INTRODUCTION
Parasites are known to be a threat to public health and as important constraints to productivity and performance in animal production [Reference Hall and Wall1–Reference Ruiz3]. Myiasis is a parasitic infestation of vertebrate animals and humans with the larval stages of true flies (Diptera). Myiasis has been known to occur at various sites of the body, such as the eyes, intestines, mouth, nose, urogenital tract and brain, where they survive by feeding on dead or living tissues, ingested food or liquid body substances [Reference Ogo2]. Female flies are attracted by a variety of organic compounds like sandy soils, rubbish, sod contaminated by urine or animal faeces, and skin injuries on animals [Reference Horen4, Reference Rice and Gleason5]. The females lay their eggs in wounds or the sleeping places of the animals, especially on straw, litter and sometimes on urine-smelling clothing [Reference Ockenhouse6]. There are various types of myiasis [Reference Palmieri, North and Santo7] due to various fly species: (a) cutaneous myiasis, the most frequent form, occurs after penetration by dipteran larvae into healthy skin. Subdivisions of cutaneous myiasis include furuncular myiasis in which a furuncle-like nodule develops with one or more maggots in it, and migratory myiasis in which the maggots migrate aimlessly through burrows in the skin and produce numerous furuncular lesions; (b) wound myiasis occurs when maggots infest open wounds such as neuropathic or vascular insufficiency ulcers, basal cell carcinoma, and psoriasis skin; (c) myiasis of body cavities is characterized by the invasion of body organs such as orbit, mouth, external ears, nasal cavities, nasopharynx, vulva, vagina, bladder, lungs, or intestines by fly larvae. Cordylobia anthropophaga (also referred to as ‘tumbu fly’, ‘mango fly’, ‘skin maggot fly’, ‘putzi fly’ or ‘vers de cayor’) is endemic in Sub-Saharan Africa [Reference Veraldi, Brusasco and Suss8], causing furuncular myiasis in domestic animals [Reference Ogo9–Reference Kouam11] and humans [Reference Dehecq12–Reference Kovaleva14].
Small rodents and dogs are considered as important reservoir hosts for the larvae of C. anthropophaga [Reference McGraw and Turiansky15]. Thus, the increase in adoption of rodents such as guinea pigs (cavies) as farm animals rather than as pets or laboratory animals, is raising concern about the potential emergence of myiasis as a result of C. anthropophaga infestation in areas where cavy farming is fast growing. The concern is related to emergence of myiasis both as an occupational disease of cavy breeders, and as an epizootic in the cavy population. In a recent study in rural areas of the western highlands of Cameroon, C. anthropophaga was reported as one of the main parasites occurring in domestic cavies [Reference Kouam11]. This parasite, with human and animal associations, is an important target of the One Health approach whose main goal is to minimize the impact of diseases of animal origin [Reference Pastoret and Vallat16]. The One Health initiative promotes agricultural practices that prevent animal, human and environmental infections. Therefore, the objectives of this study were: (1) to determine the prevalence of myiasis induced by C. anthropophaga in an area where cavy breeding is fast growing in Cameroon; and (2) to determine the agricultural related risk factors associated with this infestation.
MATERIALS AND METHODS
Study area and farms
The study was carried out between June and July 2014 in the western highlands of Cameroon. The highlands are an agro-ecological zone covering the North-West and West administrative regions of the country, located between latitude 5° 20′–7° North and longitude 9° 40′–11° 10′ East. The North-West and West regions comprise the largest cavy production zone of the country, and accounted for 94·10% of the national stock in 2012 [17]. The region is characterized by a high relief, and the climate is of Sudano-Guinean type. It has one rainy season, which lasts from mid-March to mid-November and one dry season from mid-November to mid-March. Humidity varies from 80% to 98%. Annual precipitation ranges from 1500–2500 mm while minimum and maximum temperature are 10 °C and 34 °C, respectively [Reference Bayemi18]. Originally, the vegetation of this region was of the savannah type but over the years due to intense crop production and animal rearing it has been transformed to a semi-degraded or degraded forest type. Nevertheless, the original vegetation can be observed in certain parts of the region which is characterized by an increasing human population growth, one of the highest in the country.
