Introduction
The inflammatory process underlying atherosclerosis is thought to begin in early childhood. Early atherosclerotic changes can be found in infants as young as 9 months of age [Reference Holman1–Reference Strong and Mc4]. The classical risk factors, such as exposure to tobacco smoke, high body mass index (BMI) and high cholesterol, may play an important role in the development of early atherosclerotic changes in childhood by inducing inflammation. Yet, these can only explain part of the variation in atherosclerotic changes observed between individuals [Reference Cheng5–Reference Ross8].
In the past decades the role of infectious diseases in the pathogenesis of atherosclerosis has been increasingly recognised [Reference O'Connor9, Reference Liuba and Pesonen10]. It is hypothesised that infections contribute to the inflammatory process underlying atherosclerosis by stimulating the production of inflammatory cytokines and by changing serum levels of high-density lipoprotein cholesterol (HDLC) and low-density lipoprotein cholesterol [Reference O'Connor9–Reference Liuba11]. As such, the numerous infectious disease episodes a child typically experiences in early life may have a cumulative effect on the atherosclerotic process and this may be most pronounced for infections that elicit a systemic inflammatory response [Reference Liuba and Pesonen10].
Short-term effects of acute childhood infectious diseases on vasculature and cholesterol have indeed been documented. Studies have reported reduced endothelium-dependent vasodilatation, increased intima media thickness and decreased HDLC in the weeks or months following the infection [Reference Liuba11–Reference Pesonen, Paakkari and Rapola13]. It is not known to what extent these effects persist into adolescence and adulthood and to what extent these effects are influenced by other mediating factors of atherosclerosis, including sex and socioeconomic status (SES) [Reference Burgner14–Reference Liu18].
Infection induced repeated systemic inflammatory responses are best approximated by quantifying the number of febrile infections [Reference Liuba and Pesonen10]. While this can be obtained retrospectively from parental questionnaires, such data may not be sufficiently reliable and may be subject to recall bias. Alternatively, infection-related hospitalisation data can be used but this only covers severe infections. Longitudinal general practitioner (GP) medical records may provide a suitable alternative data source, as they include prospectively recorded, doctor diagnosed infectious disease episodes as well as any antibiotic treatment [Reference Liuba11, Reference Evelein17].
In the participants of the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study, we set out to determine the role of repeated febrile infections during early childhood in inducing early atherosclerotic changes, as reflected in common carotid intima media thickness (CIMT) levels in adolescence. We hypothesise that infections that trigger a visit to a GP reflect moderate to severe infections, in particular when associated with antibiotic treatment, and are therefore more likely to be associated with an increase in common CIMT compared to parent-reported infections [Reference Liuba and Pesonen10]. This study investigated associations between common CIMT levels in adolescents and the number of (1) GP diagnosed febrile infections, (2) antibiotic prescriptions and (3) parent-reported infections in the first 4 years of life. We restricted the exposure period to the first 4 years of life as the infectious disease incidence is generally highest in these early years of childhood [Reference Chen and Kirk19, Reference Schlinkmann, Bakuli and Mikolajczyk20]. In addition, it was investigated whether these associations were dependent on sex and SES.
Methods
Study population
This study is part of the PIAMA study, a Dutch population-based birth cohort. The PIAMA study has been described in detail elsewhere [Reference Brunekreef21, Reference Wijga22]. In short, pregnant women were recruited from the general population through antenatal clinics located in the north, center and west of the Netherlands, resulting in a baseline study population of 3963 children born in 1996 and 1997. PIAMA questionnaires were sent to the parents during pregnancy, at age 3 months and thereafter annually around the birthday of the child until the age of 8 years and included questions on occurrence of infectious diseases. Medical examinations were performed at the ages of 1, 4, 8, 12 and 16 years. At age 16 years, 2159 active participants of two of the three study centres were invited for the medical examination and common CIMT was measured in one of these study centres (Utrecht). At age 18 years, all 3015 active participants in the three study centres (76% of the baseline population) were approached to consent for collection of GP data covering the full 18 years. A total of 1519 (50%) participants gave written informed consent. The medical ethics committees of the participating institutes approved the study protocol.
