Soyabean oil (SO) is extensively used in the diets of mammals to supply them with energy and essential fatty acids. It contains more than 50 % n-6 PUFA, mainly linoleic acid (18 : 2n-6). Apart from their beneficial effects, emerging evidence has revealed n-6 PUFA to be prooxidative. Indeed, PUFA are substrates for free-radical reactions and they result in lipid peroxidation( Reference Mehta, Gunnett and Harris 1 ). This process could lead to the production of peroxides, which are reactive and toxic species, as well as their decomposition products. They can form organic free radicals, causing a cascade of damages to endogenous lipids and oxidative stress( Reference Droge 2 , Reference Hennig, Enoch and Chow 3 ). In fact, a positive correlation has been observed between the amounts of dietary PUFA and the extent of lipid peroxidation( Reference Mehta, Gunnett and Harris 1 ).
There is growing evidence that intra-uterine growth retardation (IUGR) causes oxidative stress in offspring, as evidenced by increased reactive oxygen species (ROS) generation and oxidative damages( Reference Biri, Bozkurt and Turp 4 – Reference Park, Kim and Kim 6 ). This results from mitochondrial dysfunction and an impaired antioxidant defence system( Reference Liu, Yao and Yu 5 – Reference Michiels, De Vos and Missotten 8 ), which are associated with growth and developmental restriction during pregnancy because of inadequate nutrient uptake, environmental stress, diseases and other factors( Reference McMillen and Robinson 9 , Reference Wu, Bazer and Wallace 10 ). Thus, feeding IUGR offspring with a PUFA-rich diet might have long-term, low-intensity, negative effects on their health status.
Medium-chain TAG (MCT) are six- to twelve-carbon fatty acid esters of glycerol. Compared with SO, MCT are rapidly removed from the body and stored to a small degree, because medium-chain fatty acids directly enter the liver via the portal vein for energy production through mitochondrial β-oxidation, whereas long-chain fatty acids first enter the lymph system and then into a variety of tissues via the blood( Reference Wojtczak and Schonfeld 11 ). Medium-chain fatty acids are fully saturated and therefore have much greater oxidative stability. Thus, the aim of the present study was to investigate the effects of MCT on hepatic oxidative damage in weanling piglets with IUGR so as to establish new feeding strategies to improve the growth and health of IUGR offspring.
Materials and methods
Materials
MCT (consisting of caprylin and decanoin) and SO were obtained from Yihai Oils & Grains Industries Company, Limited. The fatty acid constituents of the test oils as measured by GC (GC7890, Agilent Technologies) are given in Table 1.
Table 1 Fatty acid constituents of the test oils (g/100 g total fatty acids)
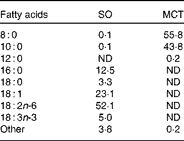
SO, soyabean oil; MCT, medium-chain TAG; ND, not detected.
Animals and treatments
The experimental protocols were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. Pregnant sows (Landrace × Yorkshire) with similar parity (second or third) were fed a commercial diet during pregnancy. At birth, the birth weight (BW) and sex of each newborn piglet (Duroc × (Landrace × Yorkshire)) were recorded carefully. A piglet was defined as IUGR when its BW was 2 sd below the mean BW of the total population( Reference Wang, Huo and Shi 12 , Reference Wang, Zhang and Zhou 13 ). In each litter, one male IUGR piglet with a mean BW of 0·95 (sd 0·04) kg and one normal same-sex littermate with a mean BW of 1·58 (sd 0·04) kg were chosen. At weaning (mean 21 (sd 1·06) d of age), twenty-four IUGR piglets and twenty-four normal-birth weight (NBW) piglets were selected according to their BW and weight at the time of weaning (IUGR: mean 5·26 (sd 0·15) kg and NBW: mean 6·98 (sd 0·19) kg) and transferred to the weaner unit. Both IUGR and NBW piglets were fed a SO diet or a MCT diet. Thus, all piglets were distributed into groups of four treatments (NBW-SO, NBW-MCT, IUGR-SO or IUGR-MCT) × four pens × four piglets per pen for 28 d. The composition of the diets is given in Table 2. Piglets were given free access to food and water until the day of sampling. The body weight of piglets was measured at the end of the experiment, and feed intake was recorded on a pen basis during the experiment to calculate the average daily gain (ADG), average daily feed intake and feed efficiency.
