Type 2 diabetes has been a growing public health problem worldwide(Reference Wild, Roglic and Green1). A large body of evidence has demonstrated that the presence of diabetes doubles the risk of a wide range of vascular diseases(Reference Sarwar, Gao and Seshasai2). In addition, diabetes is moderately associated with death from cancers of the liver, pancreas, ovary, colorectum, lung, bladder and breast(Reference Seshasai, Kaptoge and Thompson3). Preventive strategies are urgently needed to reduce the huge burden of diabetes.
Diet is widely believed to play an important role in the development of type 2 diabetes(Reference Colditz, Manson and Stampfer4, Reference Knowler, Barrett-Connor and Fowler5). Dietary glycaemic index (GI) and glycaemic load (GL) have received considerable attention for their potential contribution to the diabetes epidemic. The GI, introduced by Jenkins et al. (Reference Jenkins, Wolever and Taylor6), ranks the carbohydrate content of individual foods according to their postprandial glycaemic effects. Consumption of high-GI diets is associated with high blood glucose and insulin concentrations, thereby eventually resulting in glucose intolerance and high diabetes risk(Reference Ludwig7, Reference Willett, Manson and Liu8). The GL, which is the product of the GI of a food item and the available carbohydrate content, quantifies the overall glycaemic effect and insulin demand(Reference Salmeron, Ascherio and Rimm9, Reference Salmeron, Manson and Stampfer10).
Prospective cohort studies assessing the effects of dietary GI and GL on the risk of type 2 diabetes have yielded inconsistent results(Reference Salmeron, Ascherio and Rimm9–Reference Sluijs, van der Schouw and van der21). A previous meta-analysis of seven studies found that diets with a high GI or GL significantly increased the risk of type 2 diabetes(Reference Barclay, Petocz and McMillan-Price22). During the past few years, the number of original studies linking dietary GI and GL to diabetes has doubled. However, not all confirmed the previous findings, and the controversy on this topic continues(Reference Mosdol, Witte and Frost17, Reference Sahyoun, Anderson and Tylavsky19). With accumulating evidence, we therefore aimed to examine the associations between dietary GI and GL and the risk of type 2 diabetes by conducting an updated meta-analysis of prospective cohort studies.
Materials and methods
Literature search
We conducted a PubMed database search up to February 2011 to identify published studies of dietary GI and GL and the risk of type 2 diabetes, using the search terms ‘glycemic index’ or ‘glycemic load’ in combination with ‘diabetes’. No restrictions were imposed. In addition, we reviewed the reference lists of retrieved articles. Efforts were made to obtain additional data by contacting original authors.
Study selection
Studies were eligible for the present meta-analysis if they met the following criteria: a prospective cohort design, which is less prone to bias than a retrospective one; the exposure of interest was dietary GI or GL; the outcome of interest was incidence of type 2 diabetes; risk estimates and associated 95 % CI (or data to calculate them) were reported.
Data extraction
The following information was independently extracted by two authors: the first author's last name, publication year; study population, location, length of follow-up; number of cases and participants; assessment of exposure and outcome; most fully adjusted risk estimates with corresponding 95 % CI from the multivariable model for each category of exposure or for exposure as a continuous variable; statistical adjustment for the potential confounding factors.
Statistical analysis
The relative risk (RR) was used as the common measure of association across studies. For four studies(Reference Stevens, Ahn and Juhaeri12, Reference Hodge, English and O'Dea13, Reference Barclay, Flood and Rochtchina15, Reference Sluijs, van der Schouw and van der21) that analysed exposure as a continuous variable, RR for categories of exposure were obtained from original authors or estimated based on the exposure range (i.e. the highest and lowest categories of exposure) reported in the primary studies. A sensitivity analysis was performed by excluding these four studies to test the robustness of the results.
