefits(Reference Caughey, James and Proudman58). Asthma, another highly prevalent chronic inflammatory disease, may also positively respond to fish oil supplements(Reference Dry and Vincent59).
In spite of a high intake of fish oil, n-3 LC-PUFAs may be associated with decreased inflammation. A 12-wk randomized, double-blind placebo-controlled intervention trial in healthy subjects aged 50–70 years did not show that 3·5 g/d fish oil (1·5 g/d n-3 LC-PUFAs) significantly affected the serum inflammatory response (it did not significantly affect serum concentrations of cytokines, chemokines or cell adhesion molecules), nor did patterns of inflammatory markers(Reference Pot, Brouwer and Enneman60).
Fish oil n-3 LC-PUFAs blunted the endocrine stress response and the increase in body temperature, but had no impact on cytokine production after endotoxin challenge, which has been shown to mimic several aspects of sepsis. These findings conflict with the postulated anti-inflammatory effects of fish oil on ARA metabolism and cytokine release. These results suggest that fish oil may exert beneficial effects in sepsis though non-inflammatory(Reference Michaeli, Berger and Revelly61). However, the use of immunonutrition including fish oil in critical ill patients or patients with severe sepsis may exert an excess mortality. All of which require further research.
A high fish oil EPA and DHA intake (1·8 g EPA and DHA/d, 26 weeks) changed the expression of 1040 genes, and resulted in a decreased expression of genes involved in inflammatory- and atherogenic-related pathways, such as nuclear transcription factor kappaB signaling, eicosanoid synthesis, scavenger receptor activity, adipogenesis, and hypoxia signaling(Reference Bouwens, van de Rest and Dellschaft62).
Thirty six girls aged 18–22 years were supplemented 3 months with 15 mL fish oil daily (550 mg/d EPA; 205 mg/d) by means a cross-over clinical trial. They reduced symptoms of dysmenorrhoea, low back pain and abdominal pain, and needed significantly fewer rescue doses of ibuprofen while using fish oil(Reference Moghadamnia, Mirhosseini and Abadi63).
Pregnant women aged 18–41 years supplemented from week 22 with modified fish oil showed high thiobarbituric acid-reactive substances (TBARS), an oxidative stress index in lipids, at week 30, and minor changes of uric acid increased and beta-carotene as well as trolox-equivalent antioxidative capacity (TEAC) from week 20 to delivery. Fish oil n-3 LC-PUFAs supplementation improved infant neurological development, it causes additional increase of oxidative stress at week 30, but it also did not decrease antioxidant status during the second half of pregnancy(Reference Franke, Demmelmair and Decsi64). Maternal fish oil supplementation during pregnancy (2·2 g/d DHA and 1·1 g/d EPA from 20 weeks' gestation until delivery) was safe for the foetus and infant, and might have potentially beneficial effects on the child's eye and hand coordination(Reference Dunstan, Simmer and Dixon65).
Fish intake also plays a protective role in the development of allergic diseases in women because of its high n-3 LC-PUFAs contents. It is not understood why this association was only seen in females, but gender-related differences in metabolism of PUFA could be a possible explanation(Reference Schnappinger, Sausenthaler and Linseisen66). Supplementation of pregnant women with allergic disease with fish oil (3·7 g/d of n-3 LC-PUFAs) for the final 20 weeks of pregnancy decreased neutrophil LTB4 production, pro-inflammatory IL-6 responses and regulatory IL-10 responses by lipopolysaccharide-stimulated neonatal mononuclear cells, and a trend for less inflammatory products (LTB5) in neonates. It provides evidence that fish n-3 LC-PUFAs can influence early immune development(Reference Prescott, Barden and Mori67). Milk of lactating mothers supplemented with tuna oil had high DHA and ALA contents, which are important nutrients in the infant preterm diet(Reference Smithers, Markrides and Gibson68). The maximum DHA levels in human breast milk exceed 1 % of total fatty acids in high-fish-consuming populations. Consumption of DHA-rich human milk as sole source of nutrition provided approximately 315 mg/d in infants 1–6 months of age, and appeared to be a safe level of intake, without adverse events in infants. Daily maternal supplementation with either fish oil 1·6 g EPA and 1·1 g DHA or placebo in pregnant women affected by allergy themselves or having a husband or previous child with allergies from the 25th gestational week to average 3–4 months of breastfeeding, decreased the period prevalence of food allergy, as well as the incidence of IgE-associated eczema during the first year of life in infants with a family history of allergic disease(Reference Furuhjelm, Warstedt and Larsson69). The n-3 LC-PUFAs-status in late infancy affected heart rhythm in infants similar to that observed in adults, and influenced on brain development and CNS function, irrespectively of gender(Reference Lauritzen, Christensen and Damsgaard70).
