Impact statements
Nutrient loading (notably nitrogen and phosphorus) to coastal oceans from food production, fossil fuel burning, aquaculture operations, and wastewater from humans, livestock, and industry has accelerated during the past decades, causing over-enrichment of nutrients, or eutrophication. Eutrophication degrades coastal water quality with two most common symptoms, hypoxia and harmful algal blooms, creating profound ecological and societal consequences such as biodiversity decline, seagrass loss, coral bleaching, fish kills and marine mammal mortalities, and human health threats. Such marine pollution symptoms have persisted although billions of dollars have been invested in both research and management as well as efforts of restorations in many developed countries. Consequently, we are still witnessing trends in the expansion of coastal eutrophication and hypoxia from developed regions into developing regions. Though the limited efficacy of mitigation witnessed so far suggests the complexity of the issue, we contend that closing the knowledge gaps in the causality between eutrophication and hypoxia is essential and crucial towards making science- and evidence-based policies. We recognize that the non-linear cause–effect relationship in coastal marine ecosystem degradation caused by multi-stressors is complex. The strength and synergistic effect of multiple driving forces of coastal eutrophication is dependent on regional geographic feature, economic development, and societal management, while the long-term trends of eutrophication and hypoxia are subject to the control of the trends in nutrient loadings and physical dynamics under a changing climate. This review also examines lessons from past eutrophication management practices, and advocates for a better, more efficient indexing system of coastal eutrophication and an advanced regional earth system modeling framework to facilitate the development and evaluation of effective policy and restoration actions.
Introduction
Situated between the land and the open ocean, the coastal ocean (i.e., the areas from the shoreline to the continental shelf break influenced by the land-based runoff) possesses rich spatial, economic, and biological resources and diverse ecosystems, providing invaluable services for human society (e.g., Lu et al., Reference Lu, Yuan, Lu, Su, Zhang, Wang, Cao, Li, Su, Ittekkot, Garbutt, Bush, Fletcher, Wagey, Kachur and Sweijd2018a; Winther et al., Reference Winther, Dai, Rist, Hoel, Li, Trice, Morrissey, Juinio-Meñez, Fernandes, Unger, Scarano, Halpin and Whitehouse2020; Dai et al., Reference Dai, Su, Zhao, Hofmann, Cao, Cai, Gan, Lacroix, Laruelle, Meng, Müller, Regnier, Wang and Wang2022). The land masses adjoining the coasts are home to over 50% of the world’s population and are the world’s most economically invigorating areas, driving global economic development, especially in Asia over the past 50 years, and producing approximately 50% of the world’s gross domestic product (GDP). However, pressures on this vital region from human development and climate change have intensified ever since the Industrial Revolution, which has adversely impacted human society and impaired the regional, and by extension the global ocean, sustainability (e.g., Doney et al., Reference Doney, Ruckelshaus, Emmett Duffy, Barry, Chan, English, Galindo, Grebmeier, Hollowed, Knowlton, Polovina, Rabalais, Sydeman and Talley2012; Winther et al., Reference Winther, Dai, Rist, Hoel, Li, Trice, Morrissey, Juinio-Meñez, Fernandes, Unger, Scarano, Halpin and Whitehouse2020).
Among many other factors, nutrient pollution, primarily due to a massive increase in fertilizer usage, has resulted in worldwide expansion of coastal eutrophication, i.e., the over-enrichment of nutrients, particularly nitrogen (N) and phosphorus (P), that stimulate biological productivity and excessive algal growth. The two most acute symptoms of eutrophication are hypoxia (dissolved oxygen, DO depletion to a level of <2 mg L−1) and harmful algal blooms (HABs), which may cause changes in seawater chemistry (e.g., acidification), loss of habitat and biodiversity, even mass mortality of impacted marine organisms, and eventually impair important ecosystem services (Kristiansen et al., Reference Kristiansen, Kristensen and Jensen2002; Grantham et al., Reference Grantham, Chan, Nielsen, Fox, Barth, Huyer, Lubchenco and Menge2004; Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Cai et al., Reference Cai, Hu, Huang, Murrell, Lehrter, Lohrenz, Chou, Zhai, Hollibaugh, Wang, Zhao, Guo, Gundersen, Dai and Gong2011; Rabalais et al., Reference Rabalais, Cai, Carstensen, Conley, Fry, Hu, QuiÑOnes-Rivera, Rosenberg, Slomp, Turner, Voss, Wissel and Zhang2014; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018; Pitcher et al., Reference Pitcher, Aguirre-Velarde, Breitburg, Cardich, Carstensen, Conley, Dewitte, Engel, Espinoza-Morriberón, Flores, Garçon, Graco, Grégoire, Gutiérrez, Hernandez-Ayon, Huang, Isensee, Jacinto, Levin, Lorenzo, Machu, Merma, Montes, Swa, Paulmier, Roman, Rose, Hood, Rabalais, Salvanes, Salvatteci, Sánchez, Sifeddine, Tall, Plas, Yasuhara, Zhang and Zhu2021). Expansion of coastal hypoxic zones can also enhance the production of greenhouse gases (e.g., methane, nitrous oxide), and thus pose positive feedback to the climate system (e.g., Naqvi et al., Reference Naqvi, Bange, Farías, Monteiro, Scranton and Zhang2010; Conley and Slomp, Reference Conley, Slomp, Lffoley and Baxter2019).
