INTRODUCTION
Neglected tropical diseases (NTDs) are infections that primarily occur in regions of rural poverty in developing countries and affect more than one billion people, costing developing economies billions of dollars every year [1]. However, some of the NTDs also occur in urban slums and favelas [Reference Hotez2]. The NTDs dengue fever (DF), leptospirosis and rabies are a particular cause of urban health problems in developing countries. For both DF and leptospirosis, urban flooding, at times of natural disaster, is a key component of transmission. As global warming results in increased flooding in some regions of the developing world, the combined effects of urbanization and climate change may lead to an increased incidence of these two infections in the coming decades [Reference Hotez2]. As a result of climate change, there is also a possibility that these NTDs could emerge in urban regions of the United States [Reference Gubler3]. Therefore, DF and leptospirosis are important public health issues worldwide.
Several studies have shown that leptospirosis is often misdiagnosed as DF and underdiagnosed in endemic regions [Reference Vijayachari, Sugunan and Shriram4]. DF is transmitted by mosquitoes and leptospirosis is a zoonotic infectious disease caused by Leptospira bacteria, which can be found in fresh water contaminated by animal urine. Epidemics of DF and leptospirosis have been observed following natural disasters [Reference Watson, Gayer and Connolly5] and some of the most affected nations are disaster-prone island countries [Reference Guha-Sapir, Hoyois and Below6]. In the Republic of the Philippines, DF and leptospirosis are recognized as common disaster-related infections, in addition to diarrhoea and cholera [Reference Usuzawa7].
A clear relationship has been observed between an increase in DF cases and the southwest and northeast monsoons, particularly in Manila, in the Philippines [Reference Su8–Reference Hii10]. Moreover, leptospirosis is associated with floodwaters following monsoons [Reference Easton11–Reference Libraty13]. It has also been suggested that temperature and rainfall considerably increase the incidence of DF infections [14, Reference Bravo15]. One study that assessed climatic factors associated with DF incidence in Manila showed that heavy rainfall in the wet season from June to December was significantly associated with increased DF incidence over 1996–2005 [Reference Bravo15]. Leptospirosis outbreaks also typically occur during the typhoon season (July–October) [Reference Amilasan16].
Recently, the World Health Organization performed a case study to investigate the correlation between DF and leptospirosis incidence with meteorological factors such as rainfall, temperature and relative humidity in Sri Lanka, with the aim of developing models to predict the effects of climatic variations on disease incidence [14]. Time-series analysis revealed an association between disease epidemics and meteorological factors, which was not visually apparent. DF and leptospirosis are also serious public health problems in Manila; however, to date, time-series analysis has not been employed to examine the effect of meteorological variables on disease occurrence in the Philippines.
The present research is designed to shed light on the relationship between climate factors of rainfall, temperature and relative humidity on the occurrence of DF and leptospirosis in Manila over the period January 2013–December 2014, which encompasses Typhoon Haiyan (known in the Philippines as Typhoon Yolanda). This was one of the strongest tropical cyclones ever recorded, devastating portions of Southeast Asia, especially the Philippines, in early November 2013. It is hoped that the work will provide insight into the relationship between meteorological factors and the incidence of DF and leptospirosis [14]. The findings could also inform prevention and control of the diseases.
DATA
Study area
The Philippines comprises 7107 islands in Southeast Asia, with the three island groups of Luzon, Visayas, and Mindanao split into 17 regions [1]. The population is 92 337 852 (2010 census), with an average annual population growth rate of 1·90% over the period 2000–2010. A large proportion of the population (37·3%) lives in three regions, namely Calabarzon (16·03 million people), Manila (11·55 million people), and Central Luzon (9·72 million people) [17]. The country has a tropical marine climate, with an annual dry season from December to May and annual wet season from June to November [17]. Typhoons usually occur from June to September. We conducted the present study at San Lazaro Hospital in Manila, a national 500-bed referral infectious disease centre for Manila and neighbouring provinces, which mostly serves economically disadvantaged people and is one of the sentinel hospitals of the Philippines Integrated Disease Surveillance and Response (PIDSR) system.
DF and leptospirosis data
We used the weekly number of cases of laboratory-confirmed DF and leptospirosis from January 2013 to December 2014, collected over 104 weeks (104 data points) at San Lazaro Hospital via the PIDSR system.