Elderly people are susceptible to cardiovascular and neurological illnesses, which seem to be related in part to lower intake of n-3 fatty acids(Reference Fortier, Tremblay-Mercier and Plourde71). Furthermore, supplementation with high or low doses of fish oil n-3 LC-PUFAs for 26 weeks influenced neither the cognitive performance(Reference van de Rest, Geleijnse and Kok72), nor the quality of life of healthy older individuals, measured by means of the WHO's quality of life questionnaire(Reference van de Rest, Geleijnse and Kok73).
Subjects consuming fatty fish or with an intake of n-3 LC-PUFAs higher than 0·10 % of energy intake had a significantly low risk of depressive episode and of recurrent depressive episodes, but not of single depressive episode. These associations were stronger in men and in non-smokers, but smokers eating fatty fish had an increased risk of recurrent depression. Then, usual intake of fatty fish or n-3 LC-PUFAs may decrease the risk of recurrent depression in non-smokers(Reference Astorg, Couthouis and Bertrais74).
Few effects of n-3 LC-PUFAs on cognition and mood states, few risk-averse decisions, and improved scores on the control/perfectionism scale of the cognitive reactivity measure have been also found, but no effects on other cognitive tasks(Reference Ramel, Parra and Martinez75). A randomized, double-blind, placebo-controlled trial did not observed effect of EPA and DHA supplementation for 26 wk on mental well-being in older ( ≥ 65 years) population(Reference van de Rest, Geleijnse and Kok76). Eating oily fish at least once per week were associated with a reduction of neovascular age-related macular degeneration(Reference Augood, Chakravarthy and Young77).
Incorporating a daily fish meal rich in n-3 LC-PUFAs into a weight-loss regimen was more effective than either measure alone at improving glucose-insulin metabolism and dyslipidemia, and also reduced cardiovascular risk(Reference Mori, Bao and Burke78). Controlled trials using whole fish as a test meal were encouraged to be able to elucidate the role of different constituents of fish for human health(Reference Gunnarsdottir, Tomasson and Kiely79). Validated visual analogue scale assessment revealed low hunger sensations in volunteers (31 ± 5 years; BMI 28·3 ± 1·5 kg/m2) after an intervention (>1300 mg/d of n-3 LC-PUFAs) on the last 2 wk of an 8-wk energy-restricted balanced diet (weight loss = − 5·9 ± 3·1 %). Therefore, n-3 LC-PUFAs seems to modulate postprandial satiety in overweight and obese volunteers during weight loss, and may be considered nutritional factors with a potential to modulate food intake(Reference Parra, Ramel and Bandarra80). However, a controlled randomized dietary trial showed that dietary n-3 LC-PUFAs do not play an important role in the regulation of food intake, energy expenditure, or body weight in humans(Reference Kratz, Callahan and Yang81).
The sunburn response is markedly reduced by dietary fish oil rich in n-3 LC-PUFAs. Reduction of UV-induced inflammation by fish oil may be due, at least partially, to lowered PGE2 levels, suggesting a clinical application for fish oil n-3 LC-PUFAs(Reference Rhodes, Durham and Fraser82).
Treatment of antiretroviral treated HIV-infected patients with fish oil n-3 LC-PUFAs slightly decreased plasma TAG and induced anti-inflammatory effects by increasing formation of anti-inflammatory LTB5. No other changes were observed(Reference Thusgaard, Christensen and Mørn83).
