Myelodysplastic syndromes (MDS) are a complex and heterogeneous group of clonal haematopoietic stem cell malignancies, characterised by dysplastic and ineffective haematopoiesis, peripheral cytopenia(s) and an elevated risk of progression into acute myeloid leukaemia( Reference Tefferi and Vardiman 1 ). The average annual age-adjusted incidence rate is lower in Asians (1–2/100 000 person-years (PY))( Reference Wang, Wang and Wang 2 , Reference Chihara, Ito and Katanoda 3 ) than that in Western populations (3–4/100 000 PY)( Reference Rollison, Howlader and Smith 4 , Reference Rodger and Morison 5 ). The variation in incidence rates across populations may partly be attributed to the differences in dietary factors.
Compared with Western diets, traditional diets in Asians customarily consist of more foods from plants, particularly soya foods( Reference Messina, Persky and Setchell 6 ). The lower risk of MDS in Asians might suggest that some components from soya foods may have potential chemopreventive properties. Of the bioactive components, isoflavones are naturally occurring compounds in plants and are almost exclusively present in soya foods. Genistein and daidzein are the two major isoflavones, and glycitein is the minor one, accounting for approximately 50, 40 and 10 %, respectively, of the total soyabean isoflavone content( Reference Murphy, Barua and Hauck 7 ). Although a protective role of isoflavones has been observed for certain cancers, such as breast, prostate and gastrointestinal cancers( Reference Messina, Persky and Setchell 6 , Reference Tse and Eslick 8 ), none of the previous studies to date has reported the associations of soya foods or dietary isoflavone intake with the risk of MDS. We presented the data from a case–control study herein to investigate the association between dietary isoflavone intake and MDS risk in a Chinese population whose diet is rich in soya.
Methods
Study design and participants
A hospital-based case–control study was conducted in Hangzhou, the capital city of Zhejiang province in southeast China, between September 2012 and December 2013. Adult de novo MDS patients were identified from the medical records of the Department of Haematology at The First Affiliated Hospital of Zhejiang University. Details of the study design and participants’ recruitment have been described elsewhere( Reference Liu, Zhang and Jin 9 ). In brief, male and female patients were eligible as cases if they met the 2008 WHO MDS diagnostic criteria, and if they were ≥18 years old. Patients were excluded if they had secondary MDS or additional malignancy. Moreover, potential cases were excluded if there was the possibility of peripheral blood cytopenias associated with nutritional deficiencies, inflammation or infection, aplastic anaemia, acute myeloid leukaemia, myelodysplastic-myeloproliferative neoplasms or myeloproliferative neoplasms( Reference Tefferi and Vardiman 1 ). All relevant clinical and laboratory reports were reviewed daily. A total of 208 patients aged between 19 and 85 years were included as cases in the final analysis (response 95 %). For these cases, the median time interval between the date of diagnosis and the date of interview was 60 d, and 79 % of the cases were recruited within 1 year of diagnosis. Of the 208 cases, the major morphologic subtypes were refractory anaemia with excess of blasts (41·4 %) and refractory cytopenia with multilineage dysplasia (36·1 %). The minor subtypes were refractory cytopenia with unilineage dysplasia (12·5 %), MDS unclassifiable (6·7 %) and refractory anaemia with ring sideroblasts (3·4 %).
At the same time as the cases were recruited, each control was selected as the first attendee to match with each case on sex, birth quinquennium and residential locality (urban or rural). Potential controls were excluded if they were not matched to their corresponding cases by these predefined matching factors, or if they had a previous diagnosis of MDS or another malignancy. The controls were recruited from the outpatient departments of the participating hospital (response 91 %). Among the recruited controls, 107 (51 %) were members of the general population undergoing a routine health examination at the Medical Examination Centre; fifty-two (25 %) were outpatients from the ophthalmologic clinic; and forty-nine (24 %) were visitors who attended with family members or friends at the outpatient departments of the hospital. The study protocol was approved by the Human Research Ethics Committee of the University of Western Australia and the ethics committee of the participating hospital.
Questionnaire and interview
Participants were briefed regarding the general aims of the study to investigate dietary and lifestyle factors, confidentiality and anonymity issues. An appointment for an interview was made after obtaining their written informed consent via an initial contact. Face-to-face interviews were then conducted by the first author and a trained local research assistant. Each interview usually took 30–40 min. A structured questionnaire was used to elicit information on the following: (i) demographic characteristics and detailed lifestyle, such as education, weight and height, smoking status, tea and alcohol use, and physical activity; (ii) habitual dietary intake assessed by a quantitative FFQ; (iii) reproductive factors and family history of cancer; and (iv) chemical exposures and hair dye use. The validity and reproducibility of the FFQ have been verified previously( Reference Shu, Yang and Jin 10 , Reference Zhang, Binns and Lee 11 ), with the intraclass correlation coefficient for mean daily intake of total soya foods being 0·78 in a test–retest( Reference Zhang, Binns and Lee 11 ).