The study was conducted in privately owned farms in rural areas of the Menoua and Bamboutos border divisions (Fig.1). The farms are owned by small-scale farmers who also rear rabbits, sheep, goats, or local breeds of fowl. The housing system is either the raised floor system or in most cases, the traditional free-roaming kitchen system; in the latter system, cavies share the kitchen floor with the local fowl and/or small ruminants, and feed on kitchen waste, forage and occasionally concentrates. Forages are harvested for free as part of the natural vegetation in the compound. The breeding system is essentially semi-extensive. There are very few cases of cavy keeping being intensive. In the area, 45% of farmers keep cavies as an additional source of income, 30% for manure production for backyard crop production, 20% for meat, and 5% as pets [Reference Yiva19].

Fig. 1. Map of the West region of Cameroon showing the two divisions where sampling was carried out (Bamboutos and Menoua).
Study design and sample collection
There is no central registry of farms in Cameroon so private farms were located and visited using a snowball sampling technique whereby a farmer, when located helped to locate the next farm and so on. The animal sample size was determined based on the formula for sample size calculation [Reference Daniel20] as follows: n = Z 2 P(1 – P)/d 2, where n is the required sample size, Z is the normal deviate (1·96) at the 5% level of significance, P is the estimated prevalence of infection in cavies (50%) in absence of previous data, and d is the allowable error of estimation or precision (0·05). Thus, the computed sample size (n) was determined as 385. In this cross-sectional study, farms were visited once during which cavies were manually handled and carefully checked for any sign of Cordylobia ectoparastism. An animal was regarded as infested if after a careful and close inspection all over the body, there were characteristic furuncular lesion(s) recognizable by the posterior tip of the larva barely visible at the centre. The larvae from suspicious animals were expressed with finger pressure, then preserved in 80% ethanol and stored at room temperature (19–22 °C). When the total number of animals per farm was >5, five animals were randomly sampled by haphazardly capturing cavies one by one until a total of five was reached but when the number was <5, all the animals were sampled. In order to understand the risk factors that impact the prevalence of myiasis, animal, farm and farmer-related data were collected through a questionnaire survey (see Supplementary material). The questionnaire was administered directly to the farmer (mostly women), and the survey was conducted in French.
Identification of skin larvae
The name of the species was known already, as identified in our previous study a few months earlier [Reference Kouam11]. Briefly, the skin larvae were identified to the genus and species level based on their morphology, using a stereomicroscope (up to 100× magnification) and following the identification key provided by Zumpt [Reference Zumpt21], and Erzinclioglu [Reference Erzinclioglu22]. The larvae were distinguished and identified from other species of the genus Cordylobia, by focusing on the posterior spiracular apertures, the spines and the mouth hook. The characteristic openings (n = 3) of the posterior spiracles of C. anthropophaga are slightly curved while those of C. rodhaini are in complex curves [Reference Erzinclioglu22].
Ethical considerations
Animals were handled humanely during sample collection, and procedures complied with international laws.
Statistical analysis
Data were analysed using descriptive statistics. The χ 2 and the Exact tests were used to test the association between parasite infestation and various factors. Exact tests were used when an expected value was <5 by χ 2 analysis. The relative risk (RR) for the presence of myiasis was computed for any factor having more than two levels. A P value of <0·05 was considered significant. All statistics were performed using both the SPSS statistical package (version 13.0, SPSS Inc., USA) and Epi Info software (version 3.5.1, CDC, USA).
RESULTS
In total, 397 animals from 123 farms were sampled: 256 animals in 78 farms from Menoua Division, and 141 animals from 45 farms in Bamboutos Division. The overall prevalence of myiasis in animals was 2·80% [95% confidence interval (CI) 1·50–5·10]; myiasis was found in five (2%) and six (4·30%) animals in the Menoua and Bamboutos divisions, respectively (Table 1). Eleven farms (8·90%) in total were infested with C. anthropophaga, with five (6·40%) and six (13·3%) from the Menoua and Bamboutos divisions, respectively. Application of pressure to observed lesions (furuncle) led to larva expulsion (Fig. 2), with liquefied haemorrhagic or purulent tissue (Fig. 3). Lesions were not localized to a particular site; they were common in the limbs but could be found in areas around the genitals, the belly and the neck.

Fig. 2. Larvae expelled from a cavy's leg; note the sinuses (short arrows) after larvae extraction.

Fig. 3. Circular ulcerous furuncular lesion with haemorrhagic and purulent tissue after expulsion of a larva. The lesion is shown by the animal's owner with open hands. Note: it is common practice for farmers in the study area to remove the larvae from an infested cavy without wearing gloves.