Coastal eutrophication and associated environmental stressors have occurred over the past 50–100 years or longer in Central/Western Europe (e.g., Andersen et al., Reference Andersen, Carstensen, Conley, Dromph, Fleming-Lehtinen, Gustafsson, Josefson, Norkko, Villnäs and Murray2017), North America (e.g., Kemp et al., Reference Kemp, Boynton, Adolf, Boesch, Boicourt, Brush, Cornwell, Fisher, Glibert, Hagy, Harding, Houde, Kimmel, Miller, Newell, Roman, Smith and Stevenson2005) and part of East Asia (Japan) (e.g., Yasuhara et al., Reference Yasuhara, Hunt, Breitburg, Tsujimoto and Katsuki2012). These are recently witnessed in China and other developing countries in Asia (e.g., Dai et al., Reference Dai, Guo, Zhai, Yuan, Wang, Wang, Cai, Tang and Cai2006; Wang et al., Reference Wang, Xin, Wei and Xie2018a), and have now emerged in the rest of the world. Eutrophication, therefore, remains among the leading causes of water quality degradation and as a major threat to human sustainability (IOC-UNESCO, 2022). The persistence of these environmental threats and their adverse effects on human health and economy at the global scale, despite enormous efforts and investment, demonstrate the complexity of mitigation. Finding an effective solution remains a grand challenge, and this has been identified as one of the 10 main challenges in a recently released State of the Ocean Report (IOC-UNESCO, 2022).
Tremendous efforts over the past decades have been devoted to studying coastal eutrophication at regional and global scales, including its cascading effects such as hypoxia and HABs (e.g., UNEP, 2006; Pitcher et al., Reference Pitcher, Aguirre-Velarde, Breitburg, Cardich, Carstensen, Conley, Dewitte, Engel, Espinoza-Morriberón, Flores, Garçon, Graco, Grégoire, Gutiérrez, Hernandez-Ayon, Huang, Isensee, Jacinto, Levin, Lorenzo, Machu, Merma, Montes, Swa, Paulmier, Roman, Rose, Hood, Rabalais, Salvanes, Salvatteci, Sánchez, Sifeddine, Tall, Plas, Yasuhara, Zhang and Zhu2021; Tuholske et al., Reference Tuholske, Halpern, Blasco, Villasenor, Frazier and Caylor2021; Peñuelas and Sardans, Reference Peñuelas and Sardans2022). These research efforts span from examinations of their sources, spatial and temporal variability to their mechanisms, attributions, and interconnections. The major progress in the field has been elaborately evaluated in some critical reviews (e.g., Nixon, Reference Nixon1995; Anderson et al., Reference Anderson, Glibert and Burkholder2002; Howarth and Marino, Reference Howarth and Marino2006; Billen and Garnier, Reference Billen and Garnier2007; Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Howarth, Reference Howarth2008; Kemp et al., Reference Kemp, Testa, Conley, Gilbert and Hagy2009; Paerl et al., Reference Paerl, Hall, Peierls and Rossignol2014; Glibert and Burford, Reference Glibert and Burford2017; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018; Boesch, Reference Boesch2019; Fennel and Testa, Reference Fennel and Testa2019; Griffith and Gobler, Reference Griffith and Gobler2020; Grégoire et al., Reference Grégoire, Garçon, Garcia, Breitburg, Isensee, Oschlies, Telszewski, Barth, Bittig, Carstensen, Carval, Chai, Chavez, Conley, Coppola, Crowe, Currie, Dai, Deflandre, Dewitte, Diaz, Garcia-Robledo, Gilbert, Giorgetti, Glud, Gutierrez, Hosoda, Ishii, Jacinto, Langdon, Lauvset, Levin, Limburg, Mehrtens, Montes, Naqvi, Paulmier, Pfeil, Pitcher, Pouliquen, Rabalais, Rabouille, Recape, Roman, Rose, Rudnick, Rummer, Schmechtig, Schmidtko, Seibel, Slomp, Sumalia, Tanhua, Thierry, Uchida, Wanninkhof and Yasuhara2021; Pitcher et al., Reference Pitcher, Aguirre-Velarde, Breitburg, Cardich, Carstensen, Conley, Dewitte, Engel, Espinoza-Morriberón, Flores, Garçon, Graco, Grégoire, Gutiérrez, Hernandez-Ayon, Huang, Isensee, Jacinto, Levin, Lorenzo, Machu, Merma, Montes, Swa, Paulmier, Roman, Rose, Hood, Rabalais, Salvanes, Salvatteci, Sánchez, Sifeddine, Tall, Plas, Yasuhara, Zhang and Zhu2021), substantially advancing our mechanistic understanding of this global issue. Here, we highlight some of the major findings from prior research.
Nutrient loading and structure have dramatically changed over the past several decades (Malone and Newton, Reference Malone and Newton2020). The global N and P loads increased by 40–45% between 1980 and 2015 (Beusen et al., Reference Beusen, Doelman, Van Beek, Van Puijenbroek, Mogollón, Van Grinsven, Stehfest, Van Vuuren and Bouwman2022). The use of nutrient-rich synthetic fertilizers and their runoff into waterways, together with the planting of N-fixing crops, fossil fuels burning and wastewater inputs from treatment plants and stormwater conduits, elevates the N:P ratio of nutrient supplies to the coastal ocean (Glibert et al., Reference Glibert, Maranger, Sobota and Bouwman2014; Peñuelas and Sardans, Reference Peñuelas and Sardans2022), potentially creating P limitation and dominant algal species shifts (Lin et al., Reference Lin, Litaker and Sunda2016). Increased N loading is thus considered a primary cause of eutrophication in most estuarine and coastal environments, although phosphate pollution is also an issue receiving intense attention (Howarth and Marino, Reference Howarth and Marino2006; Howarth, Reference Howarth2008; Paerl, Reference Paerl2018).