Eligible for the present study were the participants who were invited for the common CIMT measurement at age 16 years (N = 1232), of these participants 60% did not respond or gave no informed consent for the medical examination. In 5.6% of the participants, no IMT measurement was performed due to logistical reasons or measurement errors. The population for analysis included participants with common CIMT measurements and GP data available for at least one of the first 4 years of life (N = 221, 18%) (Fig. 1).

Fig. 1. Flowchart of study population for common carotid intima media thickness (IMT) measurement.
Data collection
During the medical examination at age 16 years, IMT was measured bilaterally in the distal common carotid artery proximal to the bifurcation at six standard angles (210, 240 and 270 left side and 90, 120, 150 right side) using the automated measurement of the Panasonic CardioHealth® Station (Panasonic Healthcare) by trained research staff. The measurement region was automatically identified by the software and frozen when the accuracy was high; the common CIMT was measured in millimeters over a standard length of 10 mm in the end-diastolic phase. Mean common CIMT was calculated by averaging the common CIMT of the six measurement angles. If <6 measurements were available, mean common CIMT was calculated with the available measurements, the minimum number of measurements was 3. In addition, at the medical examination at age 16 a blood sample was taken and serum total cholesterol (TC) and HDLC were determined enzymatically using Roche automated clinical chemistry analysers (Roche Diagnostics, Indianapolis, IN, USA). The levels of TC and HDLC in mmol/l were used in the analysis to investigate whether cholesterol is a mediator in the association between febrile infections and common CIMT.
GP data were collected by sending letters to the GPs including a questionnaire to obtain participant's infectious diseases diagnoses and any medication related to infections and allergies. Alternatively, GPs could request on-site data collection by the research team or send an extract of the electronic patient file to the research team. GPs provided a start and end date for the period for which they registered the diagnoses and medication. If a start date was within 6 months after birth and the end date was after 4 years of life, the GP data was considered complete for the first 4 years of life. Complete GP data on at least one of the first 4 years of life was available for 879 (58%) of the 1519 consenting participants and complete information for the first 4 years of life was obtained for 725 (48%).
The number of parent-reported infections as well as indoor smoke exposure in the first 4 years of life were retrieved from the annual questionnaires. Information regarding education and allergies of the parents, pre-pregnancy BMI of the mother, birthweight of the participant and breastfeeding were retrieved from the questionnaires completed by the parents during pregnancy and at age 3 months.
Definition of exposure potential confounders
GP diagnosed infections
For each participant the number of GP diagnosed infections was determined by counting the number of GP diagnosed febrile infections during the first 4 years of life. Febrile infections were defined as infectious diagnoses according to the International Classification of Primary Care (ICPC) coding for which a typical disease course includes one or more days with fever, such as acute upper or lower respiratory tract infection and urinary tract infection. A list of ICPC codes and corresponding infectious diagnoses can be found in Table S1. Similarly, the number of antibiotic prescriptions in the first 4 years of life was counted including prophylactic and repeated prescriptions, when two antibiotics were prescribed on the same day this was counted as one prescription.
Parent-reported infections
For parent-reported infections, we used the number of infections reported in the annual questionnaires during the first 4 years of life. The number of parent-reported infections in the first 4 years of life was defined as the number of infections in the child over the past 12 months, including any severe respiratory tract infections (infections of the throat, nose and ears, e.g. influenza, pharyngitis, otitis media, bronchitis, pneumonia and sinusitis) and occurrence of chickenpox and physician-diagnosed measles or whooping cough. From these data, an infection count variable was created for each year of life.
Confounder definitions
Sex, parental education, birth weight, breastfeeding, pre-pregnancy overweight, allergy of the mother and indoor smoke exposure up to age 4 were included in all analyses as a priori potential confounders. The selection of confounders was based on available literature [Reference Charakida12, Reference Burgner14–Reference Liu18]. Figure S1 shows a DAG of these associations. A binary parental education variable was used as a measure of SES, defining high parental education as completed higher vocational or university education by at least one parent. Breastfeeding was categorised into no breastfeeding, ⩽16 weeks of breastfeeding and >16 weeks of breastfeeding. Pre-pregnancy overweight was defined as a maternal BMI of ⩾25 kg/m2 before pregnancy. Maternal allergy was considered positive if a mother ever had asthma, pet allergy, house dust mite allergy, or nasal allergy such as hay fever [Reference Lakwijk23]. Exposure to indoor smoke was considered present when smoking occurred within the home at least once a week at ages 3 months, 1, 2, 3 or 4 years.
