Implications
Insects and former foodstuffs (also known as ex-food) are regarded as interesting alternative protein/energy sources for animal diets and are expected to be increasingly used around the globe as a replacement for conventional feedstuffs. From a circular economy point of view, both represent a way by which food waste biomasses/streams can be upgraded to valuable feed ingredients. This is the first time that a review addresses the quality and safety of former foodstuffs, and in terms of insects, the importance of the type of metamorphosis and the tailored substrates that could lead to the production of a premium feed specialty. For both alternative ingredients the legislative framework is also discussed.
Introduction
The global population growth and the high pressure on resources is expected to double in the coming decades, resulting in a growing demand and production of food and, subsequently, of feed. The demand for cereals, for both food and feed, is expected to increase about 50% by 2050. The demand for other food products (meat and dairy products, fish and aquaculture products and vegetable oils) that are more responsive to rising incomes in developing countries is projected to grow much faster than the demand for cereals for food use (Pinotti et al., Reference Pinotti, Caprarulo, Ottoboni, Giromini, Agazzi, Rossi, Tretola, Baldi, Savoini and Đuragić2016). The global demand for fibre and fuel producing biomass is also expected to increase.
Today, agriculture is thus facing with a wide range of complex challenges. With a diminishing availability of farmland, climate changes and the threat of declining water resources, the task is to meet the growing demand for food, feed, fibre, fuel and industrial products using fewer resources. The underlying idea is to reduce the use and redisposition of resources; in other words, ‘Do more with less’.
These aspects, together with the increase in the cost of traditional feed ingredients for animal production over the last decade, have stimulated feed researchers and producers to secure a sustainable protein and energy supply for feeding animals. Insects, micro-algae, former foodstuffs, as well as duckweed are regarded as interesting alternative protein/energy sources for feed, and are expected to be increasingly used around the globe as a replacement for conventional nutrient sources (Pinotti et al., Reference Pinotti, Giromini, Ottoboni, Tretola, Cheli and Baldi2013; Spiegel et al., Reference Spiegel, Noordam and Fels‐Klerx2013; Bikker et al., Reference Bikker, van Krimpen, van Wikselaar, Houweling-Tan, Scaccia, van Hal and López-Contreras2016).
A step in this direction is the European Union (EU) Regulation 2017/893 (European Commission, 2017), which now allows the use of insect proteins as fish feed, derived from the following insect species: black soldier fly (BSF) (Hermetia illucens), common housefly (Musca domestica), yellow mealworm (Tenebrio molitor), lesser mealworm (Alphitobius diaperinus), house cricket (Acheta domesticus), banded cricket (Gryllodes sigillatus) and field cricket (Gryllus assimilis). To date, the use of insect proteins for other livestock species is not allowed in the EU.
Insects as feed ingredients have a great potential for several reasons: (i) nutrient content, since they are rich in proteins, fat (and in turn energy), vitamins and minerals; (ii) sometimes they are characterized by an adequate feed conversion efficiency compared to livestock; (iii) low space requirement; (iv) great acceptance by poultry and fish, whose diet in nature is partly represented by insects; (v) they are mostly omnivorous and can grow on different substrates (Spranghers et al., Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017; Spranghers et al., Reference Spranghers, Michiels, Vrancx, Ovyn, Eeckhout, De Clercq and De Smet2018). In this respect, insects can potentially be used to upgrade low value organic waste materials (Boccazzi et al., Reference Boccazzi, Ottoboni, Martin, Comandatore, Vallone, Spranghers and Epis2017; Spranghers et al., Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017) which can be exploited in the feed and food chain. However, according to European legislation (European Commission, 2009b), insects bred for the production of processed animal protein (PAP) should be considered as farmed animals, and are therefore subject to the feed ban rules (European Commission, 2001) as well as the rules of animal feeding (European Commission, 2009b). Thus, the use of ruminant proteins, catering waste, meat-and-bone meal and manure as feed for insects is prohibited.
Although the nutritional properties of edible insects have been reported in several studies (Veldkamp and Bosch, Reference Veldkamp and Bosch2015; Wang and Shelomi, Reference Wang and Shelomi2017; Schiavone et al., Reference Schiavone, Dabbou, De Marco, Cullere, Biasato, Biasibetti and Gai2018), to date their safety has been less investigated. The European Food Safety Authority document (EFSA, 2015) also noted the uncertainty related to possible hazards when insects are used as food and feed. The EFSA document (2015) highlighted that there are only a few studies on the potentially pathogenic microbes for vertebrates together with few published data on the hazardous chemicals used in rearing insects, underlying the lack of systematically collected data on insects consumption. Insects are also associated with various microbial and parasitical hazards. In fact, microorganisms (bacteria, fungi and viruses) may contain toxic and repellent substances, which are part of their defence mechanisms (Belluco et al., Reference Belluco, Losasso, Maggioletti, Alonzi, Paoletti and Ricci2013; Belluco et al., Reference Belluco, Losasso, Maggioletti, Alonzi, Ricci and Paoletti2015; EFSA, 2015; van Raamsdonk et al., Reference van Raamsdonk, Prins, van de Rhee, Vliege and Pinckaers2017; Wang and Shelomi, Reference Wang and Shelomi2017).
Another biomass with great potential is represented by former foodstuff products (FFPs). These are foodstuffs that have become unsuitable for human consumption for various reasons, such as production errors leading to broken or intermediate foodstuffs or surpluses caused by the logistical challenge of daily deliveries, surpluses caused by the discontinuation of a food product line, products over the perishable date, all of which are potential former food products (Pinotti et al., Reference Pinotti, Giromini, Ottoboni, Tretola, Cheli and Baldi2013; Giromini et al., Reference Giromini, Ottoboni, Tretola, Marchis, Gottardo, Caprarulo, Baldi and Pinotti2017; Tretola et al., Reference Tretola, Di Rosa, Tirloni, Ottoboni, Giromini, Leone, Bernardi, Dell’Orto, Chiofalo and Pinotti2017a; Tretola et al., Reference Tretola, Ottoboni, Di Rosa, Giromini, Fusi, Rebucci, Leone, Dell’Orto, Chiofalo and Pinotti2017b). In addition, the European Commission recently published its guidelines on the use of food no longer intended for human consumption in animal feed (European Commission, 2018), as the next key deliverable of the EU Circular Economy Action Plan on food waste. In fact, the European Commission worked in cooperation with the relevant authorities of EU member states and their document provides practical guidance that increases the legal certainty for the former foodstuff-processing sector while preserving the integrity and safety of the EU food and feed chain. The proposed scheme is reported in Figure 1.