Dietary intake assessment
Habitual food intakes were assessed using the quantitative FFQ. A ‘reference’ recall period was set as 1 year before the diagnosis in cases and 1 year before the date of interview for controls. If there were recent alterations in eating habits, information was sought on the habits before the change. The frequency of food intakes was classified into nine categories: never or hardly ever, once a month, two to three times a month, once a week, two to three times a week, four to six times a week, once a day, twice a day and ≥3 times a day. The usual amount of each food consumed per meal was estimated using the common Chinese unit liang (1 liang=50 g). The frequency and quantity variables derived from the FFQ were converted into daily intake, taking seasonal factors and market availability into account. In line with previous studies( Reference Zhang, Yang and Holman 12 , Reference Li, Zhang and Holman 13 ), soya foods included nine items of soya and soya-based foods (dry soyabean, fresh green soyabean, soyabean milk, skin of soyabean milk, fresh bean curd, textured bean curd, fried bean curd, fermented bean curd and soyabean sprouts). Daily intakes (mg) of daidzein, genistein, glycitein and total isoflavones were calculated using an updated version of the US Department of Agriculture Isoflavone Database( 14 ), as the contents of isoflavones in foods were unavailable from the Chinese Food Composition Tables. Daily intake of isoflavones was estimated on the basis of the amounts of nine-item soya foods consumed, as other food sources are very low in isoflavones and their contribution to total intake can be considered negligible.
Statistical analyses
Intakes of daidzein, genistein, glycitein and total isoflavones were divided into tertiles based on the distribution of each isoflavone variable in controls. Total energy intake derived from 107 foods was estimated using the 2009 China Food Composition Tables, accounting for seasonal factors and edible portions of foods( 15 ). Other variables were defined as follows: BMI was calculated as body weight in kilograms divided by the square of height in metres (kg/m2); metabolic equivalent task hours per week during the past year was a measure of physical activity; cigarette smoking was defined as a total consumption of twenty packs of cigarettes or more in lifetime, and the cumulative quantity of smoking exposure was indicated as pack-year (number of packs cigarettes consumed per d multiplied by number of years smoking); ever consumed liquor, beer, wine or any combination was classified as alcohol consumption; drinking tea ≥1 time/month was defined as tea consumption; ever used permanent hair dye in a lifetime was defined as hair dye use; participants self-reporting lifetime exposure to chemicals, either on a job or a hobby, at least 8 h/week for 1 year or more was defined as ever exposed( Reference Strom, Gu and Gruschkus 16 ).
Sample characteristics, soya foods and dietary intake of isoflavones were compared between cases and controls. OR and 95 % CI were calculated from conditional logistic regression models( Reference Breslow and Day 17 ) to estimate the risk of MDS according to tertiles of isoflavone intake. The lowest intake tertile served as the reference category in all models. In addition to control by design for matching factors, the multivariate analyses were adjusted for education (none, primary, secondary, tertiary), BMI (kg/m2, continuous), cigarette smoking (pack-years, continuous), tea consumption (no, yes), alcohol consumption (abstainers, ever-drinkers) and daily intakes of energy (kJ (kcal)), vegetables (g), fruits (g) and red meat (g). These variables were included in regression models because they were associated with MDS risk based on univariate analyses or previous study( Reference Ma, Lim and Park 18 ), and associated with soya food intake as a priori confounders( Reference Miettinen and Cook 19 ). Other possible risk factors for MDS, such as chemical exposure and hair dye use, appeared to be disassociated with soya food intake and did not materially alter the results after adjustment; thus, they were excluded from the final model. Variables representing isoflavone intake were subjected to tests for trend by likelihood ratio tests using the original values in continuous format. All P values were based on likelihood ratio statistics and were considered statistically significant if <0·05. The analyses were conducted with SAS version 9.3 (SAS Institute Inc.).
If the difference in the mean dietary isoflavone intake of matched pairs is 4 (sd 20), a total of 198 case–control pairs were needed with a type I error rate of 0·05 and 80 % power( Reference Dupont and Plummer 20 ). Thus, the sample size of this study provided adequate power to detect differences in dietary isoflavone intake between cases and controls.
Results
The selected characteristics of MDS cases and controls are presented in Table 1. There was no significant difference between cases and controls on matching factors, BMI, physical activity, mean daily intakes of energy, vegetables and red meat, cancer history in first-degree relatives and hair dye use. Compared with controls, cases attained less education. Cases were more likely than controls to have smoked, abstained from alcohol and to have been ever exposed to chemicals. Cases tended to consume less tea and fruits than controls.
Table 1 Selected characteristics between myelodysplastic syndromes cases and controls (Mean values with their standard errors; number of cases and controls and percentages)

MET, metabolic equivalent task.