Some in vitro and animal studies have suggested an inhibitory effect of marine n-3 fatty acids on breast cancer growth, but no significant associations between intake of total fish and breast cancer risk were observed in 310 671 women aged 25–70 years at recruitment into the European Prospective Investigation Into Cancer and Nutrition(Reference Engeset, Alsaker and Lund84). Oral nutritional supplement containing fish oil 2·0 g/d EPA and 0·9 g/d DHA had immune-modulating effects and could improve nutritional status in patients with non-small cell lung cancer (NSCLC) undergoing multimodality treatment(Reference van der Meij, Langius and Smit85). A combination of fish oil n-3 LC-PUFAs and cyclooxygenase-2 inhibitor decreased some of the signs and symptoms associated with a Systemic Immune-Metabolic Syndrome (i.e.: paraneoplastic hemopathies, hypercalcemia, coagulopathies, fatigue, weakness, cachexia, chronic nausea, anorexia, and early satiety among others) could be ameliorated(Reference Cerchietti, Navigante and Castro86). Fish oil EPA-enriched supplement (1·09 g/d) may reverse cachexia in advanced pancreatic adenocarcinoma, and showed weight-gain at both 3 (1 kg) and 7 weeks (2 kg)(Reference Barber, Ross and Voss87). Increased intakes of dietary ALA may increase the risk of advanced prostate cancer, whereas EPA and DHA intakes may reduce the risk of total and advanced prostate cancer(Reference Leitzmann, Stampfer and Michaud88).
Until now, we have listed a number of studies that have clearly remarked the benefits of fish oil n-3 LC-PUFAs. However, some concerns about potential health risks derived from the environmental pollutants and contaminants found in fish have been also raised. One of the most dangerous contaminants is methylmercury (MeHg). Mercury is emitted into the atmosphere from several sources. From the atmosphere, mercury cycles from rainwater into lakes and oceans, where it is converted by the action of microorganisms into organic MeHg, which is well absorbed and actively transported into tissues by a widely distributed carrier protein(Reference Mozaffarian89, 90). The concentration of MeHg in any given fish species depends on the degree of local environmental contamination and on the predatory nature and lifespan of the species. The concentration of MeHg in fish is increased by fish eating other fish for food. Fish that are not predatory, shorter-lived or smaller species, such as sardines, salmon, flounder, canned light tuna and shrimp, therefore have very low levels of MeHg. By contrast, longer-living and predatory fish such as shark, tuna, swordfish and orange roughly have higher levels of MeHg. Interestingly, the much-maligned farmed fish have the lowest levels of MeHg. Although MeHg per se is very neurotoxic, in fish MeHg is bound to cysteine, and this compound has a tenth of the toxicity of pure MeHg(Reference Jeejeebhoy91, Reference Harris, Pickering and George92). MeHg can bind to the sulfhydryl groups of enzymes, ion channels, and receptors, inhibiting important antioxidant systems and increasing the production of reactive oxygen species and free radicals(90, 93). Health effects of very high doses of MeHg exposure are well-documented and include paresthesias, ataxia, and sensory abnormalities in adults, and delayed cognitive and neuromuscular development in children following in utero exposure(90, Reference Gochfeld94). MeHg crosses the placenta, and exposure to the fetus is a function of maternal exposure(95). Following very high gestational exposure, severe neurodevelopmental abnormalities can occur in children. However, the health effects of chronic low level mercury exposure are scarcely well-established.
Estimated n-3 LC-PUFAs benefits outweighed cardiovascular and neurodevelopmental MeHg risks for some species (farmed salmon, herring, trout); however, the opposite was true for others (swordfish, shark). Other species were associated with a small net benefit (flounder, canned light tuna) or a small net risk (canned white tuna, halibut)(Reference Ginsberg and Toal96).