Laboratory confirmations for DF were conducted under strategies of the PIDSR system as follows: (i) isolation of the dengue virus from serum, plasma or leukocytes; (ii) demonstration of ⩾fourfold change in reciprocal IgG or IgM antibody titres to one or more dengue virus antigens in paired serum samples; (iii) detection of viral genomic sequences in serum or cerebrospinal fluid samples by polymerase chain reaction (PCR) [18]. Laboratory confirmations of leptospirosis were conducted under strategies of the PIDSR system as follows: (i) isolation (and typing) from blood or other clinical materials through cultures of pathogenic Leptospira; (ii) positive serology, preferably microscopic agglutination testing (MAT) or PCR, using a range of Leptospira strains for antigens that should be representative of local strains [18].
Meteorological data
Data on daily maximum and minimum temperatures, relative humidity, and rainfall for Manila were collected by the Philippine Atmospheric, Geophysical, and Astronomical Service Administration in Quezon City during 2013 and 2014. The daily data were gathered over a total of 730 days during the aforementioned period (730 data points). Daily average temperatures were computed as means of daily maxima and minima. Weekly means of average temperature, relative humidity and total rainfall were calculated from daily records.
Descriptive statistics for the weekly meteorological data are shown in Table 1. Mean weekly average values in Manila were: mean temperature 28·4 °C, relative humidity 74·4%, and total rainfall 48·2 mm.
Table 1. Summary statistics of weekly meteorological conditions in Manila, the Philippines

METHODS
We used time-series analysis combined with spectral analysis based on the maximum entropy method (MEM) in the frequency domain and least squares method (LSM) in the time domain, as proposed in our previous work [Reference Sumi19, Reference Harigane20]. For explanation of the time-series analysis, we used time-series data of the reported case of DF shown in Figure 1a .

Fig. 1. Comparison of least-squares-fitting curve calculated for three dominant periods (–○–) with original data (- -●- -), for: (a) dengue fever, (b) leptospirosis, (c) mean temperature, (d) relative humidity, (e) rainfall.
Spectral analysis
We assumed that the original time-series data x(t) (t = time) in Figure 1a were composed of systematic and fluctuating parts [Reference Armitage, Berry and Matthews21]:
To investigate temporal patterns of x(t), we performed MEM spectral analysis, which is useful for investigating periodicities of short time-series as used in the present study [Reference Ohtomo22]. That analysis produces a power spectral density (PSD), from which we obtain the power representing the amount of amplitude of x(t) at each frequency (note the reciprocal relationship between the scales of frequency and period). The formulation of MEM-PSD has been described previously [Reference Harigane20]. Figure 2a shows the PSD for the time-series data in Figure 1a .

Fig. 2. Power spectral density of the original data for: (a) dengue fever, (b) leptospirosis, (c) mean temperature, (d) relative humidity, (e) rainfall.
LSM
The validity of the MEM spectral analysis results was confirmed by calculation of the least squares fitting (LSF) curve to the original data x(t) (Fig. 1a ) with MEM-estimated periods. The formulation of the LSF curve in X(t) is described as follows [Reference Harigane20]:
 $$X(t) = A_0 + \sum\limits_{n = 1}^{N_p} {A_n \cos \left\{ {2\pi f_n \left( {t + \theta _n} \right)} \right\},} $$
$$X(t) = A_0 + \sum\limits_{n = 1}^{N_p} {A_n \cos \left\{ {2\pi f_n \left( {t + \theta _n} \right)} \right\},} $$
which is calculated using the LSM for x(t) (Fig. 1a ) with unknown parameters f n , A 0 and A n (n = 1, 2, 3, …, N p ), where f n (=1/T n : Tn is the period) is the frequency of the nth component, A 0 is a constant that indicates the average value of the time-series data, A n and θ n are the amplitude and phase of the nth component, respectively, and N p is the total number of components.
Figure 1a shows the LSF curve calculated using three MEM-estimated periods observed in Figure 2a . Reproducibility of the modified time-series data using the optimum LSF curve was evaluated by the Pearson correlation coefficient (ρ) with SPSS software v. 17.0J for Windows (SPSS Inc., USA). To investigate seasonality of the time-series data, their LSF curve was determined using a seasonal cycle corresponding to a 1-year period T 1 (Fig. 3a ).

Fig. 3. Normalized least-squares-fitting (LSF) curves calculated with the seasonal cycle, for: (a) dengue fever (orange), mean temperature (red), rainfall (blue) and relative humidity (grey); (b) leptospirosis (orange), mean temperature (red), rainfall (blue) and relative humidity (grey). In panel (b), LSF curves of leptospirosis (orange) and relative humidity (grey) overlap.