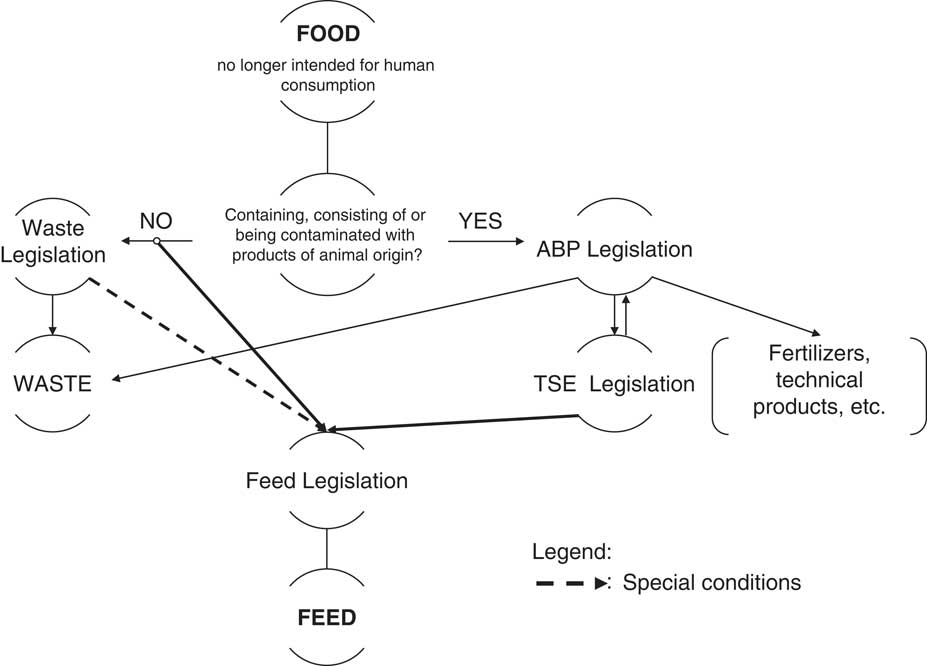
Figure 1 Flowchart from FOOD to FEED as proposed by the European Commission (2018). ABP=animal by-products; TSE=transmissible spongiform encephalopathy.
Enhancing former foodstuffs into high-quality animal feed represents an active and promising area of feed research, both in terms of assessing alternative feed ingredients and of biomass and food waste reprocessing. In particular, FFPs are considered a challenging opportunity for the feed sector as they represent innovative sources of energy for feedstuffs (Schieber et al., Reference Schieber, Stintzing and Carle2001; Pinotti et al., Reference Pinotti, Caprarulo, Ottoboni, Giromini, Agazzi, Rossi, Tretola, Baldi, Savoini and Đuragić2016; Giromini et al., Reference Giromini, Ottoboni, Tretola, Marchis, Gottardo, Caprarulo, Baldi and Pinotti2017; Herrero et al., Reference Herrero, Thornton, Power, Bogard, Remans, Fritz and Watson2017; Tretola et al., Reference Tretola, Di Rosa, Tirloni, Ottoboni, Giromini, Leone, Bernardi, Dell’Orto, Chiofalo and Pinotti2017a). Therefore, FFPs represent a way of converting surplus from the food industry into ingredients for the feed industry, thereby reducing overall food losses in the food chain (Featherstone, Reference Featherstone2014).
Although some alternative feed ingredients, such as FFPs, already constitute the raw material for the production of compound feed for animals, their use in animal nutrition is still limited. For instance, only 3.3% of the total food surplus is processed into animal feed in the EU (EFFPA, 2018), and in the case of insect materials, their large-scale production and processing is still limited. The potential of these products has not yet been fully exploited as value-added products for animal nutrition in terms of nutritional value, functional properties, and technological and biosafety issues. In terms of the target farm species, such as omnivores, pigs and poultry, they are ideally suited to convert these FFPs as well as other by-/co-products that do not fit for human consumption or insect materials into high-quality food animal protein. By-/co-products, and other alternative feedstuffs such as FFPs, can therefore be included in the non-ruminant diet to reduce/optimize the feed cost per metric tonne of feed. However, as already reported for by-products, the inclusion of alternative feedstuffs in farm animal diets does not necessarily reduce feed cost per kilogram of gain (Woyengo et al., Reference Woyengo, Beltranena and Zijlstra2014). In fact, the cost of insect protein can be very high, as highlighted in Table 1.
Table 1 Trading price of different protein sources intended for farm animal nutrition and per unit of protein expressed as times relative to soy meal 45% (=1) (adapted from All About Feed, 2016)

CP=crude protein; BSF=black soldier fly (Hermetia illucens).
The use of alternative feed ingredients in animal diets must be optimized in terms of their nutritional characterization, their safety and their technological quality. This review thus outlines the main nutritional and safety issues of insects and ex-food materials as alternative feed ingredients.