We calculated the Q and I 2 statistics to examine statistical heterogeneity across studies. I 2 is the proportion of total variation explained by between-study variation(Reference Higgins and Thompson23). Either a fixed- or, in the presence of heterogeneity, random-effects model(Reference DerSimonian and Laird24) was used to compute the summary risk estimates. In the fixed-effects model, the weight of each study is equal to the inverse variance of the natural logarithm of the RR, whereas in the random-effects model, an extra term is added to the variance according to the method proposed by DerSimonian & Laird(Reference DerSimonian and Laird24). In addition, we examined the influence of a single study on the combined risk estimates by omitting one study and analysing the remainders in each turn. Potential publication bias was assessed by visual inspection of Begg's funnel plots and by the Begg rank correlation and Egger linear regression tests(Reference Begg and Mazumdar25, Reference Egger, Davey Smith and Schneider26). All analyses were performed with the use of STATA version 11.0 (StataCorp, College Station, TX, USA). All statistical tests were two-sided and P < 0·05 was considered statistically significant, except where otherwise specified.
Results
Study characteristics
We identified sixteen potentially relevant prospective cohort studies of GI and GL and type 2 diabetes. Of these sixteen studies, three were excluded because the outcome was gestational diabetes mellitus(Reference Zhang, Liu and Solomon27) or because of overlapping publications from the same study population(Reference Hu, Manson and Stampfer28, Reference Halton, Liu and Manson29), thus leaving thirteen studies(Reference Salmeron, Ascherio and Rimm9–Reference Sluijs, van der Schouw and van der21) (twelve for GI and twelve for GL) for the final analysis. Characteristics of the selected studies are presented in Table 1. The association between GI and GL and diabetes risk was the primary outcome in all primary studies. The thirteen prospective cohort studies were published between 1997 and 2010. Among them, eight studies were conducted in the USA, two in Europe, two in Australia and one in China. The number of cases diagnosed in the original studies ranged from 138 to 8587, and the size of the cohort ranged from 1833 to 91 249. Of these thirteen studies, seven enrolled both sexes, one included men only and five included women only. The median length of follow-up ranged from 4 to 14 years. All studies used FFQ in dietary assessment. Diabetes ascertainment was based on self-report of physician diagnosis in most studies, but the majority of cases were confirmed in validation studies. The major confounding factors of interest included age, BMI, physical activity, smoking, and intakes of total energy, alcohol and dietary fibre.
Table 1 Characteristics of prospective cohort studies of dietary glycaemic index (GI) and glycaemic load (GL) and type 2 diabetes

WHR, waist:hip ratio.
Glycaemic index, glycaemic load and the risk of type 2 diabetes
The multivariable-adjusted RR for each study and the combined RR comparing the highest with the lowest categories of the GI and GL are presented in Figs. 1 and 2. Among the included studies, six studies found a statistically significant association between GI and an increased risk of type 2 diabetes. The GL was significantly associated with an increased diabetes risk in four studies. The summary RR of type 2 diabetes for the highest category of GI compared with the lowest was 1·16 (95 % CI 1·06, 1·26; n 12), with moderate evidence of heterogeneity (P = 0·02, I 2 = 50·8 %). For the GL, the summary RR was 1·20 (95 % CI 1·11, 1·30; n 12), with little evidence of heterogeneity (P = 0·10, I 2 = 34·8 %). Visual inspection of Begg's funnel plots did not show important asymmetry for either exposure. The Begg rank correlation and Egger linear regression tests did not indicate evidence of publication bias (all P>0·30).
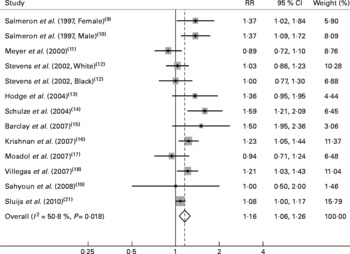
Fig. 1 Relative risks (RR) for the association between dietary glycaemic index and type 2 diabetes in prospective cohort studies.