The altered stoichiometry of nitrogen, phosphorus and silicon (N:P:Si) in addition to the overall higher nutrient concentrations exerts a profound impact on the phytoplankton communities and primary productivity (Smith, Reference Smith2003), increasing the abundance, frequency, and extent of HABs (Anderson et al., Reference Anderson, Glibert and Burkholder2002; O’Neil et al., Reference O’Neil, Davis, Burford and Gobler2012; Glibert et al., Reference Glibert, Maranger, Sobota and Bouwman2014; Glibert and Burford, Reference Glibert and Burford2017; Gobler, Reference Gobler2020; Anderson et al., Reference Anderson, Fensin, Gobler, Hoeglund, Hubbard, Kulis, Landsberg, Lefebvre, Provoost, Richlen, Smith, Solow and Trainer2021). Hypoxia is also increasingly documented in coastal waters globally at increased frequency, spatial extent, and severity that threats the ecosystem health and food security (e.g., Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Rabalais et al., Reference Rabalais, Cai, Carstensen, Conley, Fry, Hu, QuiÑOnes-Rivera, Rosenberg, Slomp, Turner, Voss, Wissel and Zhang2014; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018; Grégoire et al., Reference Grégoire, Garçon, Garcia, Breitburg, Isensee, Oschlies, Telszewski, Barth, Bittig, Carstensen, Carval, Chai, Chavez, Conley, Coppola, Crowe, Currie, Dai, Deflandre, Dewitte, Diaz, Garcia-Robledo, Gilbert, Giorgetti, Glud, Gutierrez, Hosoda, Ishii, Jacinto, Langdon, Lauvset, Levin, Limburg, Mehrtens, Montes, Naqvi, Paulmier, Pfeil, Pitcher, Pouliquen, Rabalais, Rabouille, Recape, Roman, Rose, Rudnick, Rummer, Schmechtig, Schmidtko, Seibel, Slomp, Sumalia, Tanhua, Thierry, Uchida, Wanninkhof and Yasuhara2021). However, the formation and dynamics of hypoxia are subject to a suite of complex and interactive physical, chemical, and biological processes (e.g., Rabouille et al., Reference Rabouille, Conley, Dai, Cai, Chen, Lansard, Green, Yin, Harrison, Dagg and McKee2008; Fennel and Testa, Reference Fennel and Testa2019), leading to spatiotemporal heterogeneity of hypoxia and sometimes an apparent decoupling of eutrophication-hypoxia relationship (e.g., Hetland and DiMarco, Reference Hetland and DiMarco2008; Zhao et al., Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021). Thus, such a cause–effect relationship is not straightforward and is very often non-linear, complicating most quantification efforts.
Growing evidence suggests that climate change exacerbates eutrophication and its associated negative impacts on coastal ecosystems (Doney et al., Reference Doney, Ruckelshaus, Emmett Duffy, Barry, Chan, English, Galindo, Grebmeier, Hollowed, Knowlton, Polovina, Rabalais, Sydeman and Talley2012; Altieri and Gedan, Reference Altieri and Gedan2015; Sinha et al., Reference Sinha, Michalak and Balaji2017). The increasing frequency and intensity of extreme events over the past decades (Alimonti et al., Reference Alimonti, Mariani, Prodi and Ricci2022) have and will continue to increase the magnitude and alter the timing of runoff, nutrient and terrestrial organic matter (terr-OM) delivery to the coastal ocean (Rabalais et al., Reference Rabalais, Díaz, Levin, Turner, Gilbert and Zhang2010; Diffenbaugh et al., Reference Diffenbaugh, Singh and Mankin2018), exacerbating the development of HABs (Glibert, Reference Glibert2020). Warming also decreases oxygen solubility, increases intensity and duration of stratification, and enhances microbial metabolism, resulting in an earlier onset, higher frequency, and increased severity of hypoxia in nutrient-enriched coastal systems (Altieri and Gedan, Reference Altieri and Gedan2015; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018). Furthermore, these negative consequences of increased nutrient loading and stratification might be partly, or temporarily, compromised by rising sea levels and stronger storms in low and mid-latitudes (Rabalais et al., Reference Rabalais, Turner, Diaz and Justic2009), and are further complicated by the superimposition of open ocean circulation and large-scale climate variabilities such as El Niño, the Pacific Decadal Oscillation, and the North Atlantic Oscillation (e.g., Dai et al., Reference Dai, Su, Zhao, Hofmann, Cao, Cai, Gan, Lacroix, Laruelle, Meng, Müller, Regnier, Wang and Wang2022).
