Recurrence of chronic subdural haematomas (CSDH) requiring reoperation occurs in up to 20% of patients.Reference Nakaguchi, Tanishima and Yoshimasu 1 - Reference Ernestus, Beldzinski, Lanfermann and Klug 9 Numerous risk factors for both occurrence and recurrence of CSDH have been proposed.Reference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Asano, Hasuo, Takahashi and Shimosawa 10 - Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 Despite efforts to identify and understand risk factors, the recurrence rate of CSDHs has remained relatively constant over the past few decades.Reference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Ernestus, Beldzinski, Lanfermann and Klug 9 - Reference Foelholm and Waltimo 12 , Reference Markwalder 16 - Reference Amirjamshidi, Abouzari and Eftekhar 18 In this study we evaluated clinical, operative, and radiological factors influencing haematoma recurrence in 331 consecutive cases of CSDHs in patients who underwent surgical drainage in our center. We propose a scoring system based on demographic and radiological features to identify patients at highest risk for CSDH recurrence.
Methods
Ethics approval for this study was obtained from the local institutional Healthcare Research Ethics Board. A retrospective review of 308 consecutive patients between January 2005 and December 2009 was completed, and yielded a total of 331 CSDHs that underwent drainage by 10 treating surgeons. At our institution all CSDHs are drained in an operating room with a catheter attached to a closed drain system for slow, continuous drainage post-operatively. As such, all patients were identified from operative records. Clinical and demographic information was collected via an electronic medical record, including patient age, gender, and use of anticoagulants. Prior to surgery all patients had their coagulation status normalized. Patient use of antiplatelet agents was not investigated. Factors related to operative technique were collected including the type of procedure (burr-hole or craniotomy), both initially and upon recurrence, and the number of recurrences. The picture archive and communication system (PACS) was used to gather pre-operative and post-operative radiological data from computed tomogram (CT) scans of the head for each case including: haematoma volume (calculated by the ABC/2 method previously described for CSDHsReference Gebel, Sila and Sloan 19 - Reference Sucu, Gokmen and Gelal 21 ), midline shift, parenchymal atrophy (assessed based on a three-grade system previously describedReference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Amirjamshidi, Abouzari and Eftekhar 18 ), location (left or right hemisphere), CSDH subtype (hypodense, isodense, hyperdense or mixed as previously describedReference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Amirjamshidi, Abouzari and Eftekhar 18 , Reference Stanisic, Lund-Johansen and Mahesparan 22 ), and the presence of internal haematoma septations as described in previous reports.Reference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 Intra-haematoma septations were defined as visible membranes on CT within the haematoma cavity, separating it into distinct compartments as shown in Figure 1.

Figure 1 Typical CT appearance of a CSDH without visible septations (left) and with septations (right).
Recurrence was defined as the re-accumulation of CSDH requiring drainage within three months of the initial procedure (approximate time-frame allowing at least one clinical follow-up visit and subsequent re-drainage). All patients had a routine early post-operative CT head, and as such radiological data was collected within a few days of surgery. Each variable was subject to univariate analysis using Statistical Analysis System computer software (version 9.2; SAS, Cary, North Carolina) and subsequently used for multivariate analysis using a logistical regression model in order to identify independent risk factors. Chi squared was used for determining the relationship between CSDH recurrence and categorical variables. A t-test was used for continuous variables, and analysis of variance (ANOVA) testing for continuous subgroup variables with logistical regression modeling.
The results of the analysis for each factor and its relation to CSDH recurrence were calculated as an odds ratio and 95% confidence interval. Statistical significance was set at a p-value of less than 0.05. Risk factors related to haematoma recurrence were used in a statistical selection test in order to identify and create the best model for a scoring system based on high-risk patient groups for recurrence. High-risk groups and threshold values for risk factors used in the scoring system (pre-operative haematoma volume < or ≥160 cc, patient age < or ≥80 years, and presence of intra-haematoma septations) were identified based on the 75th percentile of recurrence for each factor. The scoring system to predict CSDH recurrence was then applied to our patient database for internal validation.
Results
The baseline clinical, technical and radiological characteristics are outlined in Table 1. The overall reoperation rate was 11.8% (39 operations). The majority of patients underwent burr-hole drainage (89.4%) as opposed to craniotomy for drainage (10.6%) of their CSDH. Fifteen patients (15/39) underwent repeat burr-hole drainage and 24 patients (24/39) underwent a craniotomy as the second procedure for CSDH drainage. Four patients required an additional third operation for CSDH recurrence, two of which were repeat craniotomies as shown in Table 2.