Table 2 Composition of the basal diets (as-fed basis)
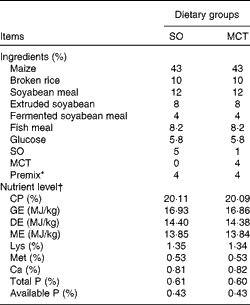
SO, soyabean oil; MCT, medium-chain TAG; CP, crude protein; GE, gross energy; DE, digestible energy; ME, metabolisable energy.
* The premix provided the following per mg/kg diet: retinyl acetate, 4·79; cholecalciferol, 0·075; all-rac-α-tocopherol acetate, 100; menadione, 3; thiamin, 3; riboflavin, 8; nicotinamide, 5; cobalamin, 0·04; biotin, 0·3; pantothenic acid, 20; niacin, 45; folic acid, 2; choline chloride, 450; Fe (as FeSO4·H2O), 180; Cu (as CuSO4·5H2O), 230; Zn (as ZnO), 65; Mn (as MnSO4·H2O), 50; I (as KIO3), 0·5; Se (as Na2SeO3), 0·2.
† All nutrient content values, except DE and ME values, were analysed values.
Sample collection
After treatment for 4 weeks, four piglets with nearly equal body weight were selected from each treatment group (one piglet per pen). Heparinised blood samples were drawn by jugular venepuncture and then centrifuged at 2000 g for 10 min at 4°C, and plasma was stored at − 80°C for further analyses. All piglets were killed by electrical stunning and exsanguination, and liver samples were collected from them within 5 min and stored in liquid N2 for further analyses. A fraction of the fresh liver samples were rapidly treated for hepatocyte isolation to determine the levels of ROS, apoptosis and necrosis.
Hepatocyte isolation
During hepatocyte isolation, the haematopoietic cell population of the liver is eliminated. More than 90 % of the cells are typical hepatocytes, and the remaining 10 % are fibroblast-like cells( Reference Lane, Crawford and Flozak 14 ). Livers were harvested from piglets and placed in ice-cold Hanks' balanced salt solution (HBSS) until the completion of harvest. Approximately 3 g of liver samples were minced and then shaken for 5 min at 37°C in 25 ml of HBSS containing 5 mm-EDTA. The minced samples were shaken for another 10 min in 25 ml of HBSS containing 0·25 % (w/v) collagenase I (Sigma-Aldrich), 0·01 % (w/v) DNase (Sigma-Aldrich) and 5 mm-CaCl2 at 37°C. The supernatant was removed, and the digestion process was repeated. The cell suspension was filtered through a 70 μm nylon mesh and centrifuged at 20 g for 1 min. The pellet was resuspended in PBS and centrifuged at 20 g for 1 min. Cell viability was determined by trypan blue exclusion.
Determination of reactive oxygen species levels
Intracellular ROS levels were determined using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich) as described previously( Reference Wang, Yang and Zhang 15 ). Inside the cells, DCFH-DA is cleaved by non-specific esterases leading to the formation of DCFH, which is in a non-fluorescent form and is oxidised to the fluorescent compound 2′,7′-dichlorofluorescein by ROS. Approximately 1 × 106 hepatocytes were washed with PBS and incubated with 10 μm of DCFH-DA in the dark for 20 min at 37°C. After washing with ice-cold PBS twice, hepatocytes were harvested and detected immediately using an FACScan flow cytometer (Beckman Coulter).
Determination of apoptosis and necrosis levels
Apoptosis and necrosis levels were determined by differential staining with Annexin V (which stains apoptotic and necrotic cells) and propidium iodide (which stains only necrotic cells) using the Alexa Fluor® 488–Annexin V/Dead Cell Apoptosis Kit (V13241; Invitrogen Life Technologies) as described previously( Reference Xu, Zhu and Wu 16 ). This method is based on the phosphatidylserine-binding property of Annexin V and the DNA-intercalating capability of propidium iodide. Briefly, the density of hepatocytes was determined, and approximately 1 × 106 hepatocytes were resuspended in a 1 × binding buffer. Then, a solution containing propidium iodide and Alexa Fluor® 488–Annexin V was added to the cell suspension. The cell suspension was gently vortexed and incubated for 15 min at room temperature in the dark. Finally, a 4-fold volume 1 × binding buffer was added to each tube and analysed immediately using an FACScan flow cytometer (Beckman Coulter).