* P value from univariate conditional logistic regression with 1 df.
Table 2 describes soya food consumption and dietary intake of isoflavones by case–control status. The vast majority of soya foods consumed in cases and controls were non-fermented products such as soyabean, soyabean milk, fresh bean curd and textured bean curd. Compared with the controls, MDS cases had lower mean daily intakes of most individual soya foods, although most differences did not reach statistical significance. With respect to isoflavones, daily intake of total isoflavones was significantly lower in cases (mean 19·0 (se 1·4)) than in controls (mean 23·0 (se 1·3)). Significantly lower intakes of daidzein and genistein were observed in cases when compared with those in controls.
Table 2 Soya food consumption and dietary intake of isoflavones between myelodysplastic syndromes cases and controls (Mean values with their standard errors)
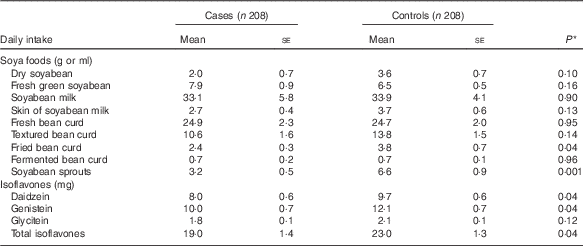
* P value from univariate conditional logistic regression.
Among cases, median daily intake of total isoflavones ranged from 5·1 mg in the lowest tertile to 36·8 mg in the highest tertile, which was less than that in controls at 7·8 and 41·0 mg, respectively. Table 3 shows the associations between dietary isoflavone intake and MDS risk. Higher dietary intake of isoflavones was associated with a decreased risk of MDS. The adjusted OR (95 % CI) for MDS in the highest tertile of intake compared with the lowest tertile were 0·43 (95 % CI 0·21, 0·85) for daidzein, 0·36 (95 % CI 0·18, 0·74) for genistein, 0·49 (95 % CI 0·25, 0·97) for glycitein and 0·40 (95 % CI 0·20, 0·81) for total isoflavones. Inverse dose–response trends were apparent for the intakes of daidzein, genistein and total isoflavones (P for trend=0·04), and a marginally significant trend was observed for the intake of glycitein (P for trend=0·10).
Table 3 Associations between dietary intake of isoflavones and myelodysplastic syndromes risk (Odds ratios and 95 % confidence intervals)

Ref., referent values.
* Median and range of each tertile intake in controls.
† Estimates from separate univariate conditional logistic regression model with sex, birth quinquennium and residential locality (urban or rural) as matching variables.
‡ Estimates from separate conditional logistic regression adjusted for education (none, primary, secondary, tertiary), BMI (kg/m2, continuous), cigarette smoking (pack-years, continuous), alcohol consumption (abstainers, ever-drinkers), tea consumption (no, yes), intakes of energy (kJ (kcal)/d, continuous), vegetables (g/d, continuous), fruits (g/d, continuous) and red meat (g/d, continuous).
§ Likelihood ratio tests for trend using the original values in continuous format.
Discussion
The present case–control study conducted in a Chinese population, whose diet is rich in soya, is the first epidemiological study to date that has examined the association between dietary isoflavone intake and MDS risk. A consistent and statistically significant reduced risk of MDS was observed with high dietary intakes of total isoflavones, and the subclasses of daidzein and genistein.
Many studies have focused on the anti-oestrogenic properties of isoflavones for preventing hormone-related cancers, such as ovarian, breast and prostate cancers( Reference Messina, Persky and Setchell 6 ). Isoflavones may also have a role in the prevention of non-hormone-mediated cancers, as they act as antioxidants and possess anti-carcinogenic properties, such as inhibiting cell growth and angiogenesis, inhibiting both protein tyrosine kinase and DNA topoisomerase II and inducing differentiation( Reference Messina, Persky and Setchell 6 , Reference Fotsis, Pepper and Adlercreutz 21 , Reference Markovits, Linassier and Fosse 22 ). Genistein, the key isoflavone present in soya foods, has exhibited anti-leukaemic activity in a number of in vivo and in vitro studies. For instance, genistein may induce the differentiation of human promyelocytic HL-60 leukaemia cells( Reference Constantinou, Kiguchi and Huberman 23 ). One study has suggested that genistein not only produces dose- and time-dependent antineoplastic activity against myeloid leukaemic cell lines but also reactivates tumour suppressor genes silenced by aberrant DNA methylation( Reference Raynal, Momparler and Charbonneau 24 ). Furthermore, researchers have observed a significant anti-leukaemic effect in mice induced by a genistein-enriched diet, the concentrations of which are observable in the plasma of high soya consumers( Reference Raynal, Momparler and Charbonneau 24 ). Although no study is available on the biological effects of isoflavones on MDS, the inferences of the laboratory work are plausible given that MDS shares several characteristics with leukaemia and is characterised by frequent (approximately 30 %) progression to acute myeloid leukaemia( Reference Corey, Minden and Barber 25 ). The findings from experimental studies on leukaemia potentially provide an underlying biological explanation for our observation that high dietary intake of isoflavones is associated with a reduced risk of MDS in a population with a soya-rich diet.