RESULTS
Temporal variations of DF, leptospirosis and meteorological data
The weekly time-series data used are depicted in Figure 1. The seasonal pattern of DF data (Fig. 1a ) indicates large values during the wet season (June–November) with a peak in September, contemporaneous with high temperature, high relative humidity and heavy rainfall (Fig. 1c – e ). A large peak in the number of leptospirosis cases (Fig. 1b ) was observed in September 2013, and a small peak in September 2014. The temporal pattern of temperature (Fig. 1c ) reveals an increase in the dry season (December–May), with peaks in April 2013 and May 2014. Thereafter, the temperature remained around 28 °C in the wet season (June–November). The seasonal pattern of relative humidity (Fig. 1d ) indicates large values during the wet season, with peaks in August 2013 and July 2014. The seasonal pattern of rainfall (Fig. 1e ) shows maxima during the wet season, with a peak in August 2013. There was a smaller maximum during the 2014 wet season, with a peak in October.
Spectral and LSF analyses
PSDs for the original time-series data in Figure 1 are shown in Figure 2. In each PSD, the most prominent spectral line was at frequency f = 1·0 [units (1/year)], corresponding to the 1-year cycle, that is, the seasonal cycle: 1·20 year, 0·91 year, 1·01 year, 0·90 year and 0·98 year for DF (Fig. 2a ), leptospirosis (Fig. 2b ), temperature (Fig. 2c ), relative humidity (Fig. 2d ) and rainfall (Fig. 2e ), respectively. In Figure 2a , a spectral peak in the low-frequency range of PSD (f < 1·0) reflects oscillations longer than the 1-year cycle.
Three dominant spectral-peak frequency modes are shown in Table 2, with corresponding periods and intensities (powers) of the peaks. In Figure 1, each LSF curve calculated with three dominant periods reproduces the modified data well. Thus, the periods detected by MEM spectral analysis for each original datum (Fig. 2, Table 2) were confirmed to be accurate. The good fit of each LSF curve to the original data was supported by the fact that the ρ values between the modified data and the LSF curve were large. They were 0·96, 0·60, 0·90, 0·88 and 0·67 for DF, leptospirosis, temperature, relative humidity and rainfall data, respectively.
Table 2. Characteristics of the three dominant spectral peaks shown in Figure 2

Seasonal cycle of DF and leptospirosis data and meteorological data
The LSF curves calculated with the seasonal cycles for DF (1·20-year) and leptospirosis (0·91-year) were normalized in amplitude, and are overlaid in Figure 3(a , b , respectively). In Figure 3, oscillation is shown in coordinate phase between the LSF curve for numbers of laboratory-confirmed DF and leptospirosis cases and the LSF curve for meteorological conditions (temperature, rainfall, relative humidity). Peaks of the LSF curves for relative humidity, rainfall and temperature data precede those for DF data by 1, 2 and 4 months, respectively, and those for leptospirosis data by 0, 1 and 3 months. The LSF curve for DF (Fig. 3a ) showed significant correlations with that for meteorological data (Table 3), i.e. −0·60, 0·45 and 0·51 for mean temperature, relative humidity and rainfall, respectively (P < 0·01). For leptospirosis, the LSF curve (Fig. 3b ) showed significant correlations with the LSF curves for mean temperature and rainfall (0·99 and 0·92, respectively, P < 0·01) but no significant correlation with mean temperature (−0·03, P = 0·74).
Table 3. Spearman's ρ calculated for original data and least-squares-fitting curves with seasonal cycles
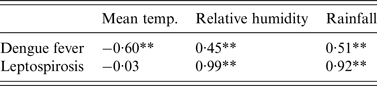
** P < 0·01.
DISCUSSION
There was a peak in temperature during May, followed by maxima in rainfall, relative humidity and number of DF patients (Fig. 3). We assumed that this result shows a relationship between monsoons and DF occurrence in the Philippines. In summer, temperature rises, rainfall increases with moist air transport from the ocean to continent, a monsoon begins and the atmosphere moistens, increasing relative humidity. This was followed by a peak in the number of DF patients. An earlier study by Su [Reference Su8] addressed the reasons for DF epidemics in terms of rainfall. We assumed that DF epidemics are correlated not only with rainfall but also relative humidity and temperature. DF during a week was related to rainfall over the prior 6–7 weeks. This can be attributed to the life-cycle duration of mosquitoes and the requirement of an adequate number of cases for spread, which is in turn affected by population density [Reference Bravo15].