More typical MeHg exposures from fish consumption are far lower. Among US women of childbearing age, the median levels of hair mercury were 0·19 ppm overall, and 0·34 ppm among women who consumed more than three servings of fish per month(Reference McDowell, Dillon and Osterloh97). These low exposure levels do not produce clinically detectable neurologic symptoms or signs in children. In studies in the Faroe Islands(Reference Grandjean, Weihe and White98, Reference Grandjean, Weihe, White and Debes99), New Zealand(Reference Kjellstrom100, Reference Crump, Kjellstrom and Shipp101), and Poland(Reference Jedrychowski, Jankowski and Flak102), higher gestational mercury exposure was associated with lower scores on some neurologic tests, but not on most of them. In the Seychelles, however, higher gestational MeHg exposure was associated with higher scores on some neurologic tests(Reference Davidson, Palumbo and Myers103, Reference Palumbo, Cox and Davidson104). Maternal fish intake during gestation was associated with better visual recognition memory scores, while maternal hair mercury was associated with lower visual recognition memory scores(Reference Oken, Wright and Kleinman105), suggesting that overall fish consumption (which provides DHA, likely beneficial for neurodevelopment) and MeHg exposure may have opposing effects. Gestational mercury exposure was not associated with neurodevelopmental scores, but it was associated with better neurodevelopmental scores in other human populations(Reference Daniels, Longnecker and Rowland106).
It should be useful in establishing advisories for a wide variety of commercially available and locally caught fish, assuming that the requisite MeHg and n-3 LC-PUFAs data are available(95, 107–112). This caution should be extended to other foods fortified with fish oil n-3 LC-PUFAs, such as eggs and milk. However, exceeding the tolerable daily intake was just noticed for heavy seafood consumers. Wild and farmed fish are generally both similar in n-3 LC-PUFAs contents but may vary in terms of potential toxins, but they affected proteins and not fatty acids.
Accordingly, the Environmental Protection Agency published a focused advisory for women of childbearing age, nursing mothers, and young children(Reference Rice113). The allowable upper limit of daily intake, for methylmercury of 0·1 μg/kg per d (approx. 50 μg/week for a 70 kg woman)(95). Four fish species (shark, swordfish, king mackerel, and tilefish) exceed this limit in a single serving. So, women of childbearing age, nursing mothers, and young children should avoid these specific species, but they could consume a variety of other fish up to 2 servings/week (including up to 1 serving/week of albacore tuna) to receive the important health benefits(112). The US Institute of Medicine recommended that pregnant women restrict their intake of fish with a higher MeHg content (shark, tuna, or swordfish) to 1 meal per 2 weeks; however, these women can eat 2–3 meals of other fish per week (sardines, salmon, or shrimp)(Reference Jeejeebhoy91). The importance of this conservative reference dose for health effects in adults remains still unclear(Reference Rice113).
The results of studies of mercury exposure and cardiovascular disease incidence in adults provide inconclusive evidence for cardiovascular toxicity of mercury exposure. Of note, in the only two studies that observed positive associations between mercury exposure and cardiovascular risk, the net effect of fish consumption was still beneficial(Reference Rissanen, Voutilainen and Nyyssonen114–Reference Virtanen, Voutilainen and Rissanen116).
Sensorimotor symptoms in adults, most commonly paresthesias, can be seen following very high methylmercury exposure from accidents(90, Reference Gochfeld94, Reference Risher, Murray and Prince117) or prolonged high intakes of mercury-containing fish (1–2 fish servings per day, including species high in mercury, for >10 years)(Reference Xiong, Zhu and Zhou54). Such symptoms are typically reversible when mercury exposure is reduced. Evidence suggests that fish consumption may favorably affect clinical neurologic outcomes in adults, including ischemic stroke(Reference He, Song and Daviglus118), cognitive decline and dementia(Reference Morris, Evans and Tangney119), and depression and other neuropsychiatric disorders(Reference Peet and Stokes120, Reference Young and Conquer121).
Other potential contaminants in fish such as dioxins and polychlorinated biphenyls could potentially increase the risk of cancer. An analysis of the potential harmful effects of these contaminants in fish versus the benefits of omega-3 fatty acids has, however, concluded that the levels of dioxins and polychlorinated biphenyls in fish are low, and potential carcinogenic and other effects are outweighed by potential benefits of fish intake(Reference Mozaffarian89, Reference Mozaffarian and Rimm122).