Statistical analysis
The incidence rates per year were calculated for the three infectious disease exposure variables investigated in this study, namely ‘number of GP diagnosed infections’, ‘number of antibiotic prescriptions’, and ‘number of parent-reported infections’. These variables were categorised into four categories of about equal size based on quartiles in order to limit the influence of outliers and to allow for a non-linear association.
The associations between each of these exposure variables and common CIMT as a continuous outcome variable were investigated using separate linear regression models. All a priori defined potential confounders were added to the models. Additionally, as previous evidence suggests that the atherosclerotic effect of infections is perhaps in part mediated through changes in serum cholesterol, we investigated this by adding TC, HDL, or both to the adjusted models [Reference O'Connor9–Reference Liuba11]. When this resulted in meaningful changes of >30%, a threshold selected by the authors, in the parameter estimate for the primary exposure, mediation was considered present. We investigated whether the association between number of infections and common CIMT was dependent on SES or sex by assessing the presence of significant interaction in the adjusted models as this has been shown by previous studies [Reference Dratva16, Reference Liu18]. In addition, in explorative post-hoc analyses it was investigated whether the other potential confounders in our model showed significant interaction with number of infections. When the P-value of the interaction term was <0.10, interaction was considered present.
To prevent bias in the parameter estimates, missing values for confounders and number of infections were imputed for participants with GP data available for at least one of the first 4 years of life and a successful common CIMT measurement (N = 185). We did not impute data for all 1232 participants invited for the medical examination at age 16, since this may introduce bias due to the large amount of missing data that would have been imputed. The imputation model included confounders, outcome and predictors of childhood infections, namely day care attendance, presence of older siblings in the household and paternal allergy. We imputed missing values using Multivariate Imputation by Chained Equations (MICE). Data were imputed using the Random Forest method.
Analyses were performed using SPSS version 24.0.0.1 (IBM Corp., Armonk, New York) and RStudio version 1.0.143 (RStudio, Boston, Massachusetts). The confidence intervals around the incidence rates were calculated using OpenEpi (Open Source Epidemiologic Statistics for Public Health, version 3.01) [Reference Dean, Sullivan and Soe24].
Results
Of 1232 PIAMA participants invited for the medical examination at age 16 years, 489 (40%) participated and IMT was successfully measured in 420 (34%). Of these participants, 221 had GP data available for at least one out of the first 4 years of life and were included in the current analysis (Fig. 1). GP data were complete for all 4 years in 185 participants and parent-reported infectious disease data were available for 207 participants (Table 1).
Table 1. Characteristics of study population with common carotid intima media thickness (IMT) measurement and general practitioner data on at least one of the first 4 years of life

GP, general practitioner; IMT, intima media thickness of common carotid artery; MI, multiple imputation; s.e., standard error.
a Variable was not imputed.
Table 1 shows the characteristics of the population for analysis; characteristics of the participants eligible for the current study can be found in Table S2. The mean common CIMT was 465 µm at a mean age of 16.3 years. Multiple imputation did not meaningfully change the distribution of the characteristics of the study population as shown in Table 1. The mean incidence of GP diagnosed infections up to age 4 years was 1.18 per year after imputation (95% CI 1.12–1.26) and 1.45 (95% CI 1.37–1.53) for parent-reported infections (Table 2). For further analyses, the imputation datasets were used.
Table 2. Prevalence of different measures of childhood infections in the first 4 years of life

MI, Multiple imputation; GP, general practitioner.
In the total study population no significant associations were found between any of the cumulative incidence measures of childhood infections and common CIMT (Table 3). The adjusted models included all a priori defined potential confounders and one of the infection measures, adding TC and/or HDLC did not result in meaningful changes of the parameter estimate for any of the infection measures (Table S3).