Tissue homogenate preparation and biochemical assay
Approximately 0·1 g of a frozen liver sample was removed quickly and placed in a 1:10 (w/v) buffer (pH 7·4) containing 10 mm-Tris–HCl, 0·1 mm-EDTA–Na2, 10 mm-sucrose and 0·8 % (w/v) NaCl according to the instructions provided with the kit obtained from the Nanjing Jiancheng Institute of Bioengineering. Liver samples were homogenised using an Ultra-Turrax homogeniser (Tekmar) at 13 500 rpm for 1 min. Then, the homogenate was centrifuged at 15 000 g for 20 min at 4°C, and the supernatant was analysed quickly. All results were normalised to total protein concentration in each sample for inter-sample comparison. Protein concentration in the homogenate was quantified according to the Bradford method( Reference Bradford 17 ).
The activities of superoxide dismutase (SOD), γ-glutamylcysteine synthetase (γ-GCS), glutathione peroxidase (GPx), glutathione S-transferase and glutathione reductase (GR) and the concentrations of malondialdehyde (MDA) and protein carbonyls were determined using colorimetric kits with a spectrophotometer according to the instructions provided with the kits obtained from the Nanjing Jiancheng Institute of Bioengineering
Briefly, the activity of SOD was determined at 550 nm using a xanthine and xanthine oxidase system according to the method of Sun et al. ( Reference Sun, Oberley and Li 18 ). One unit of SOD activity was defined as the amount of enzyme required to produce 50 % inhibition of nitrite production at 37°C. To determine the activity of γ-GCS, the formation of amino-acid-dependent ADP was monitored at 636 nm according to the method of Ruegsegger et al. ( Reference Ruegsegger and Brunold 19 ). One unit of γ-GCS activity was defined as the amount of enzyme required to produce 1 μmol of phosphorus at 37°C in 1 min. The activity of GPx was determined at 412 nm using glutathione (GSH) as a substrate by measuring the decrease in the enzymatic reaction of GSH (except for the effect of the non-enzymatic reaction). The dithionitrobenzene method of Hafeman et al. ( Reference Hafeman, Sunde and Hoekstra 20 ) was used for determining GPx activity. One unit of GPx activity was defined as the amount of enzyme required to deplete 1 μmol of GSH at 37°C in 1 min. The activity of glutathione S-transferase was determined at 412 nm using 1-chloro-2,4-dinitrobenzene as a substrate according to the method of Zhu et al. ( Reference Zhu, Gao and Starkey 21 ). One unit of glutathione S-transferase activity was defined as the amount of enzyme required to catalyse the conjugation of GSH with 1 μmol of substrate at 37°C in 1 min. The activity of GR was determined by monitoring the oxidation of NADPH at 340 nm in the presence of oxidised glutathione (GSSG)( Reference Carlberg and Mannervik 22 ). One unit of GR activity was defined as the amount of enzyme required to catalyse 1 μmol oxidation of the reduced form of NADPH at 37°C in 1 min. The concentration of MDA was measured using the thiobarbituric acid method described previously( Reference Placer, Cushman and Johnson 23 ). The concentration of protein carbonyls was determined by derivatisation using dinitrophenylhydrazine as reported previously( Reference Ganhao, Morcuende and Estevez 24 ).
The activity of glucose-6-phosphate dehydrogenase (G6PD) was determined according to a previously described method( Reference Spolarics and Navarro 25 ). A typical assay mixture contains 200 μg of protein in 1000 μl of an assay buffer (84·5 mm-Tris–EDTA (pH 8·0), 1 mm-NADP+, 25 mm-MgCl2 and 1 mm-glucose-6-phosphate). The change in absorbance was monitored at 340 nm. One unit of G6PD activity was defined as the amount of enzyme required to produce 1 nmol of NADPH at 37°C in 1 min.
The content of GSH and GSSG was determined according to a previously reported method( Reference Hissin and Hilf 26 ), and it is expressed as the number of μmol/g protein in the liver. Approximately 1 g of liver samples was ground in 1 ml of 25 % H3PO3 and 3 ml of 0·1 mm-sodium phosphate–EDTA buffer (pH 8·0). The homogenate was centrifuged at 10 000 g for 20 min. The supernatant was used for the estimation of GSH and GSSG content in a Hitachi F-7000 fluorospectrophotometer (Hitachi). The supernatant was further diluted five times with sodium phosphate–EDTA buffer (pH 8·0). The final assay mixture (2·0 ml) contained 100 μl of the diluted supernatant, 1·8 ml of phosphate–EDTA buffer and 100 μl of 0·1 % (w/v) o-phthalaldehyde. After thorough mixing and incubation at room temperature for 15 min, the solution was transferred to a quartz cuvette, and the fluorescence at 420 nm was measured after excitation at 350 nm. An aliquot of 0·5 ml of the supernatant was incubated at room temperature with 200 μl of 0·04 m-N-ethylmaleimide for 30 min to allow it to react with the GSH present in the supernatant. To this mixture, 4·3 ml of 0·1 m-NaOH were added. A 100 μl portion of this mixture was taken for measuring GSSG content using the procedure outlined for the GSH assay, except that 0·1 m-NaOH rather than phosphate–EDTA buffer was used as the diluent.