The disease leptospirosis is usually transmitted to humans through wild rats and their urine. However, bacteria in floodwater can also infect humans by entering the body through cuts and skin abrasions. Libraty et al. [Reference Libraty13] reported that the maximum occurrence of leptospirosis was in October, 2–3 months later than the peak occurrence of dengue in Thailand. However, in the present study of Manila, the peak occurrence of leptospirosis preceded that of DF by only 1 month (Fig. 3a , b ). This difference in peak month of leptospirosis cases between Thailand and Manila may be caused by epidemiological characteristics of this disease in each country. In Thailand, leptospirosis outbreaks from cultivation of rice and other agriculture have become a problem [Reference Libraty13]. In Manila, large-scale leptospirosis epidemics have occurred after heavy rains and flooding following monsoons [Reference Amilasan16]. It should also be noted that both of these diseases are caused after natural disasters, which are increasing year by year [Reference Leaning and Guha-Sapir23]. Differential diagnoses of these febrile illnesses is an important issue, and the development of point-of-care testing and metrological analysis described herein would aid such diagnoses [Reference Hattori24].
Floods, and a fortiori urban floods, have often induced outbreaks of hantavirus infection, i.e. haemorrhagic fever with renal syndrome (HFRS), whose symptoms can almost completely overlap those of leptospirosis [Reference Clement, Maes and Ranst25]. However, HFRS, which has been limited to Eurasia [Reference Bi, Formenty and Roth26], has not been targeted in the PIDSR system. This is partly because there have been no documented cases of HFRS in Filipino patients [Reference Quelapio27]. Like HFRS, hantavirus pulmonary syndrome (HPS) has not been considered in the PIDSR system, because HPS has been limited to the Americas [Reference Bi, Formenty and Roth26]. However, evidence is now mounting for clinical and pathological overlap between HPS and HFRS [Reference Rasmuson28, Reference Clement, Maes and Ranst29]. Consequently, hantavirus infections should be considered a cause of acute respiratory distress in all endemic areas worldwide [Reference Rasmuson28]. Indeed, Quelapio et al. [Reference Quelapio27] provided substantial evidence that hantavirus infection does occur in the Philippines. New hantaviruses, different hosts and human syndromes are expected to be discovered, especially in Southeast Asia. There, murid rodents and Soricomorpha small mammals [Reference Klein30] are endemic and highly diversified, and human populations are regularly exposed by contact, as in the Philippines [Reference Herbreteau31, Reference Anyamba32]. Increased awareness of hantavirus infection in the PIDSR system would make it efficient to estimate the burden of such infection and prevent it in the Philippines.
In general, meteorological phenomena are non-stationary and nonlinear, and transit from one state to another in a complicated manner. It is possible that the correlation strengths of seasonal cycles for DF and leptospirosis data with those of meteorological data (Fig. 3, Table 3) also change temporally. To elucidate the temporal evolution of such nonlinear phenomena, it is preferable to deal with shorter time-series through segment-based time-series analysis [Reference Ohtomo22]. For example, this was done in our previous studies on measles using a 56-year data duration [Reference Luo33] and influenza using a 51-year duration [Reference Sumi34]. The longer duration data (>2 years) on DF and leptospirosis in the present study (Fig. 1a, b, respectively) enabled further investigation of temporal variations for correlations of DF and leptospirosis infections with meteorological conditions, using segment-based time-series analysis. Further accumulation of DF and leptospirosis data in the PIDSR system will contribute to future investigations of meteorological factors for DF and leptospirosis transmission in the Philippines.
The present results suggest that DF and leptospirosis cases in Manila were influenced by monsoon occurrence. Further time-series analyses of the two diseases and meteorological data in other regions of the world may elucidate their potential relationships to the monsoon or other epidemiological factors.
ACKNOWLEDGEMENTS
This study was supported by the Philippines Atmospheric Geophysical and Astronomical Services Administration (PAGASA), a special research grant from the International Research Institute of Disaster Science (IRIDeS) of Tohoku University, and JSPS KAKENHI grant nos. JP16K09061, JP26257506, JP25460769, JP25305022. The first author also thanks the students of Sapporo Medical University School of Medicine (R. Inoue, M. Senda, Y. Teguri, K. Nakajima and M. Yashiro) for their help with discussion of the topic.
DECLARATION OF INTEREST
None.