Table 1. Relative risk of C. anthropophaga infestation for different risk factors
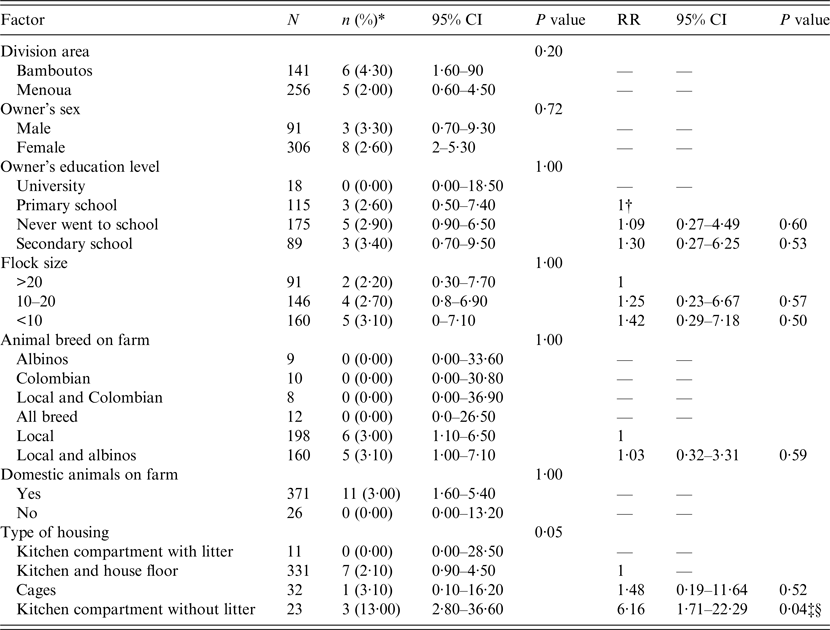
n, Number of positive animals; CI, confidence interval; RR, relative risk.
* Prevalence.
† For a given factor, the RR of 1 indicates the reference level being compared to other levels of the factor.
‡ Significant factor.
§ Factor significant even after Bonferroni correction.
Investigation of the RR of infestation within each factor having more than two levels showed that for the housing type factor, the risk of myiasis in animals kept in kitchen compartments without litter (Fig.4) was 6·16 times higher (95% CI 1·71–22·29, P = 0·04) than in animals kept in kitchens and house floors (Table 1).

Fig. 4. Types of housing: (a) kitchen compartment with litter (the litter is covered with forage); (b) kitchen compartment without litter; (c) kitchen floor; (d) cage.
DISCUSSION
Myiasis due to C. anthropophagi is known to occur in humans and other vertebrate animals. In the latter situation, cases have been reported in large mammals as well as rodents [Reference McGraw and Turiansky15, Reference Devienne, Bobard and Pinhas23], with rodents generally considered as the reservoir hosts [Reference Robbins and Khachemoune24] to sustain further infestation of humans and large mammals with the larvae. However, cases of myiasis in cavies (rodents) are seldom reported. In a previous study, we reported the occurrence of myiasis due to C. anthropophaga in domestic cavies reared in some rural areas in Cameroon, which suggests that domestic cavies are one of the hosts of this parasite in the country.
In this work, the main focus was to assess the importance of C. anthropophaga infestations in cavy stock and to determine the husbandry risk factors that sustain the infestations with this parasite. The overall prevalence of the infestation was low (2·80%), as well as the number of infected farms (8·95%). This is probably an underestimate because the infestation rate of farms in the previous study was reported to be 25·80%. It may be due to the sampling methods in both studies since no attempt was made here to sample animals with obvious signs of ectoparasitism due to myiasis (limping, difficulties in moving, inability to compete for food) as was the case in the previous study. Indeed, in this study, the simple random sampling employed to determine the prevalence of infection in animals precluded a systematic sampling of obviously diseased animals that could lead to a higher prevalence at farm level. Moreover, early infestation may not be observed due to the non-conspicuous nature of the characteristic furuncle not yet developed. The previous study was conducted over a year from March 2013 to February 2014, while the present study was conducted between June and July 2014. The time period was shorter in this work than in the previous one, suggesting that climatic conditions between the two studies could influence the infestation rate of animals. To our knowledge, the seasonal dynamics of C. anthropophaga infestations have not yet been investigated in tropical areas, although some studies suggest that C. anthropophaga infestation is extremely common during the wet season (Curtis et al. 2006, cited in Lowe et al. [Reference Lowe, Naseem and Bailey25]). The low prevalence should not be misleading and cause decision makers to consider cavy myiasis as a harmless infestation, since death of the host can occur when larvae are very close to each other; indeed where larvae are close to each other, swelling and oedema occur and the tissues may become gangrenous; the larvae may invade deeper tissues and may cause severe destruction leading to the death of the host [Reference Daniel20]. The number of cavies (individuals) is increasing in the country, from an estimate of 30 293 in 2011 to 35 256 in 2012 [17]. Therefore the continuous involvement of the rural population in cavy farming might lead to an increased prevalence and intensity of C. anthropophaga infestation of cavies in the country, hence the need to design control strategies.