Total RNA isolation and mRNA quantification
Total RNA was isolated from snap-frozen liver samples using TRIzol Reagent (TaKaRa). After the determination of RNA concentration, 1 μg of total RNA was reverse-transcribed into complementary DNA using the PrimeScript™ RT Reagent Kit (TaKaRa) according to the manufacturer's protocol. Real-time PCR was carried out on an ABI StepOnePlus™ Real-Time PCR system (Applied Biosystems) according to the manufacturer's instructions. The primer sequences of the target and reference genes (SOD1, GPX1, G6PD, thioredoxin 1 (TXN1), B-cell lymphoma/leukaemia 2 (Bcl-2), Bcl-2-associated X protein (Bax) and β-actin) used in real-time PCR are given in Table 3. Briefly, the reaction mixture was prepared using 2 μl of complementary DNA, 0·4 μl of forward primer, 0·4 μl of reverse primer, 10 μl of SYBR Premix Ex Taq™ (TaKaRa), 0·4 μl of ROX Reference Dye (TaKaRa), and 6·8 μl of double-distilled water. Each sample was tested in duplicate. PCR consisted of a pre-run at 95°C for 30 s and forty cycles of denaturation at 95°C for 5 s, followed by a 60°C annealing step for 30 s. The conditions of the melting curve analysis were as follows: one cycle of denaturation at 95°C for 10 s, followed by an increase in temperature from 65 to 95°C at a rate of 0·5°C/s. The relative levels of mRNA expression were calculated using the
![]() $$2^{ - \Delta \Delta C _{t}} $$
method after normalisation to those of β-actin as a housekeeping gene(
Reference Pfaffl
27
). The values of NBW-SO piglets were used as a calibrator.
$$2^{ - \Delta \Delta C _{t}} $$
method after normalisation to those of β-actin as a housekeeping gene(
Reference Pfaffl
27
). The values of NBW-SO piglets were used as a calibrator.
Table 3 Sequences of primers used in real-time PCR
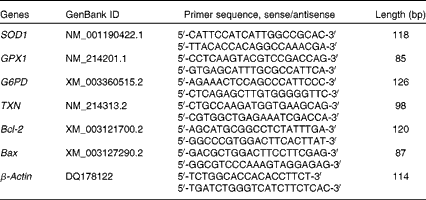
SOD1, superoxide dismutase 1; GPX1, glutathione peroxidase 1; G6PD, glucose-6-phosphate dehydrogenase; TXN, thioredoxin 1; Bcl-2, B-cell lymphoma/leukaemia 2; Bax, Bcl-2-associated X protein.
Statistical analysis
Two-way ANOVA was employed to determine the main effects (BW and diet) and their interactions using the general linear model procedure of SPSS (version 16.0; SPSS, Inc.). Differences were considered significant at P< 0·05, and P values between 0·05 and 0·1 were considered a trend.
Results
Growth performance
IUGR caused a significant decrease (P< 0·05) in the average daily feed intake and ADG of weaned piglets during the first 4 weeks after weaning (Table 4). The MCT diet improved the growth performance of piglets, which appeared to result from increased (P< 0·05) ADG and feed efficiency. Moreover, the diet did not affect the average daily feed intake of piglets (P>0·10).
Table 4 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on growth performance in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) piglets (Mean values with their standard errors, n 4)
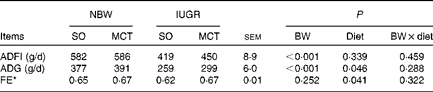
BW, birth weight; ADFI, average daily feed intake; ADG, average daily gain; FE, feed efficiency.
* FE was calculated by dividing the ADG by its ADFI.
Superoxide dismutase activity and malondialdehyde and protein carbonyl concentrations
The activities of circulating SOD (P= 0·076) and hepatic SOD (P= 0·065) tended to decrease in IUGR piglets compared with those in NBW piglets (Table 5). Piglets fed the MCT diet had significantly decreased hepatic MDA concentrations (P< 0·05) compared with their counterparts fed the SO diet. There were no significant differences in the concentrations of circulating MDA and hepatic protein carbonyls among the groups (P>0·10).