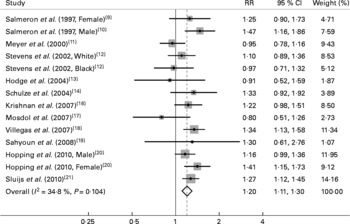
Fig. 2 Relative risks (RR) for the association between dietary glycaemic load and type 2 diabetes in prospective cohort studies.
Sensitivity analysis
We also performed sensitivity analyses to test the robustness of the findings. Restricting analysis to studies that adjusted for total fibre or cereal fibre yielded a summary RR of 1·14 (95 % CI 1·03, 1·26; n 10) for the GI and 1·16 (95 % CI 1·05, 1·29; n 9) for the GL. Restricting analysis to studies that were conducted in the USA yielded a summary RR of 1·17 (95 % CI 1·02, 1·34; n 7) for the GI and 1·19 (95 % CI 1·08, 1·31; n 8) for the GL. Restricting analysis to studies that analysed exposure as categorical variables yielded a summary RR of 1·19 (95 % CI 1·04, 1·36; n 8) for the GI and 1·22 (95 % CI 1·10, 1·36; n 9) for the GL. Further analyses investigating the influence of a single study on the overall risk estimate by omitting one study in each turn yielded a narrow range of RR from 1·13 (95 % CI 1·04, 1·22) to 1·18 (95 % CI 1·09, 1·29) for the GI and from 1·18 (95 % CI 1·09, 1·28) to 1·23 (95 % CI 1·15, 1·32) for the GL.
Discussion
The present meta-analysis of prospective cohort studies provides further evidence that higher dietary GI and GL increases the risk of type 2 diabetes. The highest GI and GL exposure compared with the lowest was associated with a 16 and 20 % increase in diabetes risk, respectively. In addition, the associations persisted and remained statistically significant in the sensitivity analyses.
A major strength of the meta-analysis is that the present findings are based on prospective cohort studies. This minimises the possibility of recall and selection biases, which are always of concern in retrospective studies. Compared with the previous meta-analysis(Reference Barclay, Petocz and McMillan-Price22), the risk estimates reported in the present study were a bit smaller. However, with accumulating evidence and enlarged sample size, we have enhanced statistical power to provide more precise and reliable risk estimates relating dietary GI and GL to diabetes risk.
Limitations of the present meta-analysis should also be acknowledged while interpreting the results. First, residual confounding is always of concern in observational studies. Although several important potential confounding factors, including age, BMI, physical activity and smoking, have been widely controlled in original studies, dietary factors were not sufficiently considered. For instance, higher GI was found to be correlated with lower intake of Mg(Reference Salmeron, Ascherio and Rimm9, Reference Salmeron, Manson and Stampfer10, Reference Schulze, Liu and Rimm14), and mounting evidence has suggested that lower Mg intake increases the risk of type 2 diabetes(Reference Schulze, Schulz and Heidemann30). Yet, few studies(Reference Schulze, Liu and Rimm14) have adjusted for Mg in the multivariable analysis. In addition, it is possible that a low-GI diet is rich in plant foods containing many phytochemicals that may protect against diabetes, which could result in attenuated associations. Therefore, we could not exclude the likelihood that unmeasured or inaccurately measured factors may be responsible for the findings.
Second, misclassification error and bias may have weakened the strength of the associations. Misclassification of dietary assessment is not avoidable and misclassification of diabetes cases was likely to occur given that the majority of diabetes ascertainments were based on self-reports. In addition, most cohort studies measured dietary intakes at baseline only, and the lack of repeated dietary assessment during the follow-up period could also result in misclassification. Third, we observed heterogeneity among studies of GI and diabetes risk. This may be partly explained by variations in characteristics of study populations, measurements of dietary intakes and ranges of dietary GI exposure. We attempted to explore possible sources of variations in the results using sensitivity analyses, but significant heterogeneity remained unsatisfactorily explained. Nevertheless, differences in risk estimates among individual studies were mainly in the magnitude rather than the direction of the association. Fourth, because GL were highly correlated with carbohydrate intake, it was difficult to determine to what extent the effect of GL on diabetes risk was explained by carbohydrate intake. Finally, publication bias could affect results of any meta-analyses. Yet formal statistical tests suggested little evidence of this bias in the present meta-analysis.