Accumulating human and climatic impacts may impair ecosystem resilience (i.e., the ability of ecosystems to absorb disturbances and preside in multiple stable states that maintain critical functionality (Elliott and Quintino, Reference Elliott and Quintino2007)) by inducing ecosystem reorganization, or “regime shifts”, defined as abrupt changes in the structure and function of ecosystems, or abrupt changes across a tipping point, a critical threshold at which a subsequent tiny perturbation can qualitatively alter the state or development of a system with irreversible consequences (Lenton, Reference Lenton2013; van Nes et al., Reference van Nes, Arani, Staal, van der Bolt, Flores, Bathiany and Scheffer2016), which have received increasing attention from either ecological or ocean-human coupled points of view (e.g., Chaparro-Pedraza and de Roos, Reference Chaparro-Pedraza and de Roos2020). The marine ecosystem, notably the coastal ocean, is likely experiencing multi-fold regime shifts due to the multiple stressors imposed on the system, yet how and whether eutrophication has induced regime shift is unclear. In the Baltic Sea, a series of threats induced by eutrophication, overfishing and climatic changes are believed to have pushed the system over a tipping point in the 1980s, from which it has yet to recover (Österblom et al., Reference Österblom, Hansson, Larsson, Hjerne, Wulff, Elmgren and Folke2007; Rabalais et al., Reference Rabalais, Díaz, Levin, Turner, Gilbert and Zhang2010; Reid et al., Reference Reid, Hari, Beaugrand, Livingstone, Marty, Straile, Barichivich, Goberville, Adrian, Aono, Brown, Foster, Groisman, Hélaouët, Hsu, Kirby, Knight, Kraberg, Li, Lo, Myneni, North, Pounds, Sparks, Stübi, Tian, Wiltshire, Xiao and Zhu2016; Möllmann et al., Reference Möllmann, Cormon, Funk, Otto, Schmidt, Schwermer, Sguotti, Voss and Quaas2021). The persistent eutrophication and continuous climate change are predicted to exacerbate coastal hypoxia (Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Fennel and Testa, Reference Fennel and Testa2019); it is identified to be a high-probability and high-impact ocean tipping point that warrants urgent attention and action (Heinze et al., Reference Heinze, Blenckner, Martins, Rusiecka, Döscher, Gehlen, Gruber, Holland, Hov, Joos, Matthews, Rødven and Wilson2021). However, the underlying mechanisms of ecosystem regime shifts involve a complex coupling of abiotic and biotic processes, making it particularly challenging to holistically understand and predict. From the perspective of nutrient–algal growth relationship (the Monod Model), every algal species has a ‘reaction norm’ to nutrient variability (characterized by the half-saturated nutrient concentration Ks and maximum growth rate μmax). Significant ‘unusual’ nutrient enrichment may favor ‘weed’ species with high Ks and high μmax, which are not suitable food for grazers, leaving high biomass (a HAB event or otherwise) for bacterial respiration in bottom waters.
Substantial efforts have been made to form task forces and set policies to abate eutrophication and its cascading consequences, from the local to the international levels (e.g., the Gulf of Mexico Watershed Nutrient Task Force, European Union directives), with billions of dollars invested in actions and implementation of conservation measures worldwide (Basu et al., Reference Basu, Van Meter, Byrnes, Van Cappellen, Brouwer, Jacobsen, Jarsjö, Rudolph, Cunha, Nelson, Bhattacharya, Destouni and Olsen2022). Although improved wastewater treatment measures have succeeded particularly in reducing point source pollution from industries mainly in North America and Europe (Boesch, Reference Boesch2019), reducing nutrient inputs from diffuse sources, such as non-point runoff from agriculture and urban landscapes, has proven to be more difficult and often fall short of goals, hindering water quality improvements and coastal ecosystem restorations from eutrophication (Macintosh et al., Reference Macintosh, Mayer, McDowell, Powers, Baker, Boyer and Rittmann2018). The hypoxic area in the northern Gulf of Mexico reached 16,000 km2 in 2015, failing to be reduced to the targeted size of 5,000 km2 (Van Meter et al., Reference Van Meter, Van Cappellen and Basu2018). The eelgrass meadows along the Swedish Kattegat in the Baltic Sea, which were lost since the 1980s, failed to recover despite a significant reduction in both nutrient loads and N concentrations (Moksnes et al., Reference Moksnes, Eriander, Infantes and Holmer2018). Various strategies have recently been proposed to resolve these issues, including dual-nutrient (N and P) reduction strategy for aquatic ecosystems, large-scale changes in agricultural management practices with technological and societal innovations, better incorporation of nutrient legacies and time lags into watershed conservation measures, economic cost-effectiveness/cost–benefit analyses, and long-term commitment at both local and regional scales (Conley et al., Reference Conley, Paerl, Howarth, Boesch, Seitzinger, Havens, Lancelot and Likens2009; Le Moal et al., Reference Le Moal, Gascuel-Odoux, Ménesguen, Souchon, Étrillard, Levain, Moatar, Pannard, Souchu, Lefebvre and Pinay2019; Malone and Newton, Reference Malone and Newton2020; Basu et al., Reference Basu, Van Meter, Byrnes, Van Cappellen, Brouwer, Jacobsen, Jarsjö, Rudolph, Cunha, Nelson, Bhattacharya, Destouni and Olsen2022).
Despite major progress, controlling eutrophication and associated hypoxia has proven to be difficult due to the major knowledge gaps concerning the complexity of the eutrophication-hypoxia causal relationship, which is often non-linear and site-specific. The complexity also lies in the non-linear drivers associated with the social-economic dimension. Thus, discerning the full spectrum of physical, biogeochemical, and socioeconomic processes that govern eutrophication and hypoxia in the coastal ocean remains a grand scientific challenge and a practical issue to be resolved, particularly from a quantitative perspective. These knowledge gaps clearly hamper the establishment of effective and science-based policy that can be transformed into action plans to remediate environmental problems. Consequently, we are still witnessing trends in the expansion of coastal eutrophication and hypoxia from developed regions into developing regions.
This review examines the evolution of coastal eutrophication to identify critical knowledge gaps in these complex cause–effect systems with the aim of providing possible solutions based on understanding the up-to-date status and mechanisms of eutrophication and hypoxia in coastal waters. In particular, we contend that the human dimension plays a key role in causing eutrophication, including its consequences, and should be fully taken into account to fill the knowledge gaps and propose remedial solutions. To do so, we examine the trajectories of eutrophic-hypoxic evolution in six model coastal systems across the globe over the last 40 years or longer and consider the social-economic impacts. Special consideration is given to the interactive/synergistic drivers of natural and anthropogenic forcing. By comparing different systems under different developmental stages, we also intend to summarize past experiences and lessons from this persistent but crucial environmental issue for regions to consider in policymaking. Finally, we put forward our perspectives on research critically needed to advance scientific solutions to coastal eutrophication and hypoxia.