Insects
Nutritional quality
Insects are regarded as an interesting alternative protein source for feed and are expected to be increasingly used in Europe as a replacement for animal-derived proteins, especially in aquaculture (Spiegel et al., Reference Spiegel, Noordam and Fels‐Klerx2013; Jo and Lee, Reference Jo and Lee2016), although they also have potential for other species (van Raamsdonk et al., Reference van Raamsdonk, Prins, van de Rhee, Vliege and Pinckaers2017) and as a fat/energy source (Wang and Shelomi, Reference Wang and Shelomi2017; Biasato et al., Reference Biasato, Gasco, De Marco, Renna, Rotolo, Dabbou, Capucchio, Biasibetti, Tarantola, Sterpone, Cavallarin, Gai, Pozzo, Bergagna, Dezzutto, Zoccarato and Schiavone2017; Schiavone et al., Reference Schiavone, Dabbou, De Marco, Cullere, Biasato, Biasibetti and Gai2018). Their nutritional and technological properties are linked to the species, rearing system adopted and especially to the substrate used (Veldkamp and Bosch, Reference Veldkamp and Bosch2015; Spranghers et al., Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017; Ottoboni et al., Reference Ottoboni, Spranghers, Pinotti, Baldi, De Jaeghere and Eeckhout2017a; Meneguz et al., Reference Meneguz, Schiavone, Gai, Dama, Lussiana, Renna and Gasco2018). Among the species reared, the BSF (Hermetia illucens), common housefly (Musca domestica) and yellow mealworm (Tenebrio molitor) have the highest potential for large-scale production (Veldkamp and Bosch, Reference Veldkamp and Bosch2015).
Spranghers et al. (Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017) investigated the efficiency of BSF larvae in converting selected organic waste substrates, namely vegetable waste, biogas digestate and restaurant waste, into edible biomass. In their study, chicken feed was used as the reference substrate. The results obtained indicated that the protein content of prepupae did not differ substantially (varying between 399 and 431 g/kg dry matter (DM)) among treatments, that is rearing substrate. No effect of substrate on protein quality was observed. In fact, differences in the amino acid content of prepupae reared on different substrates were negligible (Spranghers et al., Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017). The most prevalent essential amino acids in the BSF prepupal biomass were lysine, valine and arginine, with levels between 20 and 30 g/kg DM, while the incidence of essential amino acids was over 55% of the total amount of amino acids. These features are in line with Barroso et al. (Reference Barroso, de Haro, Sánchez-Muros, Venegas, Martínez-Sánchez and Pérez-Bañón2014) who reported that the essential amino acid profiles of insect meals, fish meal and soybean meal were very similar.
A different scenario has been observed when ether extract (EE) and ash content were considered: both can differ substantially according to the rearing substrate. Prepupae reared on digestate were low in EE and high in ash content (218 and 197 g/kg DM, respectively) compared with those reared on vegetable waste (371 and 96 g/kg DM, respectively), chicken feed (336 and 100 g/kg DM, respectively) and restaurant waste (386 and 27 g/kg DM, respectively). The prepupal fatty acid profiles were characterized by high levels of C12:0 in all treatments, which was correlated to the substrate’s fatty acid profile. It can thus be concluded that irrespective of the substrate used, the BSF larvae protein content and quality is high and comparable among insects reared on different substrates, while the lipid and ash contents may depend on the substrate.
These results were only partially confirmed in a recent study (Meneguz et al., Reference Meneguz, Schiavone, Gai, Dama, Lussiana, Renna and Gasco2018) in which similar substrates were tested. Briefly, in two different trials, Meneguz et al. (Reference Meneguz, Schiavone, Gai, Dama, Lussiana, Renna and Gasco2018) tested the effects of selected waste and agro-industrial by-products-based substrates on BSF larvae development, waste reduction efficiency and nutritional composition. In general, what has been observed is a time-dependent effect of the substrate: time needed to reach the prepupae stage was reduced from 20 to 22 days for fruit waste, wine by-products and vegetable waste, to 8 days in the case of beer agro-industrial by-products. Furthermore, BSF also showed the ability to process efficiently substrate high in moisture and fibre. Specifically, wet substrate (high moisture content) has reduced BSF larvae’s mortality, increasing the opportunity for processing high moisture materials (i.e. vegetable-fruit waste and beer agro-industrial by-products) with limited pre-processing, such as drying. Considering the fibre content, it has been observed that BSF larvae are also able to efficiently bioconvert wastes and by-products characterized by high fibre content, such as beer agro-industrial by-products, without any detrimental effect on their growth performance. This is also relevant from a circular economy point of view, since it implies that insect rearing can potentially be used to upgrade low-value organic food waste streams. It has been estimated that food waste accounts for 23% of arable land, 24% of freshwater resources used for crop production and an amount of food per capita of roughly 625 kcal/cap/day, including large quantities of nutrients, micronutrients and minerals (Spiker et al., Reference Spiker, Hiza, Siddiqi and Neff2017).
From a technological point of view, Ottoboni et al. (Reference Ottoboni, Spranghers, Pinotti, Baldi, De Jaeghere and Eeckhout2017a) investigated the inclusion of Hermetia illucens larvae or prepupae in an experimental extruded feed, together with the impact of this technological process on the organic matter and protein digestibility. The results indicated that of the various mixtures containing cereals+pre-pupae/larvae±added oil, those containing wheat and larvae (with no oil addition) were the best for extrusion. This was due to the presence of naturally occurring fat, provided by the young insect state (i.e. larvae), which was enough to guarantee an adequate extrusion process (i.e. technological quality), without any effect on the digestibility of both organic matter and proteins. However, this study (Ottoboni et al., Reference Ottoboni, Spranghers, Pinotti, Baldi, De Jaeghere and Eeckhout2017a) considered a very simple blend (two ingredients), and the results require further investigation with a wider panel of blends and conditions.
This was done recently by Irungu et al. (Reference Irungu, Mutungi, Faraj, Affognon, Kibet, Tanga, Ekesi, Nakimbugwe and Fiaboe2018), who investigated the effects of substituting freshwater shrimp meal with BSF larvae meal or adult cricket meal on the physico-chemical properties of hot-extruded fish feed. Substituting freshwater shrimp meal up to 75% with both insect meals was possible without a substantial detrimental effect on aquafeed technological quality, although the moisture content of the formulated blends needs to be adjusted before extrusion. Overall, it was observed that extruded pellets with 75% BSF larvae meal or 75% adult cricket meal at 30:100 g feed moisture, were of a good quality. Adopting these inclusion rates, the floatability, expansion rate, bulk density, durability index, water absorption index, water solubility index and water stability of the extruded pellets were maintained. Up to a 75% insect protein substitution level is therefore possible, however, the moisture content of the blends needs to be gradually increased in order to obtain good quality pellets with regard to physical quality parameters.