Table 3. Difference in common carotid intima media thickness (IMT) at age 16 years between exposed and reference group after multiple imputation
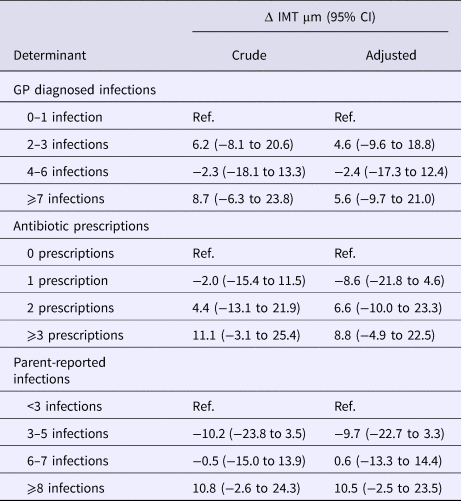
Models adjusted for sex, education of parents, birthweight, overweight of the mother before pregnancy, breastfeeding, allergy of the mother and indoor smoke exposure.
GP, general practitioner; IMT, intima media thickness of common carotid artery.
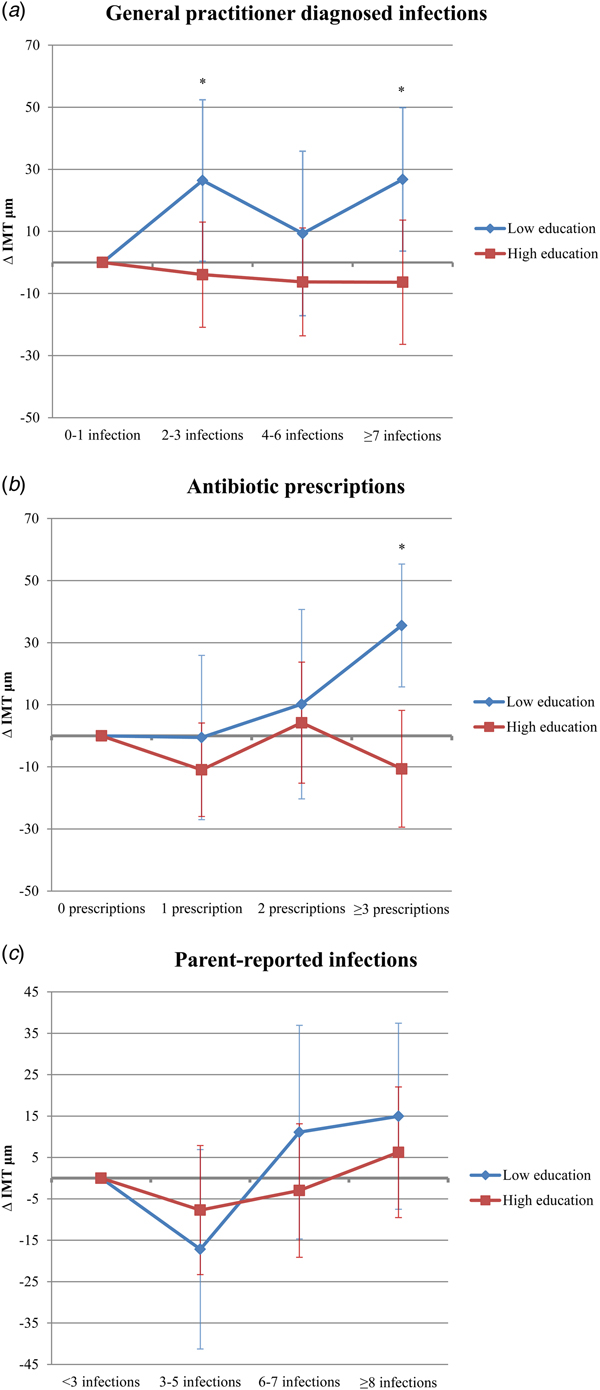
The number of antibiotic prescriptions and GP diagnosed infections showed statistically significant interaction with parental education for the association with common CIMT, therefore, an exploratory analysis was performed stratifying the results according to education level (Fig. 2). The number of GP diagnosed infections and antibiotic prescriptions during the first 4 years were positively associated with common CIMT in adolescence in participants with a low level, but not in those with a high level of parental education. Low parental education participants with 2–3 or ⩾7 GP diagnosed infections had a +26.4 µm and +26.8 µm higher common CIMT, respectively, than those with 0 or 1 GP diagnosed infections. Also, participants with ⩾3 prescriptions had a +35.5 µm higher common CIMT than those without antibiotic prescriptions. No significant association between 4 and 6 GP diagnosed infections and common CIMT was observed for low parental education participants. None of the infectious disease exposures showed statistically significant interaction with sex. P-values of the interaction terms for the other confounders can be found in Table S4.