Table 5 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on superoxide dismutase (SOD) activity and malondialdehyde (MDA) and protein carbonyl concentrations in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) piglets (Mean values with their standard errors, n 4)
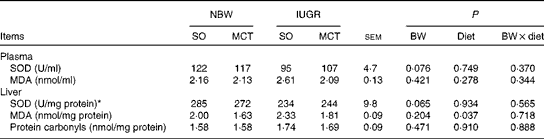
BW, birth weight.
* One unit of SOD is defined as the amount of SOD required to produce 50 % inhibition of the rate of nitrite production at 37°C.
Reactive oxygen species, apoptosis and necrosis levels
IUGR piglets had higher (P< 0·05) ROS levels in hepatocytes compared with NBW piglets (Table 6). However, the diet did not affect ROS levels in hepatocytes (P>0·10). IUGR significantly increased hepatocyte death, as evidenced by the significantly increased (P< 0·05) percentage of apoptotic cells and necrotic cells. The MCT diet significantly decreased (P< 0·05) the levels of apoptosis and necrosis compared with the SO diet.
Table 6 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on reactive oxygen species (ROS) and apoptosis levels in hepatocytes in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) piglets (Mean values with their standard errors, n 4)
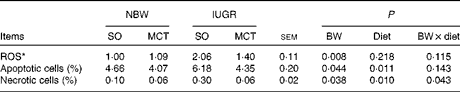
BW, birth weight.
* Expressed in arbitrary units. The ROS levels of each piglet in the NBW-SO group were assigned a value of 1.
Hepatic oxidative status
Parameters related to hepatic oxidative status are summarised in Table 7. IUGR induced a significant decrease in hepatic G6PD activity (P< 0·05). IUGR piglets had decreased (P< 0·05) hepatic GSH concentrations and a lower (P< 0·05) ratio of GSH:GSSG content compared with NBW piglets. The MCT diet increased (P< 0·05) the hepatic activities of GR and G6PD compared with the SO diet. In addition, a significantly increased (P< 0·05) ratio of GSH:GSSG content was also observed in MCT diet-fed piglets compared with their SO diet-fed counterparts. Moreover, there were no significant differences in the activities of γ-GCS, GPx and glutathione S-transferase as well as the content of GSSG among the groups (P>0·10).
Table 7 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on hepatic oxidative status in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) piglets* (Mean values with their standard errors, n 4)
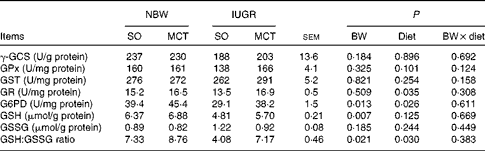
BW, birth weight; γ-GCS, γ-glutamylcysteine synthetase; GPx, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase; G6PD, glucose-6-phosphate dehydrogenase; GSH, glutathione; GSSG, oxidised glutathione.
* One unit of γ-GCS activity is the amount of enzyme forming 1 μmol of P at 37°C in 1 min. One unit of GPX activity is defined as the amount of enzyme depleting 1 μmol of GSH at 37°C in 1 min. One unit of GST activity is defined as the amount of enzyme catalysing the conjugation with GSH of 1 μmol of substrate at 37°C in 1 min. One unit of GR activity is defined as the amount of enzyme catalysing 1 μmol oxidation of the reduced form of NADPH at 37°C in 1 min. One unit of activity was expressed as the amount of enzyme producing 1 nmol NADPH at 37°C in 1 min.
Gene expression
The expression levels of genes related to hepatic oxidative status and cell death are summarised in Table 8. IUGR piglets had a lower (P= 0·060) mRNA abundance of Bcl-2 compared with NBW piglets. In addition, a tendency towards a decreased (P= 0·084) expression of GPX1 was observed in IUGR piglets compared with NBW piglets. The MCT diet significantly increased (P< 0·05) the expression of G6PD compared with the SO diet. The mRNA abundance of GPX1 increased numerically (P= 0·063) in MCT diet-fed piglets compared with that in SO diet-fed piglets. Moreover, no alterations were observed in the mRNA abundances of SOD1, TXN1 and Bax among the groups (P>0·10).