A further aspect that needs to be considered in reviewing insects as an alternative feed ingredient is the type of development/metamorphosis that different species encounter during their life. Basically, there are two types of development/metamorphosis for insects, complete and incomplete. In complete metamorphosis (indirect development) species, the larva goes through different stages, finally transforming into a pupa, before becoming an adult. The immature stages of the insect (larvae and pupae) do not morphologically resemble the adult, nor its feeding habits. Insects that go through complete metamorphosis include moths, flies and beetles. Species with the highest potential for large-scale production, such as Hermetia illucens, Musca domestica and Tenebrio molitor are all characterized by complete metamorphosis and their larvae are usually selected as the best source of nutrients.
Other insect species, such as crickets, are characterized by an incomplete metamorphosis (direct) from nymph to adult. In the latter case, nymphs morphologically resemble adults and usually feed on the same host. Insects with ‘incomplete’ metamorphosis are a polyphyletic assemblage, which includes cockroaches, grasshoppers, dragonflies and true bugs (Truman and Riddiford, Reference Truman and Riddiford1999). The impact of the various types of development/metamorphosis on the nutritional composition of the insect material is likely to be significant, although as yet it has been not extensively addressed.
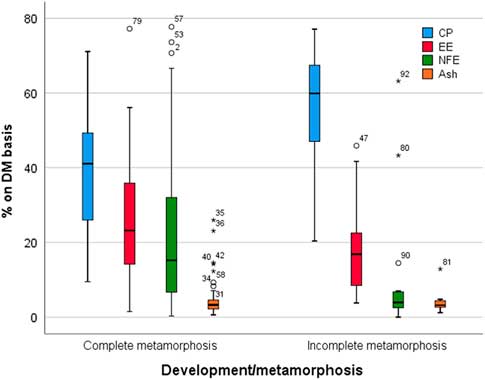
Figure 2 reports the nutrient composition in insect meal obtained from insect species with complete and incomplete metamorphosis. This issue has been reported by Sánchez-Muros et al. (Reference Sánchez-Muros, Barroso and Manzano-Agugliaro2014), who found that the crude protein (CP) content seems to be higher in direct metamorphosis insects (>60%) than in complete metamorphosis insects (>40%). This is in line with Barroso et al. (Reference Barroso, de Haro, Sánchez-Muros, Venegas, Martínez-Sánchez and Pérez-Bañón2014), in which the Orthoptera order (incomplete metamorphosis) was characterized by a higher CP content compared to Coleoptera and Diptera orders, which was related to the harvesting stage. In general, the order Orthoptera exhibits between 60% and 70% of CP content (on a DM basis), although all the samples of this order were adults, that is with more chitin (and chitin nitrogen) and less fat which enhances protein levels. Although it is difficult to draw solid conclusions related to differences between taxonomic orders, the results of this study (Barroso et al., Reference Barroso, de Haro, Sánchez-Muros, Venegas, Martínez-Sánchez and Pérez-Bañón2014) clearly indicated that two major groups or clusters of insect as protein sources can be defined: (1) Diptera in the same cluster as fish meal and (2) Orthoptera and Coleoptera in the same group as soybean meal. It has been suggested that Diptera is similar to fish meal in terms of its amino acid composition, especially Hermetia, Musca and Eristalis larvae, whereas Coleoptera is different. Soybean meal is closer to Orthoptera (Barroso et al., Reference Barroso, de Haro, Sánchez-Muros, Venegas, Martínez-Sánchez and Pérez-Bañón2014). A further key point that it must be considered in addressing insect as protein source is the true protein content in these materials. The protein content is usually calculated from total nitrogen using the nitrogen-to-protein conversion factor (Kp) of 6.25. This factor, however, tends to overestimate the protein content due to the presence of non-protein nitrogen in insect’s material. The calculated non-protein nitrogen content in whole larvae of three insect species was between 11% and 26%. The authors (Renske et al., Reference Renske, Vincken, van den Broek, Fogliano and Lakemond2017) in justifying these results proposed that besides the analytical procedures, differences in composition and recovery might also be caused by different diets (substrates) fed to the insects. In the same study (Renske et al., Reference Renske, Vincken, van den Broek, Fogliano and Lakemond2017), a specific Kp of 4.76±0.09 was calculated for larvae from Tenebrio molitor, Alphitobius diaperinus and Hermetia illucens, using amino acid analysis. After protein extraction and purification, a Kp factor of 5.60±0.39 was found for the larvae of three insect species studied. Accordingly, it has been proposed to adopt these Kp values for determining protein content of insects to avoid overestimation of the protein content (Renske et al., Reference Renske, Vincken, van den Broek, Fogliano and Lakemond2017).
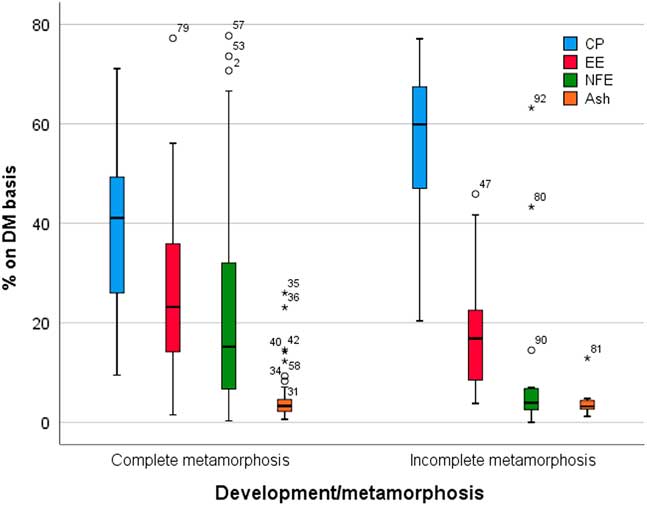
Figure 2 Mean, median, quartiles, minimum and maximum observations and outliers for nutrient composition (expressed on DM basis) in complete and incomplete metamorphosis insect species. Data are expressed as percentages on a DM basis (data from Sánchez-Muros et al., Reference Sánchez-Muros, Barroso and Manzano-Agugliaro2014). DM=dry matter; CP=crude protein %; EE=ether extract; NFE=nitrogen-free extracts.