Current status
Nutrient loading and eutrophication
The global human population has increased from 3 billion in 1960 to 7.8 billion in 2020, along with rapidly growing GDPs that appear to hinge on the steadily increasing urbanization rate and amount of synthetic fertilizer use (Figure S1 in the Supplementary Material). The human footprint on Earth, as an index of the pressure imposed on the eco-environment, is presently remarkably concentrated in Asia, Europe and North America, followed by some regions of Africa and South America (Figure 1a). Human development and activities have thus imposed intense pressure on the coastal ocean. Excessive loads of N- and P-containing nutrients (Figure 1b), largely associated with the increasing human population and activities, have caused worldwide occurrences of eutrophication, especially in the coastal ocean (Steffen et al., Reference Steffen, Richardson, Rockström, Cornell, Fetzer, Bennett, Biggs, Carpenter, de Vries, de Wit, Folke, Gerten, Heinke, Mace, Persson, Ramanathan, Reyers and Sörlin2015; Malone and Newton, Reference Malone and Newton2020; Liu et al., Reference Liu, Stock, Dunne, Lee, Shevliakova, Malyshev and Milly2021a).
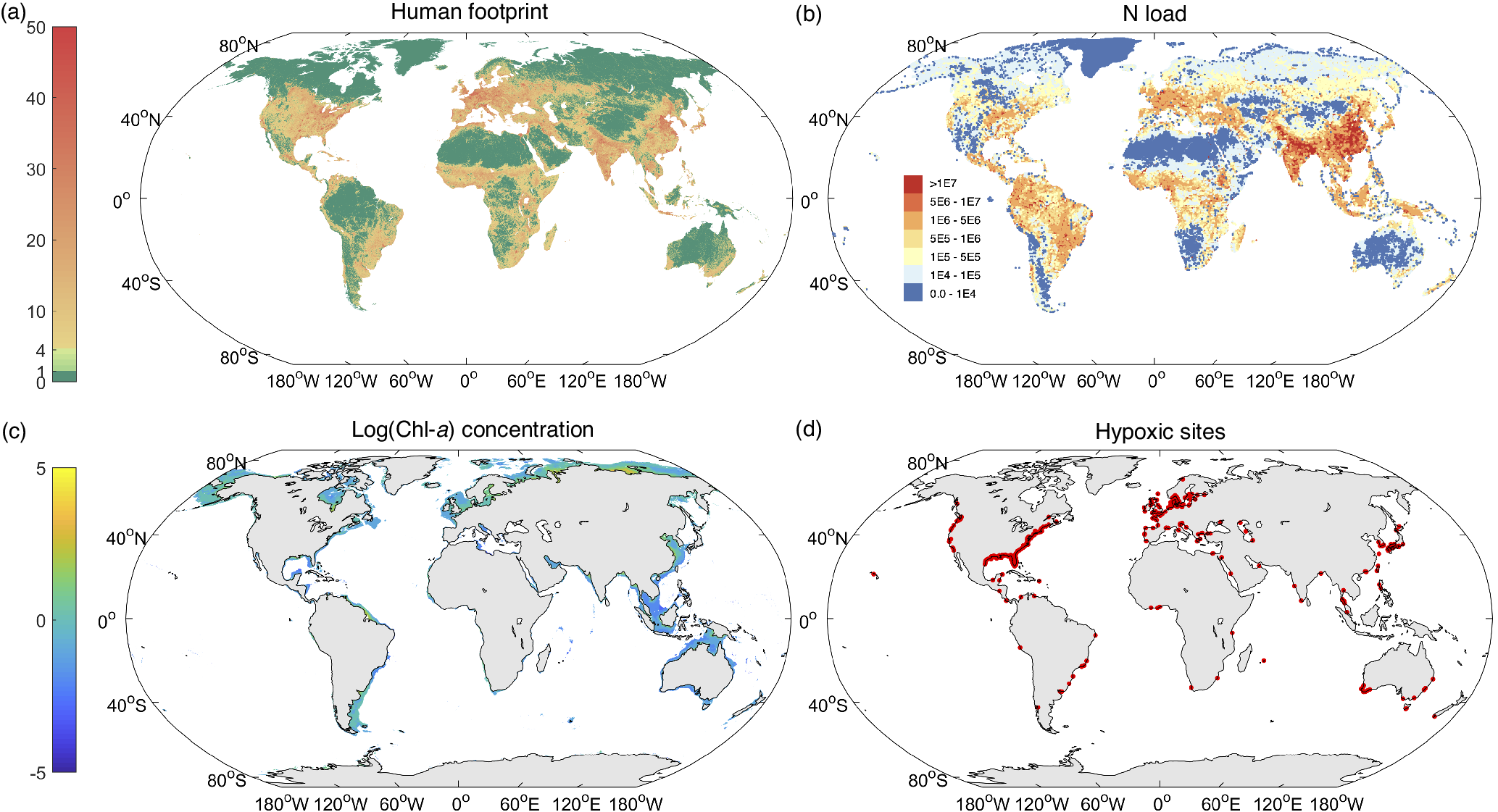
Figure 1. Global distributions of (a) the human footprint index in 2018, (b) N loads in 2020 (kg N yr−1), (c) log(Chl-a) concentration (μg L−1), and (d) hypoxic sites. Data on the human footprint are from Mu et al. (Reference Mu, Li, Wen, Huang, Du, Su, Miao and Geng2022); data on N loads are from Beusen et al. (Reference Beusen, Doelman, Van Beek, Van Puijenbroek, Mogollón, Van Grinsven, Stehfest, Van Vuuren and Bouwman2022); data on Chl-a concentrations are derived from the remote sensing satellite images from the Climate Change Initiative-European Space Agency project (http://www.esa-oceancolour-cci.org/); Data of hypoxic sites (where the dissolved oxygen (DO) concentration is less than 2 mg L−1) were downloaded from World Resources Institute (https://www.wri.org/data/eutrophication-hypoxia-map-data-set) (Diaz et al., Reference Diaz, Selman and Chique2011).