Table 8 Effects of soyabean oil (SO) and medium-chain TAG (MCT) on the expression levels of genes related to hepatic oxidative status and cell death in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) piglets (Mean values with their standard errors, n 4)
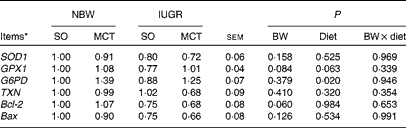
BW, birth weight; SOD1, superoxide dismutase 1; GPX1, glutathione peroxidase 1; G6PD, glucose-6-phosphate dehydrogenase; TXN, thioredoxin; Bcl-2, B-cell lymphoma/leukaemia 2; Bax, Bcl-2-associated X protein.
* Expressed in arbitrary units. The mRNA level of each target gene in the NBW-SO group was assigned a value of 1 and normalised to that of β-actin.
Discussion
Approximately 15–20 % of newborn piglets suffer from IUGR because of the selection for high litter size in commercial swine production, which significantly hinders postnatal growth and greatly affects health status( Reference Quiniou, Dagorn and Gaudre 28 , Reference Su, Lund and Sorensen 29 ). Piglets have been widely used as an animal model for human IUGR studies owing to their biological similarity to humans. When the fetus is exposed to malnutrition, the organism diverts the limited nutrient supply to favour the survival of vital organs such as the brain at the expense of growth and other organs such as the liver( Reference Hales and Barker 30 ). Early insults at critical stages of development can lead to permanent alterations in the structure and function of organs( Reference Fowden, Giussani and Forhead 31 ). Therefore, innovative feeding strategies during the early periods after weaning should be finely balanced to guarantee the appropriate development of IUGR offspring.
BW is correlated with the growth performance of weaned piglets( Reference Dwyer, Fletcher and Stickland 32 , Reference Gondret, Lefaucheur and Louveau 33 ). Many studies have confirmed that IUGR piglets have decreased body weight gain and feed intake compared with their heavier counterparts during the post-weaning period( Reference Michiels, De Vos and Missotten 8 , Reference Jones, Gabler and Main 34 , Reference Bruininx, van der Peet-Schwering and Schrama 35 ), and these findings are in agreement with the results of the present study. In the present study, the MCT diet was found to increase the ADG and feed efficiency of weanling piglets. Similar results were obtained by Dove( Reference Dove 36 ), who confirmed the beneficial effects of a MCT diet, as indicated by the significant increase in body weight gain and a greater feed conversion ratio in pigs during the first 2 weeks after weaning, compared with SO or animal fat. Hong et al. ( Reference Hong, Hwang and Kim 37 ) also showed that a MCT diet increased the ADG and nutrient digestibility of newly weaned pigs. Compared with SO, MCT are easily digested and absorbed to supply energy through mitochondrial β-oxidation in the liver, and several studies have shown that MCT could act as effective energy sources in weanling piglets( Reference Bach, Storck and Meraihi 38 – Reference Odle, Benevenga and Crenshaw 40 ). However, few studies have focused on the effect of MCT on the oxidative status of piglets.
The antioxidant defence system controls the redox balance( Reference Durak, Bingol and Avci 41 ). However, the concentrations of ROS (such as superoxide anions, H2O2 and hydroxyl radicals) exceeding the antioxidant protection levels of cells can cause widespread damage to DNA, proteins and endogenous lipids( Reference Yu 42 ). SOD is generally recognised as one of the main antioxidant enzymes; the superoxide anion is converted to H2O2 by SOD, which is then removed by GPx or catalase. A reduction in SOD activity is usually considered as decreased antioxidant capacity to clear out ROS. In the present study, IUGR was found to decrease the activity of SOD in both the plasma and the liver, which provides an explanation for the higher ROS levels in hepatocytes. These findings are in agreement with those of a previous study, in which IUGR piglets were found to have decreased MnSOD activity in the liver and increased MDA and protein carbonyl concentrations( Reference Liu, Yao and Yu 5 ). The concentrations of MDA or protein carbonyls reflect the degree of lipid peroxidation or protein oxidation, respectively. In a previous study, IUGR was also found to increase cerebral lipid peroxidation in rats( Reference Lane, Ramirez and Tsirka 7 ). In addition, a lower circulatory antioxidant capacity was observed in low-BW piglets, which appeared to result from decreased GPx activity and ferric reducing ability compared with NBW piglets( Reference Michiels, De Vos and Missotten 8 ). These observations confirm that IUGR offspring have an impaired antioxidant defence system and exhibit more severe oxidative damages. Thus, it is important to develop feeding strategies to decrease the risk of oxidative stress in IUGR offspring.