Conversely, the fat content seems to be higher in complete metamorphosis insects than in incomplete metamorphosis species (Figure 2), with several exceptions. The lipid content of most of the insects full fat meals is about 20%, although the lipid content can reach 30%, such as in Locusta migratoria, Musca domestica larvae and Tenebrio molitor, up to 38.0% lipids in Zophoba morio (Barroso et al., Reference Barroso, de Haro, Sánchez-Muros, Venegas, Martínez-Sánchez and Pérez-Bañón2014). When fat quality (fatty acid profile) is considered, the situation is extremely variable. It has been reported (van Broekhoven et al., Reference van Broekhoven, Oonincx, van Huis and van Loon2015) that diets/substrates that allow for fast larval growth and low mortality seem to lead to a less favourable n-6/n-3 ratio. Controlling fatty acid composition is, however, difficult to achieve when using organic by-products and waste streams.
From a nutritional point of view, another interesting factor is the nitrogen-free extractive content, which is about three times higher in complete than in incomplete metamorphosis species. The nitrogen-free extractive content can be considered as an indirect measure of nitrogen-free components, such as carbohydrates. In this comparison (complete v. incomplete metamorphosis), however, chitin is not considered. Chitin is a polysaccharide that is present exclusively in the exoskeleton of arthropods. Specifically, chitin is a polymer of β(1-4) joined by a β(1-4) glycosidic bond, which is a crude fibre, and therefore is not digestible by non-ruminant species. The chitin content may interfere with protein use/digestibility. Chitin in commercially farmed insects has been found to range from 2.7 to 49.8 mg/kg of fresh weight (from 11.6 to 137.2 mg/kg of DM) (Finke, Reference Finke2007). The main sources of variability are the insect species and the development phases: generally, the adult form of complete metamorphosis insects contains higher chitin (Finke, Reference Finke2007). On the other hand, a slight variability in the chitin content related to insect rearing substrate was also observed (Spranghers et al., Reference Spranghers, Ottoboni, Klootwijk, Ovyn, Deboosere, De Meulenaer and De Smet2017).
Scaling up tailored feed specialties represents a further approach in insect production. In fact, given that BSF larvae meal and oil have already been considered as an animal-grade alternative to fish meal and fish oil in animal diets and formulas (Sánchez-Muros et al., Reference Sánchez-Muros, Barroso and Manzano-Agugliaro2014; Veldkamp and Bosch, Reference Veldkamp and Bosch2015), a further step in their production and use is to obtain selected premium products. BSF larvae meal have been used to feed carnivorous fish and other animals, due to their high protein and lipid contents even when larvae have been fed with plant-based waste streams. An interesting approach might be to shape the insect material composition as proposed by Sealey et al. (Reference Sealey, Gaylord, Barrows, Tomberlin, McGuire, Ross and St‐Hilaire2011), experimentally producing fish offal-enriched black soldier fly (EBSF) prepupae.
The importance of fish meal and especially fish oil in aquaculture is well known, but competition with the demand for fish for human consumption along with fisheries becoming depleted has meant that supplies of fish meal and oil have gone down and costs have gone up. Fisheries are thus now searching for new sources of marine proteins and oil alternatives such as vegetable oils. BSF larvae can accumulate lipids in their bodies if fed a lipid-rich diet, and are generally more palatable than vegetable oils. Accordingly, n-3 fatty-acid-enhanced prepupae can be produced when the larval diet is supplemented with fish offal, as reported by Sealey et al. (Reference Sealey, Gaylord, Barrows, Tomberlin, McGuire, Ross and St‐Hilaire2011). Such ‘enriched’ prepupae are suitable aquafeed, and do not differ in fish growth and development compared to normal fish meal for feeding rainbow trout. The sensory analysis of trout fillets also showed no differences among fish-fed fish meal, BSF larvae or EBSF larvae diets (Sealey et al., Reference Sealey, Gaylord, Barrows, Tomberlin, McGuire, Ross and St‐Hilaire2011).
Although in the EU, material of animal origin is not allowed as feed substrate for rearing insects, Sealey et al. (Reference Sealey, Gaylord, Barrows, Tomberlin, McGuire, Ross and St‐Hilaire2011) highlighted the great potential for designing innovative specialty feed ingredients. The effects of specific insect material preparations (e.g. partially defatted BSF, lauric acid in BSF meal) on animal wellbeing, microbiota and gut morphology are under investigation in several farm species (Renna et al., Reference Renna, Schiavone, Gai, Dabbou, Lussiana, Malfatto, Prearo, Capucchio, Biasato, Biasibetti, De Marco, Brugiapaglia, Zoccarato and Gasco2017; Schiavone et al., Reference Schiavone, De Marco, Martínez, Dabbou, Renna, Madrid, Hernandez, Rotolo, Costa, Gai and Gasco2017; Dabbou et al., Reference Dabbou, Gai, Biasato, Capucchio, Biasibetti, Dezzutto, Meneguz, Plachà, Gasco and Schiavone2018; Spranghers et al., Reference Spranghers, Michiels, Vrancx, Ovyn, Eeckhout, De Clercq and De Smet2018).
Safety aspect
Although the potential nutritional contribution of edible insects to the feed chain has been reported, to date the safety implications have been less investigated (Veldkamp and Bosch, Reference Veldkamp and Bosch2015; Verbeke et al., Reference Verbeke, Spranghers, De Clercq, De Smet, Sas and Eeckhout2015; Allegretti et al., Reference Allegretti, Talamini, Schmidt, Bogorni and Ortega2018). Furthermore, safety implications can be exacerbated by the organic waste streams used as a substrate for mini-livestock. Additional hazards may include a wide range of contaminants, such as bacteria, mycotoxins, heavy metals as well as pesticide residues, present or originated in the rearing substrate.