Fig. 2. Difference in common carotid intima media thickness (IMT) at age 16 stratified by parental education for number of general practitioner diagnosed infections (A), antibiotic prescriptions (B) and parent-reported infections (C). *Indicates a P-value of <0.05.
Discussion
Overall, we observed no association between number of infections or antibiotic prescriptions during the first 4 years of life and common CIMT in adolescence. Analysis in strata of low and high parental education showed that a higher frequency of GP diagnosed childhood infections and antibiotic prescriptions were associated with an increased common CIMT at age 16 years only for participants with a low parental education level.
We hypothesised that repeated febrile infections severe enough to warrant a GP consultation, and in particular when requiring antibiotic treatment, would be associated with increased common CIMT in adolescence due to the repeated inflammatory responses elicited by the infections [Reference Liuba and Pesonen10, Reference Burgner15–Reference Evelein17]. This hypothesis was not confirmed in the total study population of the current study and findings of previous studies have been inconsistent [Reference Liuba11, Reference Burgner15–Reference Evelein17, Reference Burgner25–Reference Qanitha27]. Studies relating infection-related hospitalisations (IRH), tonsillectomy, appendectomy, or infections with 3 days of fever in childhood (before 20 years) to cardiovascular diseases, such as acute coronary syndrome and acute myocardial infarction, in adulthood reported an increased risk [Reference Burgner25–Reference Qanitha27]. Yet the study by Burgner et al., did not find an association between IRH and common CIMT in adulthood [Reference Burgner15]. However, IRH include only the infections sufficiently severe to warrant hospitalisation and therefore do not reflect the total burden of repeated (febrile) infections, while the repeated character of the infection induced inflammation as such, might be related to the development of atherosclerosis [Reference Liuba and Pesonen10]. Previous studies investigating repeated infections and IMT in adolescence have reported inconsistent results. The study of Evelein et al., did not observe an association between number of GP diagnosed infections and common CIMT at age 5 years [Reference Evelein17]. As atherosclerosis is the result of an inflammatory process developing over time, the effect of infections in the first 5 years of life on CIMT might not be measurable at age 5 years [Reference Libby6–Reference Ross8, Reference Liuba and Pesonen10, Reference Evelein17]. Another study investigating the effect of repeated childhood infections (reported by parents) did find an association with common CIMT, the analysis was restricted to severe infections (bronchitis, pneumonia, tonsillitis, otitis, mononucleosis, meningitis, appendicitis, salmonellosis and scarlet fever), supporting the hypothesis that repeated (severe) infections might stimulate the development of atherosclerosis [Reference Dratva16]. The parent-reported infections in the current study were not restricted to severe infections and focused mostly on the respiratory tract which might explain why we did not observe a similar association. The negative findings in our study are in line with the study of Evelein et al., suggesting that repeated mild and moderate infections in general have little or no impact on the early atherosclerotic process [Reference Evelein17].
The current study however, suggests that the association between repeated infections and common CIMT may be present in specific subgroups, i.e. persons with a low parental education level, which is associated with lower SES. This is in line with the findings of Liu et al. [Reference Liu18], who observed a differential effect of infections in low vs. high SES subjects on flow-mediated dilatation (FMD), a measure of endothelial dysfunction and thought to precede development of atherosclerosis and associated increase in IMT [Reference Ross8, Reference Liu18, Reference Halcox28–Reference Ross30]. Yet, Liu et al., did not find any significant differential effect of infections on common CIMT in low vs. high SES individuals [Reference Liu18]. Low SES has been related to higher levels of inflammatory markers. Perhaps, infections only affect vasculature in persons who are already in a (low-grade) inflammatory state due to lifestyle factors related to SES, resulting in an increased CIMT after the inflammation is boosted by the infectious disease [Reference Liu18, Reference Fraga31–Reference Koster33]. This could explain the reported differential effects on common CIMT and FMD in low vs. high SES individuals. Another explanation might be that the inflammatory response following infection is generally stronger in persons with low SES, for instance due to differences in immune functioning and therefore results in increased vascular effects [Reference Liuba and Pesonen10, Reference Cohen34, Reference Cohen35]. Differences in the effects of early childhood infections on CIMT between persons with low and high SES could potentially explain the inconsistent findings of previous studies if they did not assess such interactions.