The risk of cordylobiasis spread was significantly higher (sixfold) in cavies housed in kitchen compartments without litter than in those moving freely on kitchen and house floors. Ecological conditions in kitchen compartments without litter are likely to be more conducive to egg laying than in other types of housing reported. Adults are known to feed on decaying fruits, decomposing animal tissues and excreta [Reference Taylor, Coop and Wall26]. For egg laying, the female is attracted to dry sand or areas contaminated with urine or faeces [Reference Rice and Gleason5, Reference Taylor, Coop and Wall26]. ‘Kitchen and house floor’, ‘cages’ and ‘kitchen compartment without litter’ provide such a favorable environment for egg laying because urine will either evaporate or be absorbed by the empty soil. Conversely, the environment is moist in ‘kitchen compartment with litter’ due to the presence of litter-keeping urine. In kitchen compartments without litter, there is a limited space for animals to move, leading to the accumulation of faeces and smell of urine on the compartment floor. This might attract more females to the compartment where forage leftover offers a dry spot to lay eggs. This is not the case in kitchen compartments with litter which, although also having a confined space, is often moist. On kitchen and house floors, even though forage leftovers may be more important, faeces and urine are not concentrated in a small area, which is attractive to females, as in kitchen compartments. Nevertheless, some entomological studies to demonstrate the relative attractiveness of different environments to female flies need to be carried out to confirm these hypotheses. The key to reducing the infestation risk will likely involves keeping females away from the living area of animals and destruction of any laying spots, through appropriate measures. These include, among others, cleaning of the animal living areas, daily removal of faeces and forage leftovers, extraction of infesting larvae and proper care of infested animals, protecting animal kitchen compartments, cages or pens with gauze wire, using mosquito bed-net materials over kitchen compartments. Unfortunately, some of these measures are difficult to implement, like the daily removal of faeces, simply because for farmers, whose main objective in cavy keeping is the production of manure [Reference Yiva19], faeces are intentionally accumulated and removed after 1 or 2 weeks. This group of farmers in particular should be sensitized regarding the necessity to clean and remove faeces from animal housing on a daily basis
As C. anthropophaga infests both humans and animals, cordylobiasis is likely to be reported as an occupational disease in cavy keepers in the forthcoming years as a result of the increased interest in cavy rearing, aided by the habits of some farmers (manure producers) who disregard sanitation and preventive measures on the farms. In the present study, the human interface of the infestation was not investigated in cavy keepers, but this would be welcome. A sporadic case of C. rodhaini infestation has already been reported in the Centre region of the country [Reference Vanhecke27] suggesting that both C. rodhaini and C. anthropophaga should be suspected in future investigations in humans.
CONCLUSION
The prevalence of the C. anthropophaga infestation was low, but is believed to be underestimated; hence the need to assess the burden of cordylobiasis in cavy flocks, and to assess the magnitude of infestation in cavy keepers. The relative risk of C. anthropophaga was more than sixfold higher for cavies in kitchen compartments without litter compared to cavies kept in kitchens and house floors. Although the human interface of the disease was not investigated, it is assumed that the risk for humans acquiring the disease is higher in those farmers keeping cavies in kitchen compartments without litter. As required by the One Health strategy, farmers need to be educated regarding control measures suggested to reduce the risk of infestation, which include both sanitation (good hygienic practices) and medical (extraction of larvae) measures.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816002466.
ACKNOWLEDGMENTS
The field work of the current research work was sponsored by Cavy Project ILRI-CSIRO CSI002-GUI led by the University of Dschang and in collaboration with its partners, namely Commonwealth Scientific and Industrial Research Organization (CSIRO) and BecA-ILRI (Bioscience in eastern and central Africa – International Livestock Research Institute).
DECLARATION OF INTEREST
None.