In Europe, traceability and safety should be guaranteed at each stage of the food and feed chain according to the General Food Law principles (European Commission, 2002) and the Feed Hygiene requirements (European Commission, 2005). An assessment of the potential microbial, chemical (including allergens) and physical hazards specifically related to the consumption of insects by both humans (entomophagy) and livestock animals was carried out by EFSA (2015). From a food safety point of view, safe food is free not only from toxins, pesticides and chemical and physical contaminants but also from microbiological pathogens such as bacteria and viruses that can cause illness in both humans and animals.
The main concern in this context is that hat intensive insect rearing with high insect densities is likely to be associated with health issues, since insects can serve as reservoirs for pathogens as observed in conventional livestock production (Kelemu et al., Reference Kelemu, Niassy, Torto, Fiaboe, Affognon, Tonnang and Ekesi2015). Several studies, however, have highlighted that a safety margin exists when good mini-livestock practices are adopted as well as the post-harvest biomass treatment and processing (Boccazzi et al., Reference Boccazzi, Ottoboni, Martin, Comandatore, Vallone, Spranghers and Epis2017; Ottoboni et al., Reference Ottoboni, Spranghers, Pinotti, Baldi, De Jaeghere and Eeckhout2017a). Insects for feed are processed with their intestinal content, which can harbour different species of transmissible microorganisms. In addition, insect mycobiota and microbiota can be enriched/modulated during farming and processing. However, the same is true for chemical hazards, such as pesticides, fluorine, heavy metals and dioxins. Thus it has been suggested that: (i) the quali-quantitative presence of some genera (both of microorganisms and fungi) in edible insects is strictly dependent on the diet provided to the growing insect; (ii) dietary/substrate exposure time also has an influence in defining the biodiversity of both micro/mycobiota, which are probably both transient and environment-dependent (Boccazzi et al., Reference Boccazzi, Ottoboni, Martin, Comandatore, Vallone, Spranghers and Epis2017). These findings seem to underline the importance of substrate on which the insects have been maintained in shaping the structure and taxonomy of the associated microbial/fungal communities.
However, in the case of the feed of animal origin, including insects, an extra set of requirements following the ban on the use of PAP needs to be considered, that is compliance with the legislation for eradicating transmissible spongiform encephalopathy, which limits or prohibits the use of PAP. This last point is essential: insects as PAP are subject to a few general bans (the same as their substrates), for which there are ranges of exemptions and derogations (Van Raamsdonk et al., Reference van Raamsdonk, Prins, van de Rhee, Vliege and Pinckaers2017). In the EU, similar to other farmed animals, authorized insect species may only be fed with ‘authorized substrates’; the obtained insect material is now allowed in EU only for aqua feed formulations.
However, despite the extensive food regulatory systems, it is clear that both quality and safety problems can occur when insects are used as feed/foods. Feed/food authorities can prevent most of these problems by applying good practice systems that include traceability. Traceability implies that the ingredients of all products can be traced throughout the entire manufacturing process so that if any safety problem arises, its source can be located and the problem can be resolved. These good practice systems at all levels of the feed/food chain should enable all food and feed ingredients to be traced along with the presence of undesirable substances (European Commission, 2017; van Raamsdonk et al., Reference van Raamsdonk, Prins, van de Rhee, Vliege and Pinckaers2017; Ottoboni et al., Reference Ottoboni, Spranghers, Pinotti, Baldi, De Jaeghere and Eeckhout2017b).
Ensuring that insect materials traded in the EU conform to quality and traceability standards is a major undertaking. This explains why the definition of the substrates that can potentially be used for rearing insects (EFSA, 2015), up to the identification and traceability of these material in feeding stuffs, is in strict in accordance with the transmissible spongiform encephalopathy legislation.
In addition, special attention needs to be paid to non-EU countries that export feed and mini livestock (insects) to the EU, and in particular developing countries often have climates that potentially favour not only an optimal insect biomass production but also microbial and fungal contamination. They may also have less structured supply chains, and limited resources to conduct monitoring and testing, so that health and safety problems may be more likely to arise in products from these countries. Better knowledge of the main routes of feed into Europe, identification of the main feed suppliers and monitoring of final livestock destinations are important not only for maintaining feed quality but also for providing information for the consumer/stakeholders to make informed buying decisions.
Former food products
Nutritional quality
FFPs are defined as ‘foodstuffs other than catering reflux, which are manufactured in full compliance with EU food law but are no longer intended for human consumption for practical and logistical reasons or due to problems in manufacturing or packaging which are unlikely to cause any health risks when used as feed’ (European Commission, 2013). They represent a way for these high-quality unintentional food losses to return to the food chain. FFP ingredients can be divided into two main categories: leftovers from the food industry mainly composed of bakery products (e.g. bread, pasta) and leftovers principally composed of confectionery products (e.g. chocolates, biscuits). To date, in the EU the reprocessing of FFPs into feedstuffs is limited compared to the total food waste (5 million tonnes/year compared to 89 million tonnes/year of total food waste in the EU) and FFPs are mainly reprocessed for monogastric nutrition (Pinotti et al., Reference Pinotti, Giromini, Ottoboni, Tretola, Cheli and Baldi2013; Stenmarck et al., Reference Stenmarck, Jensen, Quested, Moates, Buksti, Cseh and Scherhaufer2016; Featherstone, Reference Featherstone2016).
The growing use of FFPs represents an interesting opportunity also in terms of the circular economy, which involves recycling food wastage using innovative approaches and solutions. FFP reprocessing is thus one way to convert food losses into ingredients for animal diets, reducing the biomass caused by food waste incineration and landfilling, and represents an important campaign for food waste control.