Similarly, in participants with lower educated parents we found that the number of infections treated with antibiotics in the first 4 years of life was positively associated with common CIMT at age 16 years. An antibiotic prescription could be a marker of more severe infection, which might explain the larger effect estimate for antibiotic prescriptions compared with GP diagnosed infections. This is in line with the study of Evelein et al., which reported an association between antibiotic prescriptions and an increase in common CIMT [Reference Evelein17].
The current study has some limitations, for instance parent-reported infections were mostly limited to respiratory tract infections. However, as respiratory tract infections are the most common infections in childhood, we consider it unlikely that this limitation has influenced the results. The study population of the current study consisted mostly of Dutch participants. The development of atherosclerosis is suggested to be influenced by ethnicity, but whether the effects of infections, if any, are different for different ethnic groups is unknown [Reference Tattersall36, Reference Tzou37]. The current study investigated a subsample of the PIAMA study population due to data availability limitations, however when comparing Table 1 with Table S2, the differences in participant characteristics are limited. Therefore, we consider it unlikely that our results would have been different when we would have been able to analyse the entire eligible population. The percentage of participants with a high SES, as defined by educational level of the parents, was higher than the percentage with low SES in this study. As a low SES is associated with a higher prevalence of childhood infections and an increased susceptibility to infections, this might have influenced the strength of the associations reported in this study and could explain the absence of a significant association for the 4–6 GP diagnosed infections category in participants with low parental education [Reference Liu18, Reference Cohen34, Reference Cohen35, Reference Ruijsbroek38]. Due to sample size restrictions the subgroup analyses have limited statistical power and should be interpreted with caution. There is a chance for false positive findings in small samples, however the chance of not finding any interaction would presumably be higher. In addition, statistically significant interaction was observed for multiple infectious disease exposures, therefore we consider it unlikely that the reported interaction is a chance finding, however, the results of the current study need to be confirmed in future studies.
Conclusion
The study suggests that at population level the number of early childhood infections is not associated with the development of early atherosclerotic changes in adolescence. In participants with lower parental education, GP diagnosed and antibiotic treated infectious diseases, probably indicative of more severe infections were associated with increased common CIMT at age 16 years. Although it is based on relatively small number, this suggests that infections might contribute to other (inflammatory) processes, ultimately resulting in increased common CIMT and atherosclerosis, only in subgroups of the population. As the development of atherosclerosis can start in early childhood, identifying determinants of this early development is important for the recognition of potential risk populations as well as potential targets for prevention.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026881800287X
Author ORCIDs
Annemarijn Prins-van Ginkel http://orcid.org/0000-0002-0767-736X.
Acknowledgements
The authors gratefully thank the contribution of all participating children and parents or caregivers of the PIAMA study. They thank the general practitioners for their participation in the collection of the infectious disease data. They also thank Ada Wolse, Marjan Tewis, Marieke Oldenwening and Linda Pluymen for their contribution to the data collection and data management. They thank Barbara Strijbosch, Simone Ruijs, Jonathan Eindhoven, Willem Miellet, Lisanne Verbruggen, Kirsty Verheggen, Maxime de Jong and Rowan van Rooijen for their contribution to the general practitioner data collection. The PIAMA study is supported by The Netherlands Organization for Health Research and Development, The Netherlands Organization for Scientific Research, The Netherlands Asthma Fund, The Netherlands Ministry of Spatial Planning, Housing and the Environment and The Netherlands Ministry of Health, Welfare and Sport.
Conflict of interest
None.