The degree of lipid peroxidation is proportional to the number of double bonds in unsaturated fatty acids( Reference Mehta, Gunnett and Harris 1 ). The observation that MCT diet-fed piglets had lower hepatic MDA concentrations than SO diet-fed piglets supports the findings of Diniz et al. ( Reference Diniz, Cicogna and Padovani 43 ), who reported that the ratio of PUFA:SFA in diets played an important role in lipid peroxidation. Thus, the consumption of a SFA-rich diet decreases the risk of lipid peroxidation due, in part, to the higher saturation of dietary fat.
Indeed, the use of oxygen in the oxidative metabolism of fuel could lead to the generation of ROS( Reference Esposito, Melov and Panov 44 , Reference Masoro 45 ). Mitochondria not only play a major role in energy metabolism but also serve as the major production sites of intracellular ROS, because ROS are by-products of oxidative phosphorylation. Thus, some investigators believe that increasing the efficiency of oxidative metabolism in mitochondria would intensify the production of ROS( Reference Masoro 45 , Reference Rea and Johnson 46 ). Hart et al. ( Reference Hart, Dixit and Seng 47 ) reported that alterations in food constituents or fuel used in energy generation might be associated with oxidative stress. However, the MCT diet was found to have no effects on ROS levels in the hepatocytes of piglets in the present study, although previous studies have shown that MCT could enhance the efficiency of mitochondria-based oxidative metabolism by improving the activity of succinate dehydrogenase and by increasing the contents of adenosine triphosphate in the liver( Reference Balietti, Giorgetti and Di Stefano 48 , Reference Ooyama, Kojima and Aoyama 49 ). It is worth noting that the MCT diet increased the metabolic efficiency of the hepatic GSH redox cycle, as evidenced by the greater ratio of GSH:GSSG content and the increased GR activity. Oxidative stress shifts the GSH oxidative status towards lower GSH content and higher GSSG content( Reference Park, DiNatale and Chung 50 ). GSH is one of the predominant endogenous antioxidants responsible for the detoxification of ROS, removal of hydrogen and lipid peroxides, and repair of oxidatively damaged proteins through a reaction catalysed by GPx( Reference Fang, Yang and Wu 51 ). In the present study, the MCT diet was found to partially improve hepatic GSH concentrations compared with the SO diet, which is similar to the results of a previous study in which MCT were found to increase the reduced GSH levels in the liver compared with long-chain TAG( Reference Wollin, Wang and Kubow 52 ), possibly due, in part, to the ketogenesis of MCT. All the extrahepatic tissues can use the ketone bodies supplied by the blood, and a modest elevation of ketone body levels has been reported to be not dangerous( Reference Yeh and Zee 53 ). A physiological level of ketone bodies might decrease mitochondrial ROS production by oxidising co-enzyme Q, because co-enzyme Q is a source of intracellular ROS when it is in the reduced state( Reference Veech, Chance and Kashiwaya 54 ). Moreover, ketone bodies could reduce mitochondrial NAD+ and cytoplasmic free NADP+ levels. Cytoplasmic NADPH favours the reduction reaction of GSH catalysed by GR( Reference Veech, Chance and Kashiwaya 54 ). GR is an important cellular antioxidant enzyme, and it protects cells from oxidative stress. Consequently, GSH is oxidised to GSSG, which in turn is rapidly reduced back to GSH by GR at the expense of NADPH, thereby forming a closed system( Reference Mahmoud and Edens 55 ). In the present study, MCT diet-fed IUGR piglets exhibited higher hepatic GR activity than their SO diet-fed counterparts, which was observed alongside the up-regulation of the expression of GPX1 (encodes cytosolic GPx). These results might explain the improvement observed in the oxidative status after MCT treatment. Furthermore, no alterations were detected among the groups with regard to the activity of γ-GCS, a key enzyme involved in the control of the de novo synthesis of GSH. Therefore, the MCT diet improved hepatic oxidative status by improving the metabolic efficiency of the GSH redox cycle rather than the de novo synthesis of GSH.