Based on the nutritional evidence reported for humans, FFPs are generally extremely rich in carbohydrates and, depending on their origin, also in fats. FFPs thus still have a high value for feeding animals because they often contain a lot of energy (Table 2). These aspects have been extensively addressed by Giromini et al. (Reference Giromini, Ottoboni, Tretola, Marchis, Gottardo, Caprarulo, Baldi and Pinotti2017) who reported that FFPs have a nutritional composition similar to wheat grain, although with a higher metabolizable energy (ME) content. The ME value reported for FFPs was 16.95 MJ/kg. Fats and starch are the main contributors in such energy content. In fact, FFPs are important sources of fat, as demonstrated by the EE content of about 10%, that is six times higher than in wheat (<2%). The FFP starch content is also valuable (compared to common cereals), reaching about 52.4% on a DM basis. FFPs are also highly digestible, ranging from 79 up to 93% DM, depending on the mixture used in their preparation. The overall mean protein content in FFPs has been found to be about 10%; therefore, FFPs cannot be considered as a valuable protein source. Thus, although these results should be interpreted with care since they are case sensitive, that is they represent just a few examples of different former foodstuffs that could be present on the feed market – FFPs are energetic feed ingredients with a high value for feeding animals, as also demonstrated by the high energy content (Pinotti et al., Reference Pinotti, Giromini, Ottoboni, Tretola, Cheli and Baldi2013; Bouxin, Reference Bouxin2016; Giromini et al., Reference Giromini, Ottoboni, Tretola, Marchis, Gottardo, Caprarulo, Baldi and Pinotti2017; Table 2). Their composition, however, might vary and some nutritional features (i.e. free sugar content) should be studied in order to be correctly included in animal diet.
Table 2 Nutrient composition expressed in % on DM basis

FFPs=former foodstuff products; CP=crude protein; EE=ether extract; CF=crude fibre; NDF=neutral detergent fibre; ADF=acid detergent fibre; ash and energy content (ME, MJ/kg) of FFPs and conventional feed ingredients (wheat and barley) intended for pig feed formulation.
†Adapted from Giromini et al. (Reference Giromini, Ottoboni, Tretola, Marchis, Gottardo, Caprarulo, Baldi and Pinotti2017); ‡Adapted from Bouxin (Reference Bouxin2016).
By using a balanced by-product combination, it is possible to substitute partially ‘classic’ and traditional sources of energy and protein in animal diets (Pinotti and Dell’Orto, Reference Pinotti and Dell’Orto2011; Pinotti et al., Reference Pinotti, Krogdahl, Givens, Knight, Baldi, Baeten and Luten2014). This substitution can be obtained without major changes in the diet composition, and can sometimes reduce feed cost compared to traditional ingredients. Obviously, this approach is not without its drawbacks. In addition to economics and marketing issues, further aspects need to be considered when including FFPs in food for animal diets. This implies a functional evaluation with special emphasis on the FFP impact on animal welfare in general and the gastro-intestinal tract (i.e. gut health), in particular.
Gut health is essential for optimal health and production. The gut of piglets, for instance, is a complex environment. In particular, in new-borns and around the time of weaning, the pigs’ gut rapidly changes in size, has high protein turnover rates, undergoes rapid changes in microbiota and digestive changes, and quickly alters its digestive and immune functions. The gut health and the complex interactions between microbiota and gut maturation are influenced by host and many environmental factors with feeding strategies and husbandry practices being the most significant. Understanding what a healthy microbiota looks like and how former foodstuffs can influence the composition of the gut microbial population, improving eubiosis and/or reducing dysbiosis, provides fundamental information for efficiently reconverting ex-food into value-added products for animal nutrition (Lalles et al., Reference Lalles, Bosi, Smidt and Stokes2007; Pluske, Reference Pluske2013).
In addition, FFPs contain a notable amount of free sugars, giving these ingredients a great glycaemic index potential (Ottoboni et al., Reference Ottoboni, Tretola, Cattaneo, Luciano, Giromini, Fusi, Rebucci and Pinotti2018). Ottoboni et al. (Reference Ottoboni, Tretola, Cattaneo, Luciano, Giromini, Fusi, Rebucci and Pinotti2018) reported that compared to conventional feed ingredients, such as corn, FFPs are characterized by a higher (+40% in FFP v. corn) glycaemic index. A glycaemic index potential has been proposed in equine nutrition (Kronfeld et al., Reference Kronfeld, Treiber and Geor2005) for disorders associated with carbohydrate metabolism as well as in the nutrition of performance horses. The same concept, however, has not been adequately addressed in other farm animals such as pigs. In fact, practical experience and research has demonstrated that in pigs, soft faeces are produced when animals are fed a high-sugar diet, thus these materials need to be assessed in terms of gut health (Mavromichalis Reference Mavromichalis2012). Overall, the valuable nutritional characteristics, together with the sustainability of controlling food losses, make the reprocessing of FFP biomass a highly attractive sustainable and abundant source of nutrients for the feed sector. The widespread application of FFPs in animal nutrition also has an important advantage in terms of feed manufacturing: the lipid fraction is embedded in the FFP matrix, thus facilitating its manipulation and processing for feed production.
However, as also reported by Lemenager et al. (Reference Lemenager, Applegate, Donkin, Johnson, Lake, Neary and Sutton2006), several challenging aspects need to be considered when adding alternative feed ingredients to conventional feed such as the variation in nutrient content and nutrient availability between batches, the effects on animal performance, end-product quality, as well as their handling, storage and processing. Furthermore, as previously mentioned, the high amounts of free sugars in FFPs may negatively affect the animal gut health. Optimal gut health is an essential requirement to ensure food digestibility and nutrient bioavailability, as well as to achieve optimal growth and production.