To sum up, the balance of benefit vs. risk is most favourable for oily fish species which contain higher amounts of n-3 LC-PUFAs, compared with lean fish, which are generally lower in n-3 LC-PUFAs.
Plant omega-3 fatty acids
To achieve recommended alpha-linoleic acid (ALA) intakes, food sources including flaxseed and flaxseed oil, walnuts and walnut oil, and canola oil are recommended. Short-term trials (6–12 wk) in healthy participants mostly showed no or inconsistent effects of ALA intake (1·2–3·6 g/d) on blood lipids, LDL oxidation, lipoprotein A, and apolipoproteins A-I and B. There was a protective effect against nonfatal myocardial infarction(Reference Kaul, Kreml and Austria123–Reference Dodin, Cunnane and Mâsse128). However, no protective associations were observed between ALA status and risk of heart failure, atrial fibrillation, and sudden death(Reference Campos, Baylin and Willett129–Reference Lemaitre, King and Sotoodehnia134). Dietary ALA and EPA+DHA had different physiologic effects on fasting TAG concentrations, and susceptibility of LDL to oxidation(Reference Finnegan, Minihane and Leigh-Firbank135). Findings from long-term trials of ALA supplementation were awaited to answer the question whether food-based or higher doses of ALA could be important for cardiovascular health in cardiac patients and the general population. ALA derived from plant sources decreased the risk for mild dementia among elderly people(Reference Malgeunsinae, Jung Hyun and Dong Hoon136). Plant sources of dietary n-3 LC-PUFAs may have a protective effect on bone metabolism via a decrease in bone resorption in the presence of consistent levels of bone formation(Reference Griel, Kris-Etherton and Hilpert137).
Flaxseed is a rich source of ALA (35 % of its mass as oil, of which 55 % is ALA), fibre and lignans, making it a potentially attractive functional food for modulating cardiovascular risk. Flaxseed oil intake increases ALA and EPA plasma levels, but not DHA, did not affect glycaemia(Reference Taylor, Noto and Stringer138), had an hypotensive effect(Reference Paschos, Magkos and Panagiotakos139), a modest but short lived LDL-cholesterol lowering effect, yet reduced lipoprotein A, improved insulin sensitivity in hyperlipidemic adults(Reference Bloedon, Balikai and Chittams127), had no effect on plasma adiponectin concentration in dyslipidemic men(Reference Paschos, Zampelas and Panagiotakos140), did not affect serum lipids, except for a slight reduction in serum TAG, did not decrease CVD risk by altering lipoprotein particle size or plasma concentrations, and did not compromise antioxidant status(Reference Harper, Edwards and Jacobson141, Reference Cunnane, Hamadeh and Liede142). Flaxseed oil did not have antioxidant activity except they suppressed oxygen radical production by white blood cells. An intake of ≤ 9·5 g/d flaxseed oil ALA did not alter the functional activity of neutrophils, monocytes, or lymphocytes, but it changed the fatty acid composition of mononuclear cells. Flaxseed oil ALA doses ≤ 14 g/d did not affect inflammatory mediators/markers, but ≥ 14 g/d reduced inflammatory mediators/markers and platelet aggregation, and increased platelet activating inhibitor-1 and bleeding time(Reference Kew, Banerjee and Minihane143). Therefore, flaxseed and its components improve cardiovascular health. Fibre contents of flaxseed increased bowel movements per week(Reference Cunnane, Hamadeh and Liede142), and suppression of atherosclerosis wa just due to its lignan content(Reference Kew, Banerjee and Minihane143).
Feeding healthy term infants' soy-based formula DHA and ARA supplemented at concentrations similar to human milk significantly increased circulating levels of DHA and ARA in total red blood cells and plasma phospholipids. Supplementation did not affect the tolerance of formula or the incidence of adverse events(Reference Hoffman, Ziegler and Mitmesser144).