The main sources of anthropogenic nutrient loading to coastal ecosystems include synthetic fertilizer use, manure application, biological N-fixation by legume, emissions to the atmosphere, sewage discharge, and aquaculture operations. The global use of synthetic fertilizers has increased by more than five-fold since 1961 to 200 Tg yr−1 (1 Tg = 1012 g) in 2020, and becomes the major contributor, at about an order of magnitude greater than the other sources (Table 1). Of the current global fertilizer use, South Asia accounts for 55.1% and South America contributes 13.3%, with the remaining use divided among North America and Europe (approximately 12% each), Africa (3.5%) and Oceania plus Central America (totaling 3.3%) (Figure S1c in the Supplementary Material). Among synthetic fertilizers, N fertilizer is the most used, accounting for 56%, while P fertilizer makes up a proportion of 24% (Table 1).
Table 1. Global sources of anthropogenic nitrogen and phosphorus
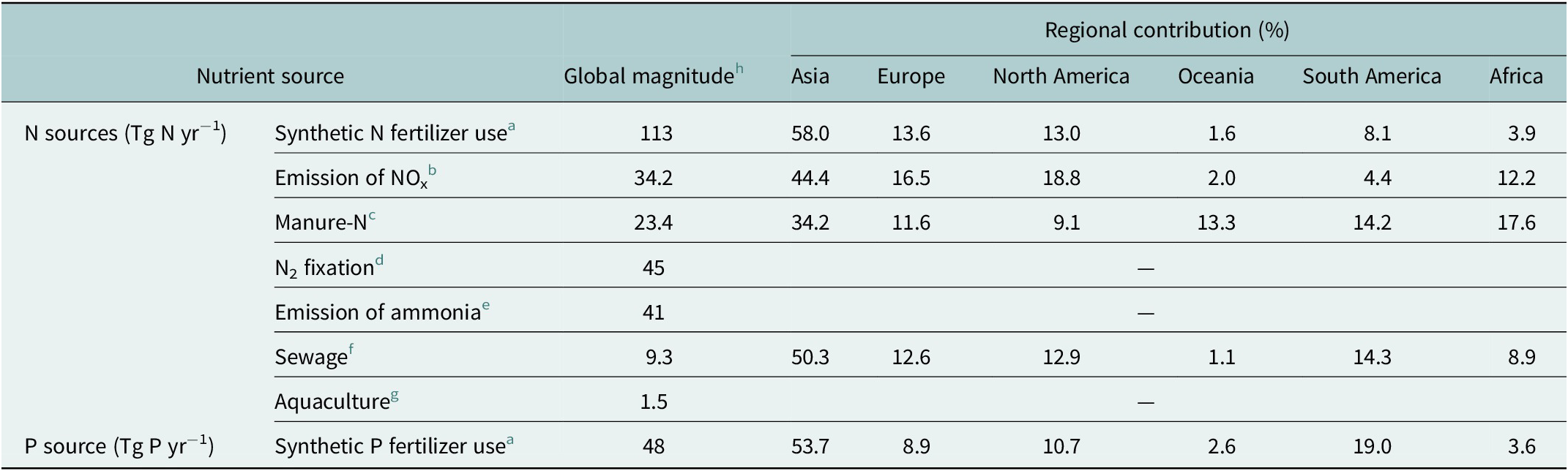
a FAOSTAT: https://www.fao.org/faostat/en/#data/RFN.
b The global top-down estimates of NO+NO2 emissions mainly from fossil-fuel combustion, biomass burning, oil and gas production, industry, agriculture, and biogenic activities using an Ozone Monitoring Instrument NO2 retrieval (NASAv3) through a hybrid 4D-Var/mass balance inversion (Qu et al., Reference Qu, Daven, Owen and Jessica2020).
c The manure nitrogen applied to cropland averaged over the result of Zhang et al. (Reference Zhang, Tian, Lu, Dangal, Yang and Pan2017a) reconstructed based on the dataset from the Global Livestock Impact Mapping System (GLIMS) in conjunction with country-specific annual livestock populations and Tian et al. (Reference Tian, Bian, Shi, Qin, Pan, Lu, Pan, Tubiello, Chang, Conchedda, Liu, Mueller, Nishina, Xu, Yang, You and Zhang2022) reconstructed based on different data sources in a spatiotemporally consistent way;
d Biogeological nitrogen fixation in agroecosystems linearly interpolated based on the results of Bouwman et al. (Reference Bouwman, Goldewijk, Van Der Hoek, Beusen, Van Vuuren, Willems, Rufino and Stehfest2013b) which predicted N2 fixation from 39 Tg N yr−1 in 2000 to 54 Tg N yr−1 by 2050.
e The volatilization of ammonia from agricultural lands (29 Tg N yr−1) and ammonia emission from animal houses and storage systems (12 Tg N yr−1) into the atmosphere as of 2020 linearly interpolated based on the results of Bouwman et al. (Reference Bouwman, Goldewijk, Van Der Hoek, Beusen, Van Vuuren, Willems, Rufino and Stehfest2013b) which predicted total ammonia emission from (24 + 10) Tg N yr−1 in 2000 to (36 + 15) Tg N yr−1 by 2050.