To determine the mechanism underlying the improvement of the GSH redox cycle, the activity and expression levels of G6PD were measured. G6PD is the first and rate-limiting enzyme involved in the control of the flux of glucose-6-phosphate through the pentose phosphate pathway, which produces NADPH to meet the cellular needs for reductive biosynthesis and maintenance of the reduction levels of GSH( Reference Cramer, Cooke and Ginsberg 56 ). A separate control mechanism for the activity of G6PD v. other lipogenic enzymes, such as malic enzyme, which is the main supplier of NADPH for lipogenesis in adipocytes, has been found in a previous study( Reference Gondret, Lefaucheur and Louveau 33 ). In the present study, the up-regulation of G6PD levels induced by the MCT diet was found to occur at both the activity and transcriptional levels compared with that induced by the SO diet, which is in accordance with the results of previous studies that reported that MCT feeding significantly improves the activity of G6PD in the liver of rats compared with long-chain TAG feeding( Reference Chanez, Bois-Joyeux and Arnaud 57 , Reference Shinohara, Ogawa and Kasai 58 ). These results indicate that the consumption of the MCT diet might enhance the channelling of glucose metabolites through the pentose phosphate pathway, leading to the greater metabolic efficiency of the hepatic GSH redox cycle observed after MCT treatment. In addition, in our previous study, the activity of succinate dehydrogenase was found to be significantly increased and that of pyruvate kinase was found to be significantly decreased in the liver of piglets after MCT treatment, possibly because MCT provided sufficient fuel for energy metabolism and then decreased the expenditure of glucose as a source of energy.
There is growing evidence that ROS play an important role in the induction of apoptosis. It has been shown that H2O2 could induce apoptosis, which is prevented by catalase( Reference Pierce, Parchment and Lewellyn 59 ). The release of cytochrome c from the mitochondria is a crucial event in mitochondria-initiated apoptosis and can trigger the formation of the apoptosome complex, leading to caspase activation and subsequent cell death( Reference Wang 60 ). In the present study, IUGR was found to increase the levels of hepatocyte apoptosis and necrosis, which is similar to the results of previous studies in which IUGR was found to enhance apoptosis in vital organs such as the brain, kidney, small intestine and placenta( Reference Burke, Sinclair and Cowin 61 – Reference Baserga, Bertolotto and Maclennan 64 ). This indicates that IUGR might affect the development and metabolism of the liver by up-regulating the apoptotic pathway. Notably, this process appeared to be largely mediated by the direct or indirect action of ROS. A previous study has shown that the ROS-induced oxidation of the mitochondrial pores disrupts the mitochondrial membrane potential and then contributes to cytochrome c release( Reference Zamzami, Marchetti and Castedo 65 ). ROS also trigger the oxidation of cytochrome c, thereby rendering it capable of caspase activation( Reference Vaughn and Deshmukh 66 ). In fact, the oxidation of cytochrome c is important for apoptosis( Reference Brown and Borutaite 67 , Reference Li, Wang and Xu 68 ). Importantly, GSH could lead to the inactivation of cytochrome c by keeping it in its reduced state( Reference Vaughn and Deshmukh 66 ). Thus, the increased percentage of apoptotic cells in IUGR piglets was primarily due to the higher ROS levels and lower GSH concentrations in the liver, whereas the lower levels of hepatocyte apoptosis in MCT diet-fed piglets were the result of the improvement of the GSH redox cycle in the liver.
In the present study, IUGR was also found to induce the down-regulation of the mRNA expression of Bcl-2 in the liver; Bcl-2 has been suggested to antagonise the pro-apoptotic function of Bax by blocking its activity( Reference Oltvai, Milliman and Korsmeyer 69 ). Bax, a pro-apoptotic member of the Bcl-2 family, can directly cause mitochondria to release cytochrome c by forming ion channels and opening pores in the outer mitochondrial membrane( Reference Antonsson, Conti and Ciavatta 70 , Reference Green and Reed 71 ). Thereby, this observation also supports the greater incidence of apoptosis and necrosis in the hepatocytes of IUGR piglets.
In conclusion, the results of the present study indicate that MCT attenuate hepatic oxidative damage in IUGR weanling piglets and add to the understanding of how a MCT diet alters hepatic oxidative status, which could help in the development of new feeding strategies for IUGR offspring to decrease the risk of oxidative stress during the early periods after weaning.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451400155X
Acknowledgements
The authors thank the staff at the Free Radical Regulation Research Centre of Fudan University for their assistance. They are also grateful to Pengying Li and Dongyun Shi for their dedicated and skilful technical support.
The present study was supported by the National Natural Science Foundation of China (no. 31272454).
The authors' contributions are as follows: H. Z., J. W. and T. W. designed the study; H. Z., Y. C. and L. Y. carried out the animal experiment; H. Z., Y. C., Y. L. and L. Y. conducted the study; H. Z. and Y. C. analysed the data; H. Z. discussed the results and wrote the article. All authors read and approved the final manuscript.
None of the authors has any competing interests to declare.