The exact mechanisms by which high-GI foods may increase the risk of type 2 diabetes are uncertain. A high-GI diet produces high concentrations of blood glucose and increased insulin demand. This could result in glucose intolerance and insulin resistance and eventually lead to a higher risk of type 2 diabetes(Reference Willett, Manson and Liu8). Furthermore, a high-GI diet could increase postprandial NEFA release, thereby directly increasing insulin resistance(Reference Ludwig7). In addition, there is evidence that higher dietary GI is positively associated with subsequent gain in waist circumference(Reference Du, van der and van Bakel31), which is a strong predictor of insulin resistance(Reference Racette, Evans and Weiss32) and diabetes risk(Reference Vazquez, Duval and Jacobs33).
On the other hand, metabolic studies suggest that a low-GI diet may improve glycaemic control. A recent meta-analysis of seven randomised controlled trials conducted in diabetes has shown that a low-GI diet, compared with a control diet, significantly decreased glycated HbA1c, a long-term measure of blood glucose levels(Reference Thomas and Elliott34). Although the reported decrease in glycated HbA1c was only 0·4 % (95 % CI 0·2, 0·7) in that meta-analysis(Reference Thomas and Elliott34), such a reduction has potential clinical importance as lowering glycated HbA1c by 0·6 % was demonstrated to contribute to risk reductions of 32 % for diabetes-related clinical endpoints, 42 % for diabetes-related deaths and 36 % for all-cause mortality(35).
The adverse effects of a high-GI diet may increase with an individual's underlying degree of insulin resistance(Reference Willett, Manson and Liu8). Thus, several factors related to insulin resistance as well as diabetes risk, such as cereal fibre intake, physical activity and BMI, may have impacts on the associations between GI and GL and diabetes risk. Epidemiological studies have provided compelling evidence that lower cereal fibre intake is associated with an increased diabetes risk(Reference Schulze, Schulz and Heidemann30). Previous studies(Reference Salmeron, Ascherio and Rimm9, Reference Salmeron, Manson and Stampfer10, Reference Schulze, Liu and Rimm14) have consistently suggested that the positive associations between GI and GL and diabetes risk were more pronounced among those with a lower intake of cereal fibre, with significant interaction. Physical activity has strong effects on glucose tolerance and insulin sensitivity(Reference Goodyear and Kahn36) and can significantly reduce the risk of developing type 2 diabetes(Reference Jeon, Lokken and Hu37). As expected, the associations of GI and GL with diabetes risk appeared to be more evident in participants with low physical activity levels(Reference Schulze, Liu and Rimm14, Reference Villegas, Liu and Gao18). As for BMI, an important determinant of insulin resistance, results were inconsistent. For example, two studies(Reference Schulze, Liu and Rimm14, Reference Villegas, Liu and Gao18) observed a stronger effect of GI and GL in participants with higher BMI, whereas another study(Reference Krishnan, Rosenberg and Singer16) showed a higher risk for GI and GL in those with lower BMI. Of note, the numbers of cases were relatively small in these stratified analyses, and therefore chance may, at least in part, account for these findings. Nevertheless, as potential effect modifiers, these variables need to be closely considered in subsequent studies for a better understanding of the relationships of dietary GI and GL to diabetes risk.
In conclusion, the present meta-analysis of prospective cohort studies provides further evidence in support of significantly positive associations between dietary GI and GL and the risk of type 2 diabetes. Given the wide exposure and the huge burden of diabetes worldwide, reducing the intake of high-GI foods, particularly refined carbohydrates, may bring potential benefits in diabetes prevention among the general population.
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (no. 30771808). None of the authors had a conflict of interest. J.-Y. D. and L.-Q. Q. were responsible for the study design, data acquisition, statistical analysis and the interpretation of the results. J.-Y. D. prepared the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.