Dietary intake of rapeseed ALA, EPA or DHA for 3 weeks led to a significant enrichment these fatty acids in the LDL particles, with dietary EPA preferentially incorporated. ALA enrichment did not enhance LDL oxidizability, whereas the effects of EPA and DHA on LDL oxidation were inconsistent, possibly in part due to further changes in LDL fatty acid composition(Reference Egert, Somoza and Kannenberg145).
Omega-3 fatty acids enriched dairy products
The consumption of 500 mL/d for 6 wk of an enriched semi-skimmed milk (400 mg of EPA and DHA) decreased TAG and increased HDL-cholesterol serum levels(Reference Visioli, Rise and Plasmati146). An 8-wk supplementation of 500 mL/d enriched semi-skimmed dairy products (60 mg/100 mL EPA and DHA) decreased LDL-cholesterol and TC serum levels(Reference Baró, Fonollá and Peña147, Reference Carrero, Baró and Fonollá148). The consumption of 3 g/d n-3 LC-PUFAs-supplemented dairy products for fifteen weeks decreased cardiovascular risk factors (TC, TAG, high HDL-cholesterol, low LDL/HDL ratio)(Reference Dawczynski, Martin and Wagner149). The consumption of n-3 LC-PUFAs milkshake providing 2·0 g EPA and 2·7 g DHA (ratio 2:3) had an attenuating effect on augmentation index and stiffness index(Reference Chong, Lockyer and Saunders150). Seven-month consumption of 500 mL/d of a PUFA enriched dairy drink (60 % olive oil, 20 % peanut, and 20 % sunflower), containing a quarter of the saturated fat present in standard whole milk, decreased serum levels of total cholesterol and LDL-cholesterol, without reducing caloric intake, in 3–9 year-old children(Reference Estévez-González, Saavedra-Santana and Betancor-León151). These effects were not observed after administration of EPA and DHA capsules(Reference Cobiac, Clifton and Abbey152), showing that the vehicle of administration (milk) also plays a role in the produced effects.
The consumption of a PUFA enriched dairy 500 mL/d of the test milk for 1 year in 297 25–65 y-o subjects with moderate CV risk increased serum HDL-cholesterol levels, and decreased TG, TC, and LDL-cholesterol(Reference Fonollá, López-Huertas and Machado153). When this intervention was carried out in patients with peripheral vascular disease, TC apolipoprotein B levels decreased, mainly in patients with high cholesterol values, but also increased the walking distance before the onset of pain, a method to measure the intensity of this illness(Reference Carrero, López-Huertas and Salmerón154). Similar results were obtained in patients with history of myocardial infarction(Reference Carrero, Fonollá and Marti155).
Finally, 3-month consumption of 186 mg/d EPA and DHA in skimmed milk reduced TC, LDL-cholesterol, and TAG serum levels(Reference Benito, Caballero and Moreno156). The average inclusion of 300 mg of EPA and DHA in the milk produced 25–50 % enrichment in the plasma levels of the fatty acids after a minimum period of 6 weeks, because milk is a very efficient carrier for fat absorption, enhancing the bioavailability of n-3 LC-PUFAs(Reference Lopez-Huertas7, Reference Cunnane, Hamadeh and Liede142, Reference Kew, Banerjee and Minihane143, Reference Benito, Caballero and Moreno156). The intake of ALA, EPA or DHA-supplemented margarine led to a significant enrichment of the LDL with the respective n-3 LC-PUFAs. ALA, EPA, or DHA intake did not affect fasting serum concentrations of total and LDL-cholesterol, but fasting serum TAG concentrations significantly decreased. DHA intake significantly increased serum HDL cholesterol, whereas no changes were found with ALA or EPA intake(Reference Egert, Kannenberg and Somoza124).
These intervention studies in patients show that the inclusion of n-3 LC-PUFAs enriched dairy products in the usual dietary pattern increases the ability to control the CVD risk factors, and also improve clinical outcomes.