f Human N emission in wastewater by households and industries to surface water linearly interpolated based on results of Van Drecht et al. (Reference Van Drecht, Bouwman, Harrison and Knoop2009), which predicted the global sewage emissions from 6.4 Tg N yr−1 in 2000 to 12.0–15.5 Tg yr−1 by 2050.
g N release to the freshwater (1.2 Tg N yr−1) and marine (0.3 Tg N yr−1) aquatic environments due to feeds in aquaculture (Bouwman et al., Reference Bouwman, Beusen, Overbeek, Bureau, Pawlowski and Glibert2013a).
h Data for Emission of NOx in 2016, Manure-N in 2014, Aquaculture in 2010, and for the rest in 2020.
The global anthropogenic N input to the terrestrial ecosystems is now estimated to be 267 Tg N yr−1 (Tian et al., Reference Tian, Bian, Shi, Qin, Pan, Lu, Pan, Tubiello, Chang, Conchedda, Liu, Mueller, Nishina, Xu, Yang, You and Zhang2022), exceeding the natural N fixation in the entire ocean by 87.7% (Wang et al., Reference Wang, Moore, Martiny and Primeau2019). In addition to N fertilizer inputs on cropland and pasture, biological N-fixation by legume and manure N application contribute to 45 Tg N yr−1 and 23.4 Tg N yr−1, respectively, of nutrient inputs in the watershed, while emissions of nitrogen oxides (NOx) mainly from fossil fuel combustion and biomass burning and of ammonia from agricultural lands, animal houses and storage systems account for 34 Tg N yr−1 and 41 Tg N yr−1, respectively (Table 1). As the most prevalent urban source of nutrients, estimated human sewage discharge into the environment reached 9.3 Tg N yr−1 by 2020 (Table 1), of which 6.2 Tg N yr−1 enters coastal waters accounting for approximately 40% of total N from agriculture based on a high-resolution geospatial model (Tuholske et al., Reference Tuholske, Halpern, Blasco, Villasenor, Frazier and Caylor2021). Of total wastewater N, 63% (3.9 Tg N yr−1) comes from sewered systems, 5% (0.3 Tg N yr−1) from septic, and 32% (2.0 Tg N yr−1) from direct input. Despite a relatively small magnitude of wastewater N release compared to other sources, human sewage impacts most coastlines globally, with sewered, septic, and untreated wastewater inputs varying greatly across watersheds and by country. Nearly half of all wastewater N loadings come from 25 watersheds (Tuholske et al., Reference Tuholske, Halpern, Blasco, Villasenor, Frazier and Caylor2021). The nutrient release from the aquaculture into aquatic environment was estimated to be 1.5 Tg N yr−1 in 2010 (Table 1), but its global contribution to nutrient loading of rivers is small (Bouwman et al., Reference Bouwman, Beusen, Overbeek, Bureau, Pawlowski and Glibert2013a).
The N emissions from fertilizer, livestock waste and fossil fuel combustion, which are closely related to population and economic growth, were the highest in Europe and North America in the 1980s and earlier, accompanied by the emergence of coastal eutrophication in these regions since the 1950s (Nixon, Reference Nixon1995). From the 1960s to the 2010s, hotspots of total anthropogenic nitrogen inputs, especially from synthetic fertilizer use, shifted from Europe and North America to East and South Asia (Tian et al., Reference Tian, Bian, Shi, Qin, Pan, Lu, Pan, Tubiello, Chang, Conchedda, Liu, Mueller, Nishina, Xu, Yang, You and Zhang2022), associated with a mirrored human population and economic development pattern (Figure S1 in the Supplementary Material). Galloway et al. (Reference Galloway, Dentener, Capone, Boyer, Howarth, Seitzinger, Asner, Cleveland, Green, Holland, Karl, Michaels, Porter, Townsend and Vöosmarty2004) predicted that by 2050 the percentage of the anthropogenic N input in coastal watersheds exported to coastal ecosystems would increase by 40–50% from approximately 20% in 2000. South Asia, in particular, is projected to account for nearly half of this increase (Lee et al., Reference Lee, Seitzinger and Mayorga2016). Also, of the total estimated human sewage discharge in 2020, Asia (mainly South and East Asia) accounts for 50.3%, followed by South America, North America, Europe, and Africa with contributions varying from 8.9–14.3% (Table 1). Consequently, coastal eutrophication has emerged as a major environmental concern in the developing countries of Asia, South America and North Africa (Wang et al., Reference Wang, Li, Humphries, Chinni, Uthaipan, Dai, Urban and Ittekkot2022).