Table 1 Baseline clinical, technical, and radiological characteristics from patients with 331 chronic subdural haematomas
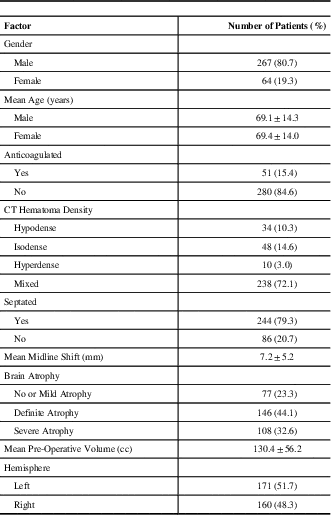
CT=computed tomogram
Table 2 Frequency of chronic subdural haematoma recurrence

CSDH=chronic subdural haematoma
Univariate and multivariate analysis results of the relationship between post-operative residual haematoma volume and each risk factor are shown in Table 3. Multivariate analysis demonstrated that increased pre-operative haematoma volume (p<0.01), a greater amount of parenchymal atrophy (p=0.04), and presence of septations (p<0.01) all to be statistically significant predictors for increased post-operative haematoma volume.
Table 3 Univariate and multivariate analysis results of factors related to increased post-operative residual haematoma volume
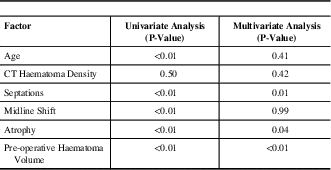
CT=computed tomography
Data from patients with CSDH recurrence requiring reoperation is presented in Table 4. Upon multivariate analysis, the presence of CSDH septations pre-operatively (p=0.04) was found to be a predictor for requiring a second operation for CSDH drainage. Patients with larger residual haematomas following their initial surgical drainage were also more likely to require a second operation for re-drainage of a haematoma (p<0.01).
Table 4 Univariate and multivariate analysis results of factors related to haematoma recurrence requiring repeat drainage
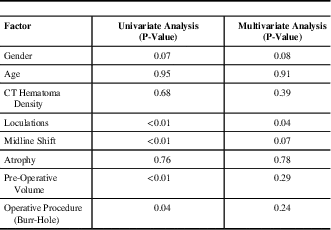
CT=computed tomography
A scoring system predicting CSDH recurrence was created using statistical selection methods, as shown in Table 5 and Figure 2. Patients at highest risk for reoperation were stratified based on age (< or ≥80 years), pre-operative haematoma volume (< or ≥160cc) and presence of septations on pre-operative CT (yes or no). For each risk factor identified, an additional point is added to the total score (for example, a patient who is ≥80-years-old, has a haematoma volume ≥160cc, and has a septated CSDH on CT would be attributed a total score of 3). This scoring system was found to be strongly associated with predicting post-operative residual haematoma volume. There was also a significant trend for predicting reoperation (p=0.02) on chi-square testing. Based on this scoring system and our dataset, the rate of reoperation based on haematoma recurrence for patients was 4.8%, 11.7%, 13.8%, and 20.7% for scores of 0, 1, 2, and 3, respectively (Figure 2).

Figure 2 Patient CSDH recurrence risk based on their cumulative risk factor score.
Table 5 Risk factor scoring system for chronic subdural haematoma recurrence requiring repeat surgical drainage
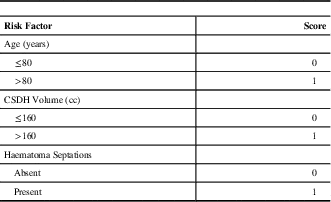
CSDH – chronic subdural haematoma; cc=cubic centimeters
Discussion
Several risk factors for CSDH recurrence have been previously reported, including: advanced age, bleeding tendency, alcohol abuse, brain atrophy, hematoma density, bilateral CSDHs, among others.Reference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Asano, Hasuo, Takahashi and Shimosawa 10 - Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Krupp and Jans 23 - Reference Okada, Akai, Okamoto, Iida, Takata and Iizuka 24 However, results identifying consistent risk factors have been difficult to reproduce.Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 , Reference Mori and Maeda 4 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Torihashi, Sadamasa, Yoshida, Narumi, Chin and Yamagata 25 Through multivariate analysis and logistic regression we sought to identify risk factors for CSDH recurrence requiring repeat drainage and increased post-operative subdural haematoma volume. Outlined are the risk factors found to be associated with increased post-operative haematoma volume and CSDH recurrence requiring repeat drainage. A simple scoring system for predicting CSDH recurrence for patients is also proposed.
Septations
The presence of septations within a CSDH has been previously related to recurrence.Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 , Reference Stanisic, Lund-Johansen and Mahesparan 22 , Reference Tanikawa, Mase and Yamada 26 However, separate statistical evaluation of septations within the clot has rarely been done. In the present study, the presence of septations was statistically significant for higher residual haematoma volume and CSDH recurrence requiring re-drainage. Septated haematomas are more difficult to drain as disruption of each compartment is necessary for complete drainage.
