To the Editor—Streptococcus pneumoniae is a leading cause of community-acquired bacterial infections, but an unusual source of neonatal sepsisReference Hoffman, Mason and Schutze1 and nosocomial cases have rarely been described. We report 2 original cases of neonatal S. pneumoniae infection due to the same serotype 14 pneumococcal strain that occurred in the same pediatric intensive care unit (PICU) with a highly probable nosocomial contamination from one to the other.
Patient 1 was a 13-day-old boy admitted to the PICU for respiratory distress due to respiratory syncytial virus (RSV)–induced bronchiolitis on November 11. He developed apnea with bradycardia and a deep decrease in blood oxygen requiring noninvasive ventilation using bilevel positive airway pressure. His respiratory distress worsened 24 hours after admission due to left pulmonary pneumoniae, and a blood culture was positive with a serotype 14 S. pneumoniae isolate with decreased susceptibility to penicillin G (Table 1) and resistance to macrolides and cotrimoxazole. The patient was treated with cefotaxime (100 mg/kg/day). Evolution was favorable with spontaneous ventilation on day 4, and he was discharged from the PICU on day 5 (November 15).
Table 1. Characteristics of Streptococcus pneumoniae Strains
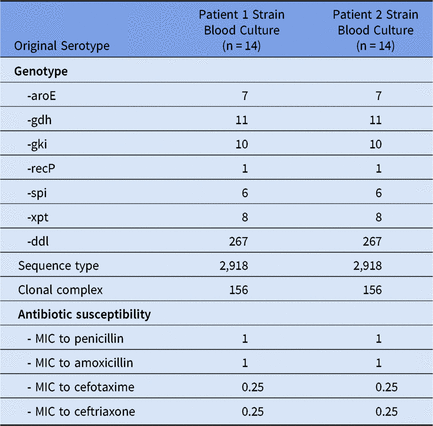
Note. MIC, minimum inhibitory concentration.
Patient 2 was a 18-day-old healthy full-term boy also admitted to the PICU for respiratory distress due to RSV-induced bronchiolitis on November 9. He had a right superior lobe pulmonary atelectasis, no signs of bacterial infection, and negative blood culture. Noninvasive ventilation using continuous positive airway pressure was performed, followed 2 days later by invasive ventilation because of the onset of acute respiratory distress syndrome (ARDS). On November 15, his clinical state worsened, with bilateral pneumonia, and a tracheal sample yielded S. pneumoniae with the same pattern of antibiotic susceptibility as case 1. Central line insertion, prone positioning, high-frequency ventilation, nitric oxide, corticotherapy, and antibiotic treatment with cefotaxime (100 mg/kg/day for 5 days) were performed. Fever stopped 2 days after the initiation of antibiotherapy, and the patient was removed from mechanical ventilation on November 22. Fever reappeared on November 24 due to central-line–associated bloodstream infection (CLABSI). Streptococcus pneumoniae was detected in qualitative peripheral and catheter blood cultures performed November 25, as well as in a quantitative catheter central-line blood culture with 104 colony-forming units (CFU)/mL. The catheter culture after removal was positive, with 106 CFU/mL. This serotype 14 S. pneumoniae harbored the same profile of susceptibility to antibiotics as case 1 (Table 1). Cefotaxime (150 mg/kg/day) was introduced and continued for a total duration of 7 days after the first sterile blood culture. The patient’s fever disappeared, and he was discharged from the PICU on November 27. Considering the antibiotic susceptibility pattern, the serotype of the isolates of both cases, and the fact that both neonates were cared for by the same caregivers, multilocus sequence typing (MLST) was performed on the S. pneumoniae detected in blood cultures of both patients. Both strains were of the same ST2918 belonging to the clonal complex (CC) 156 (Table 1).
Reports of large series of neonatal sepsis in the post-penicillin era have shown that S. pneumoniae is a rare cause of neonatal infection (1%–11%).Reference Hoffman, Mason and Schutze1, Reference Westh, Skibsted and Korner2 The predominant clinical presentation in neonates is bacteremia, but other clinical manifestations have been reported.Reference Kaplan, Rudensky and Beck3 We report here 2 cases of S. pneumoniae infection occurring in the same PICU in 2 neonates, including 1 CLABSI. Both infections were due to a serotype 14 S. pneumoniae, a serotype included in the 7-valent pneumococcal conjugate vaccine (PCV7) introduced in France in 2003. PCV7 introduction resulted in a dramatic decrease of PCV7 invasive pneumococcal disease (IPD). Serotype 14 represented 15% of IPD in 2001–2002 but represented <1% after 10 years.Reference Batah and Varon4 Although most infants <60 days of age are too young to receive any direct protection from vaccination, it has been observed in the United States and England that they benefited from PCV7 through indirect (herd) protection.Reference Ladhani, Andrews, Waight, Borrow, Slack and Miller5, Reference Soto-Noguerón, Carnalla-Barajas and Solórzano-Santos6 This herd protection also resulted in a drastic decrease of S. pneumoniae included in the PCV7 nasopharyngeal carriage (−97%) in children.Reference Cohen, Biscardi and Levy7 Therefore, IPD due to serotype 14 in neonates should be exceptional, and the occurrence of 2 infections due to this serotype in our ICU in <12 days strongly suggests a nosocomial transmission. Although whole-genome sequencing of the 2 strains was not performed, the sequence type determination revealed a single locus variant of ST156 (CC156) that corresponded to the highly successful clone Spain9V-3.Reference McGee, McDougal and Zhou8 Interestingly, ST2918 is a very rare sequence type among the CC156, and only 1 strain among 2,266 serotype 14 S. pneumoniae isolates available on the PubMLST public databases (https://pubmlst.org/spneumoniae/) belonged to ST2918. Thus, considering the rarity of IPD of serotype 14 and the rarity of ST2918 among serotype 14 S. pneumoniae, it is highly probable that healthcare transmission of the pathogen occurred between the two patients.
In addition, nosocomial pneumococcal bloodstream infections (NPBI) are rarely described in the literature,Reference Bouza, Pintado and Rivera9 with pneumonia being the major portal of entry (70.1%), whereas CLABSI represented only 3.9%. Therefore, our second case is of particular interest; it demonstrates that although CLABSI due to S. pneumoniae is rare,Reference Bouza, Pintado and Rivera9, Reference Canet, Juan, Xercavins, Freixas and Garau10 clinicians should be aware of this potential source of bacteremia.
In conclusion, we report 2 original cases of neonatal S. pneumoniae infection with a highly probable healthcare-associated contamination from one patient to another. These 2 infections were likely due to a single serotype 14 S. pneumoniae strain in neonates that were too young to receive pneumococcal conjugate 13-valent vaccine, and one of these patients developed secondary CLABSI.
Author ORCIDs
Michael Levy 0000-0003-2766-5777
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