The strengths and limitations of the study should be considered. A major strength of the study was that extensive information on soya food intake, as well as other dietary factors, was sought from face-to-face interviews using a validated and reliable questionnaire specifically developed for adult Chinese( Reference Shu, Yang and Jin 10 , Reference Zhang, Binns and Lee 11 ). The FFQ used in the study included nine-item soya foods, which were commonly consumed in China and were the major sources of dietary isoflavones. The average daily intake of total isoflavones in the controls was comparable to that in our previous study (23·0 v. 19·5 mg/d)( Reference Zhang, Yang and Holman 12 ). Moreover, the bioavailability of self-reported usual intake of soya isoflavones has been assessed in previous literature, from which there were strong correlations between plasma concentrations, urinary excretion of isoflavones and self-reported soya intake measured by FFQ( Reference Wu, Yu and Tseng 26 , Reference Maskarinec, Singh and Meng 27 ), suggesting that the participants’ self-reported usual soya intake derived from the FFQ should have provided a reliable measure of isoflavone intake. Another characteristic was that the multivariate conditional logistic regression analyses were adjusted for a variety of dietary and non-dietary factors, such as the intakes of vegetables, fruits, red meat, energy and cumulative exposure to cigarette smoking. Thus, residual confounding seems unlikely to have had a substantial impact on the risk estimates. Although the univariate analyses show that lifetime exposure to chemicals was significantly associated with MDS risk, it was not significantly associated with dietary isoflavone intake in the control series. Thus, there is no a priori or empirical justification for viewing chemical exposures as a potential confounder in this analysis; if we nevertheless proceed to ‘over-adjust’ by inclusion of chemical exposures as a covariate, the price paid is an unwarranted loss of precision in estimates concerning our primary research question.
The study is thought to have introduced little selection bias. The cases were identified from medical records at the participating hospital. Relevant medical records were reviewed daily and eligible patients diagnosed in this MDS Centre during the study period were invited to participate in the study with a 95 % response proportion. Thus, the systematic identification procedure ensured that the ascertainment of cases was maximised and near complete. The controls selected from outpatient departments of the same hospital were considered as a valid study base sample, as evidenced by the remarkably similar distributions of dietary isoflavone intake, as well as demographic characteristics, lifestyle factors between hospital outpatient controls and community controls in China( Reference Li, Zhang and Holman 13 , Reference Li, Zhang and Holman 28 , Reference Li, Zhang and Holman 29 ).
With regard to the likelihood of information bias, the potential protective role of soya foods against MDS had not received attention before nor at the time of interview, and thus information bias from that source was unlikely. Dietary habits may change because of disease status in cases. Nevertheless, it appears unlikely that disease status materially affected self-reported responses, as participants’ usual dietary intake was measured using a ‘reference’ recall period. If non-differential misclassification of dietary intake occurred, the resultant bias would have been towards the null and could not have accounted for the inverse association reported here. We made an effort to minimise recall bias by asking study participants to report their usual dietary intake during the 1 year before the diagnosis in cases and 1 year before the interview date for controls. In this study, 92·8 % of the cases and 99·0 % of the controls reported that their diets in the previous year were typical of their usual diets. Other personal life habits as confounding factors that were adjusted for in regression analyses, including cigarette smoking, tea consumption and alcohol consumption, were relatively easier for participants to recall with reasonable accuracy. Last, it was impractical to evaluate the effect of isoflavone intake by sex and by MDS subtype because of the limitations of the study sample size.
In conclusion, the study suggests an inverse association between the intake of dietary isoflavones and MDS risk in a Chinese population. Future studies are needed to confirm or refute these results and to characterise the underlying biological mechanisms, which may have longer-term therapeutic potential.
Acknowledgements
The authors are grateful to the participants of this study. The authors thank Hong-Yan Tong, Li-Hong Cao and other staff from The First Affiliated Hospital of Zhejiang University for their kind assistance with fieldwork. P. L. was supported by the Scholarship for International Research Fees and the University Postgraduate Award of The University of Western Australia.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors’ contributions are as follows: P. L., C. D’A. J. H., J. J. and M. Z. designed the research project; P. L., J. J. and M. Z. managed the data; P. L. carried out the statistical analyses; P. L. wrote the article; C. D’A. J. H. and M. Z. critically revised the article; P. L., C. D’A. J. H. and M. Z had primary responsibility for the final content.
There are no conflicts of interest to declare.