Atrophy
A greater amount of parenchymal atrophy was found on multivariate analysis to be a predictor of increased post-operative haematoma volume. It has been well described as being a risk factor for both occurrence and recurrence of CSDH.Reference Nakaguchi, Tanishima and Yoshimasu 1 , Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Amirjamshidi, Abouzari and Eftekhar 18 , Reference Stanisic, Lund-Johansen and Mahesparan 22 , Reference Torihashi, Sadamasa, Yoshida, Narumi, Chin and Yamagata 25 This has been explained by examining brain elastance. Higher elastance is associated with increasing age, atrophy and persistence of a subdural space.Reference Mori and Maeda 4 , Reference Fukuhara, Gotoh, Asari, Ohmoto and Akioka 14 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Amirjamshidi, Abouzari and Eftekhar 18
Pre-operative Haematoma Volume
Increased pre-operative haematoma volume was a significant predictor of higher post-operative residual haematoma volume. Larger CSDHs have a lower surface to volume ratio, which has been suggested to result in decreased absorption of the haematoma fluid once a particular threshold is passed.Reference El-Kadi, Miele and Kaufman 11 Larger haematomas are also more difficult to completely drain (particularly through a burr hole). As a result, incomplete drainage and less promotion of thrombosis and re-absorption may occur.
Post-Operative Haematoma Volume
Interestingly, increased post-operative haematoma volume was significantly associated with requiring a second drainage procedure. Within a CSDH there is a micro-environment of hyperfibrinolysis and hypercoagulation.Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 Haematoma resolution is determined by the difference between re-absorption and microvascular leakage.Reference Markwalder 16 , Reference Ito, Komai and Yamamoto 27 - Reference Weir 31 If there is a larger residual haematoma, then the balance may not have been shifted beyond this therapeutic threshold.
Scoring System
The combination of increased pre-operative haematoma volume, advanced patient age, and the presence of intra-haematoma septations was found to have the greatest statistical influence on predicting increased residual haematoma volume and operative recurrence. By dichotomizing patients into high- and low-risk groups based on these variables, it was possible to quantify the increased risk of recurrence for patients from our database. For example, we found that patients with a score of ‘3’ had a more than four-fold risk of recurrence compared to a patient with a score of ‘0’ (≤80-years-of-age, no septations, and haematoma volume ≤160cc), and just under a two-fold risk compared to patients with a score of ‘1’. This offers important prognostic information pre-operatively regarding an individual patient’s risk of recurrence, as well as post-operatively with respect to following patients clinically and/or radiologically.
Limitations
This is a single-observer, retrospective study and, as such, is subject to bias. Despite a large patient cohort, a relatively lower rate of recurrence was found. Although it is ideal to have a recurrence rate as low as possible, this was also a limiting factor for identifying risk factors in our multivariate analysis model. Specifically, technical factors including the number of burr holes, lysis of subdural space septations, irrigation of the subdural space, the size of the craniostomy for drainage, amount of post-operative pneumocephalus, local or general anaesthetic use, among others, have been debated as affecting recurrence rates and neurological outcomes.Reference Yamamoto, Hirashima, Hamada, Hayashi, Origasa and Endo 2 , Reference Mori and Maeda 4 , Reference Oishi, Toyama, Tamatani, Kitazawa and Saito 17 , Reference Amirjamshidi, Abouzari and Eftekhar 18 , Reference Stanisic, Lund-Johansen and Mahesparan 22 , Reference Santarius, Kirkpatrick and Ganesan 32 - Reference Mondorf, Abu-Owaimer, Gaab and Oertel 36 In addition, although previous studies have found a difference in CSDH recurrence rates based on the initial procedure, here this was not found to be an independent risk factor upon multivariate analysis. However, it is possible that the number of patients in either cohort was not large enough to detect such as difference. Furthermore, the use of a drainage system has been previously shown to reduce recurrence and six-month mortality.Reference Santarius, Kirkpatrick and Ganesan 32 Although it is routine practice at our institution to use a drain in CSDH drainage, other technical factors may have varied between treating surgeons affecting recurrence rate. For example, some surgeons will prefer to do a larger burr-hole or “mini-craniotomy” if septations are present, or prefer to use different types of craniostomy drills resulting in variable burr-hole sizes. Follow-up studies with the creation of a similar scoring system focusing more on clinical and technical factors such as those listed and less on radiological factors could prove useful. Moreover, patient use of antiplatelet agents was not accounted for. Increasing use of anti-platelet agents amongst patients is a potential limitation that should be accounted for in future studies. The creation of a scoring system for CSDH recurrence is novel, however further testing is required with larger sample sizes and/or recurrence rates from other datasets to validate this system. A larger sample size and/or higher recurrence rate may also allow for the inclusion of more factors. Application of this scoring system to prospectively collected datasets of clinical and radiological factors, in addition to its application to datasets collected from other institutions, is necessary for its validation.
Conclusion
Increased pre-operative haematoma volume, a greater amount of brain atrophy, and the presence of intra-haematoma septations were all risk factors on multivariate analysis for a larger post-operative haematoma collection. The presence of loculations and higher post-operative subdural haematoma volume were both predictors of CSDHs requiring reoperation. When septations are clearly visible within a CSDH on CT, craniotomy might be more suitable as a primary procedure as it allows more complete drainage of a septated subdural collection. A scoring model based on patient age, haematoma volume, and presence of intra-haematoma loculations may serve as a clinically useful way to help identify patients at risk for recurrence.
Disclosures
The authors of this paper have nothing to disclose.