Animal-derived food omega-3 fatty acids
Poultry meat contributes small but worthwhile amounts of EPA and DHA. Studies on EPA and DHA contents of animal-derived foods mainly use fish oil to enrich these diets. This enrichment has the potential to provide a daily intake of EPA and DHA of about 230 mg to the Western adult diet, with poultry meat providing the largest amount (74 mg)(Reference Givens and Gibbs157). A significant increase in n-3 LC-PUFAs levels in beef from cattle fed rations supplemented with flaxseed has been demonstrated(Reference Kronberg, Barcelo-Coblijn and Shin158).
Available literature indicates that the levels of EPA and DHA in food products may be increased more, if the animals' diet was supplemented with fish products rather than seed products. Sometimes, organoleptic properties of food products may be compromised. It has been suggested that omega-3 fatty acids may be enriched in pork by feeding swine with tuna oil, but sensory properties and shelf life decreased(Reference Kjos, Skrede and Overland159, Reference Jaturasitha, Khiaosa-ard and Pongpiachan160). However, adverse effects could not appear, i.e. addition of fish oils to Bruehwurst sausages increased the n-3 LC-PUFAs contents without changes on sensory properties, and just showed off-flavours, not always described as ‘fishy’(Reference Muench and Watzl161).
A standard egg contains a ratio of n-3 LC-PUFAs to total fat less than 1 %. By feeding laying hens with grains, soybean and ?axseed rich in ALA, n-3 LC-PUFAs content per egg can be increased to 6 times than the standard eggs. Three n-3 LC-PUFAs-enriched eggs provided approximately the same amount of n-3 PUFA as one meal with fish(Reference Cachaldora, Garcia-Rebollar and Alvarez162). Consumption of n-3 LC-PUFAs-enriched eggs reduced systolic blood pressure, but had no effect on BMI, WHR, waist circumference and diastolic blood pressure, with no change in the daily intake of energy, protein, carbohydrate, total fat, SFA and MUFA, but increased PUFA and TC blood levels, and decreased plasma fasting insulin and CRP levels. Reasonable consumption of n-3 LC-PUFAs enriched eggs (hen feed supplemented at 5 % tuna oil, and enriched eggs contained nine times more n-3 PUFA than usual eggs, mainly DHA) was associated with a significant decrease in 16–18 % decrease in serum triglycerides, but with no significant difference in serum LDL- and HDL-cholesterol. These eggs could be a palatably acceptable source of n-3 LC-PUFAs(Reference Bovet, Faeh and Madeleine163). Feeding hens with microalgae-rich diet, an improvement in DHA contents was obtained, avoiding unpleasant flavours associated with fish oil supplementation(Reference Rizzi, Bochicchio and Bargellini164).
It is interesting, however, to know the impact of the chow formulation used on farms and breeding centres on the nutritional value of the animal products, and their effect on the health of consumers. The consequences of modifications in the composition of animal foods on the value of derived products consumed by humans are more marked when single-stomach animals are concerned than multi-stomach animals, because hydrogenating intestinal bacteria of the latter group transform a large proportion of PUFA in their food into SFA, among others, thus depriving them of any biological interest(Reference Bourre165).
Krill oil omega-3 fatty acids
Antarctic krill, Euphausia superba, is a marine crustacean that has not been a traditional food in the human diet. Krill is a rich source of high-quality protein, with the advantage over other animal proteins of being low in fat and a rich source of EPA and DHA. Antioxidant levels in krill are higher than in fish, suggesting benefits against oxidative damage. Finally, the waste generated by the processing of krill into edible products can be developed into value-added products(Reference Tou, Jaczynski and Chen166).
Plasma EPA and DHA concentrations increased significantly, and blood urea decreased after overweight and obese men and women received capsules containing 2 g/d of krill oil for 4 weeks. Nor other changes, neither adverse effects were detected(Reference Maki, Reeves and Farmer167). Patients treated 3 mo with 1 g/d and 1·5 g/d krill oil demonstrated that krill oil is effective for the management of hyperlipidemia by significantly reducing total cholesterol, LDL, and triglycerides, and increasing HDL levels. At lower and equal doses, krill oil was significantly more effective than fish oil for the reduction of glucose, triglycerides, and LDL levels(Reference Bunea, El Farrah and Deutsch168). Neptune Krill Oil may significantly reduced dysmenorrhea and the emotional symptoms of premenstrual syndrome and showed to be significantly more effective than omega-3 fish oil(Reference Sampalis, Bunea and Pelland169).