Safety aspect
In addition to the nutritional evaluation, the use of FFPs in animal feeding also involves an evaluation of safety issues. The quality, traceability and safety of former foodstuffs are keys to guarantee the safe re-use of biomass, according to a biosecurity approach. In terms of safety, one major concern regarding feed ingredients is their microbiological quality. Tretola et al. (Reference Tretola, Di Rosa, Tirloni, Ottoboni, Giromini, Leone, Bernardi, Dell’Orto, Chiofalo and Pinotti2017a) recently evaluated the microbiological load in different FFPs. The results obtained indicated that all FFPs analysed were not only safe from a microbiological point of view but were also Salmonella spp. free. In addition, all other microbiological contaminants were limited or not detectable, and therefore always within the threshold levels established by Health Protection Agency and European Regulation No. 142/2011 (European Commission, 2011). These results highlighted the high quality of the FFPs analysed, and thus the negligible microbiological risk of the production process.
Former foodstuffs, although nutritious and safe from a microbiological point of view, may generate other safety issues, such as those related to the presence of packaging remnants. Ex-food, in fact, is un-packaged automatically in order to process a larger amount of product. Processing methods that convert former foodstuffs into feed ingredients do not usually include the pre-removal of packaging materials. Feed processors routinely remove the packaging from ex-food mechanically in the feed plant, however, despite the removal of most of the packaging, small amounts of wrapping materials can remain in the resulting feed. As a result, a small amount of packaging remnants in the final product (feed) appear to be unavoidable (Tretola et al., Reference Tretola, Di Rosa, Tirloni, Ottoboni, Giromini, Leone, Bernardi, Dell’Orto, Chiofalo and Pinotti2017a). Classical remnant residues in FFPs are plastics, paper and aluminium foil. The typical un-packaging process of FFPs can be summarized as follows: (1) the packaging is broken and reduced in size, (2) the now accessible FFPs are processed to produce a ready product and (3) the remains of packaging materials are finally removed by several procedures such as sieving, magnetic attraction, eddy current separation or density methods (van Raamsdonk et al., Reference van Raamsdonk, Rijk, Schouten, Mennes, Meijer, Van der Poel and De Jong2011; Amato et al., Reference Amato, Desiato, Giovannini, Pinotti, Tretola, Gili and Marchis2017; Tretola et al., Reference Tretola, Ottoboni, Di Rosa, Giromini, Fusi, Rebucci, Leone, Dell’Orto, Chiofalo and Pinotti2017b). Despite these processes, some packaging remnants such as plastic, resin, aluminium and pressed paperboard can remain as residue in the final product. Packaging materials are not accepted as a feed ingredient according to Regulation (EC) No. 767/2009 (European Commission, 2009a), which prohibits the use of feedstuffs on the market containing packaging materials from the agri-food industry.
A large range of materials, often characterized by complex compositions, are used in food packaging. The European Regulation (EC) 1935/2004 (European Commission, 2004) covers the general requirements for all types of packaging materials. It requires that packaging materials should not release their constituents at a level that could endanger human health. Specific EU directives regulate the composition of plastics and regenerated cellulose. Other packaging materials (i.e. paper, coatings or aluminium foil) are regulated in detail at a national level. EFSA has evaluated a few of these components concluding that the risk is limited (EFSA, 2008).
Besides the nutritional and safety subjects, the use of FFPs in animal feeding also requires the evaluation of sustainability issues. Sustainability-environmental studies and lifecycle assessments have shown that feed production is a significant contributor to the environmental footprint of animal products, and therefore an important element to take into account when considering mitigation. Without a comprehensive sustainability analysis of the impacts of animal feed, it is almost impossible to establish the Product Environmental Footprint of animal products such as meat, eggs, dairy products and fish. The most exhaustive example is the Vandermeersch et al. (Reference Vandermeersch, Alvarengaa, Ragaert and Dewulf2014) study, which directly compared the processing of former foodstuffs and biogas production of ‘bread waste’. The authors highlighted that exploiting food losses for animal feed seems the best option, especially for those fractions of food losses/waste with a low water content (e.g. bread waste). Although their results should be analysed carefully, since they are case-sensitive (i.e. they represent the situation of a company from the retail sector and two food waste companies in Belgium), they provide some clear evidences; that is food waste has a great potential for conversion into animal feed ingredients, especially for dry biomass.
A different scenario is the use of FFPs as premium substrate, for insects. van Broekhoven et al. (2015) investigated the effects of a low protein/high starch substrate compared to a high protein or a commercial diet. The study indicated that feed conversion efficiency was lower in a low protein/high starch diet, compared to high protein or commercial diets. In fact, high larval mortality was observed when high starch diets based on cookie leftovers, that is FFPs, were used as feeding substrate in mini livestock (van Broekhoven et al., 2015). In the light of these results, low protein/high starch diets, especially those based on cookie leftovers, might contain compounds that are harder to digest for mealworms.
Conclusions
The increasing global need to find alternative protein/energy sources has promoted research in the field of non-conventional feed ingredients. Insects contain high protein and fat whereas former foodstuffs contain high energy in the form of carbohydrates and fats. Both should therefore be considered as promising alternative feed ingredients for livestock production. In addition to their interesting nutritional features, they also represent a way of upgrading food waste biomasses/streams to valuable feed ingredients. In insect meals, the protein content seems to be consistent irrespective of the type of waste material the insects were offered, while the fat and ash concentration appear to be dependent on the rearing substrate. The type of insect metamorphosis and substrate time of exposure are also key variables.
Within insect production, the selection of an appropriate and tailored substrate could lead to the production of a premium feed specialty, thus providing new opportunities for raw materials and diet formulations. This implies that a standardization of the rearing protocols is needed. FFPs are considered as ‘a fortified version of cereals’ as they are extremely rich in carbohydrates, and depending on their origin, also in fats. As a consequence, former foodstuffs still have a high nutritional value for animal feed because they often contain a lot of energy.
In addition, an essential prerequisite for the use of these alternative feed ingredients in animal feeding is their safety. Based on the available data, when produced in line with the major feed/food authority’s criteria, they are characterized by a high quality and safety standards. This makes them comparable to other feed materials and ingredients currently available on the market, although their full nutritional, functional, safety and sustainability evaluation cannot be considered as complete.
Acknowledgement
None.
Declaration of interest
The authors declare that they have no competing interests.
Ethics statement
Not applicable.
Software and data repository resources
None of the data were deposited in an official repository.