Seal oil omega-3 fatty acids
Seal oil supplementation in healthy, normocholesterolemic subjects decreased the n-6/n-3 ratio and increased EPA, DHA, and DPA and the ratio of EPA/AA and DHA/AA in the serum, while exhibited a modest beneficial effect on fibrinogen and CRP levels(Reference Conquer, Cheryk and Chan170). No change was observed in body weight, fasting blood glucose, renal function and blood cells of patients with nonalcoholic fatty liver disease associated with hyperlipidemia after an intervention with 2 g n-3 LC-PUFAs from seal oils, three times a day, 24 wk. Liver alanine aminotransferase and TAG blood levels decreased after the intervention. Fatty liver regression was observed in 19·7 % of the patients, and an overall reduction was found in 53·0 %. No serious adverse events occurred in all the patients who completed the treatment(Reference Zhu, Liu and Chen171).
Discussion
In this review, findings were classified according to the dietary source of the omega-3 fatty acids, and their benefits and the risks for the public health.
Algal omega-3 fatty acids are DHA and DPA, and their main effects are a decrease of TAG and VLDL and a slightly increase of HDL and LDL-cholesterol plasma levels, as well as Factor VII coagulant activity. Up to date, no adverse effects have been observed.
Fish oils are the most common source of source of omega-3 fatty acids, mainly EPA and DHA. It has been pointed out protective and beneficial effects of these fatty acids on hearth health, CVD, blood lipid profile, T2DM, inflammatory and renal diseases, maternal and child health, CNS function, elderly, psychiatric disorders, several cancers, and other illnesses. Several studies suggested an increased risk of T2DM with the intake of marine n-3 LC-PUFAs, especially with higher intakes. Another potential health risk derived from the environmental contaminants found in fish.
Plant omega-3 fatty acids are the main source of ALA, which increases blood DHA and ARA levels, improves insulin sensitivity, has a very small hypotensive effect, and a protective effect on bone metabolism. Other benefits are still inconsistent. The main question is whether dietary intake of ALA can provide enough EPA and DHA amounts.
Enriched dairy products are a good vehicle to provide omega-3 fatty acids. The benefits are addressed to improve the blood lipid profile, arterial stiffness, inflammation, and oxidative stress markers, and to decrease CVD risks. No adverse effects have been yet described.
Animal-derived food omega-3 fatty acids contribute to EPA and DHA levels. Enriched eggs are one of the most common sources of animal-derived food omega-3 fatty acids. The benefits and risks on the public health depend on the chow formulation used in farms, and the type of fats fed by the animals. The only adverse effects may be decreased meat sensory properties and shelf life.
Krill is a rich source of high-quality protein, also low in fat and a rich source of EPA and DHA. The benefits are effects against oxidative damage, increase of HDL, EPA and DHA blood levels, decrease of LDL, TAG, and urea levels, as well as dysmenorrhea and premenstrual symptoms, and the waste generated by its processing into edible products can be developed into value-added products. No adverse effects have been described.
Seal oil contributes to increase EPA, DHA, DPA, and TAG blood levels. No adverse effects have been described. and disclosures
Acknowledgements and disclosures
The preparatory meetings for this series of reviews on fat and health were funded by Puleva Food. Neither Josep A. Tur nor Maria del Mar Bibiloni, Antoni Sureda or Antoni Pons have conflicts of interest to disclose. Josep A. Tur and Maria del Mar Bibiloni contributed to the design of the strategy for the literature search, double screened and selected the retrieved documents. Authors acknowledge Angel Gil from the University of Granada the support provided to select and retrieve several documents. Antoni Sureda and Antoni Pons provided previous literature searches and analysis. Josep A. Tur prepared the main outline of the manuscript and all authors contributed to the preparation of the manuscript.