Pathways of nutrient inputs into the coastal ocean mainly include river runoff (e.g., Große et al., Reference Große, Fennel and Laurent2019), submarine groundwater discharges (e.g., Santos et al., Reference Santos, Chen, Lecher, Sawyer, Moosdorf, Rodellas, Tamborski, Cho, Dimova, Sugimoto, Bonaglia, Li, Hajati and Li2021), direct waste discharge (e.g., Tuholske et al., Reference Tuholske, Halpern, Blasco, Villasenor, Frazier and Caylor2021) and atmospheric deposition (Codispoti et al., Reference Codispoti, Brandes, Christensen, Devol, Naqvi, Paerl and Yoshinari2001). The total riverine N loading was estimated to be 57.9 Tg N yr−1 in 2020 (Liu et al., Reference Liu, Stock, Dunne, Lee, Shevliakova, Malyshev and Milly2021a), a 40% increase since 1961. The global flux of P to the ocean has reached about 22 Tg P yr−1 (Bennett et al., Reference Bennett, Carpenter and Caraco2001). These nutrient loadings from diverse sources are expected to enhance nutrient concentrations and inventory in the coastal waters, which however remains difficult to be fully assessed due to the lack of systematic observational data in the global coastal ocean. Numerical modeling studies show an increase in nitrogen inventory of 5.8% (~16.6 Tg N) from 1960 to 2010 in the global coastal ocean, accompanied by enhanced net primary productivity by 4.6% in coastal oceans during the same period (Liu et al., Reference Liu, Stock, Dunne, Lee, Shevliakova, Malyshev and Milly2021a). Regional monitoring programs have meanwhile shown the concentration of dissolved inorganic nitrogen (DIN, the sum of ammonium, nitrate and nitrite) in coastal waters (excluding regions of coastal upwelling) varies from 1.67 to 66 μmol L−1 in the US and Europe and is around 1.50 μmol L−1 in African coastal waters (Lu et al., Reference Lu, Yuan, Lu, Su, Zhang, Wang, Cao, Li, Su, Ittekkot, Garbutt, Bush, Fletcher, Wagey, Kachur and Sweijd2018a). Relatively high values up to over 200 μmol L−1 appear mainly in South and East Asia, while the lowest value, 0.3 μmol L−1, is observed in Moreton Bay, Australia. Most of the global nutrient-enriched systems have experienced an increase in DIN concentrations over the past few decades (Malone and Newton, Reference Malone and Newton2020).
The concentration of chlorophyll-a (Chl-a) is commonly used as an indicator of eutrophication (Woodland et al., Reference Woodland, Thomson, Mac Nally, Reich, Evrard, Wary, Walker and Cook2015): a system is defined as eutrophic with satellite Chl-a concentrations greater than 2.21 μg L−1, or oligotrophic with the concentrations less than 0.1 μg L−1 (Karydis and Kitsiou, Reference Karydis and Kitsiou2019). Based on MODIS ocean color data, the Chl-a concentration has increased over time along the coasts of East and South Asia, East Africa, South America, and Europe in the 21st century (Figure 1c) (Elsworth et al., Reference Elsworth, Lovenduski, McKinnon, Krumhardt and Brady2020). The total area of global coastal waters (depth ≤ 200 m) that show increasing trends in satellite-derived Chl-a concentration is 7.99 × 105 km2, approximately 30% greater than areas with an oligotrophic potential (Maúre et al., Reference Maúre, Terauchi, Ishizaka, Clinton and DeWitt2021).
HABs in coastal waters
International research programs GEOHAB (1998–2013) and GlobalHAB (2017-) have been in place for decades to advance scientific knowledge about the causative species, outbreak and decline dynamics, and environmental drivers. In the latest global HAB report, toxic bloom events accounted for nearly half of the global bloom events, and this percentage continues to increase gradually over time (Hallegraeff et al., Reference Hallegraeff, Anderson, Belin, Bottein, Bresnan, Chinain, Enevoldsen, Iwataki, Karlson, McKenzie, Sunesen, Pitcher, Provoost, Richardson, Schweibold, Tester, Trainer, Yñiguez and Zingone2021). Anthropogenic nutrient loading and climate change are the two major environmental drivers of increasing HABs (Hallegraeff et al., Reference Hallegraeff, Anderson, Belin, Bottein, Bresnan, Chinain, Enevoldsen, Iwataki, Karlson, McKenzie, Sunesen, Pitcher, Provoost, Richardson, Schweibold, Tester, Trainer, Yñiguez and Zingone2021), but how these factors interact with the algae is very complex (Glibert, Reference Glibert2020). Most algal phyla have HAB-forming species, but dinoflagellates are particularly notorious for their greatest contribution of species, diversity of toxins and bloom events. On the global scale, HABs formed by dinoflagellates have increased in frequency and range due to climate change and eutrophication. For example, in Chinese coastal waters, the frequency and area of dinoflagellates-dominant HABs substantially increased from the 1980s onwards when the ratio of total N and P in inputs exceeded the critical values of 25–30 (Wang et al., Reference Wang, Bouwman, Liu, Beusen, Van Dingenen, Dentener, Yao, Glibert, Ran, Yao, Xu, Yu, Middelburg and Yu2021), resulting in more frequently reported fish mortality, shellfish poisoning, economic loss, and even human death (Wang et al., Reference Wang, Tang, He, Fukuyo and Azanza2008; Yu et al., Reference Yu, Lü, Liang, Glibert, Berdalet, Burford, Pitcher and Zhou2018). Advances in monitoring technology and greater public awareness can also contribute to the increasing record of HAB outbreaks (Gobler, Reference Gobler2020; Hallegraeff et al., Reference Hallegraeff, Anderson, Belin, Bottein, Bresnan, Chinain, Enevoldsen, Iwataki, Karlson, McKenzie, Sunesen, Pitcher, Provoost, Richardson, Schweibold, Tester, Trainer, Yñiguez and Zingone2021).
A HAB outbreak represents a sharp regime shift, from a genetically (taxonomically) diverse algal community evolving into a single or few species-dominated assemblage. Such a rapid and dramatic process conceivably involves the surpassing of an ecosystem tipping point, which is likely intimately related to the genetics of species in the community dictating their abilities to acquire energy and nutrients, defend and proliferate sexually as well as asexually (Zhang et al., Reference Zhang, Lin, Shi, Lin, Luo, Li and Lin2019b; Yu et al., Reference Yu, Zhang, Li, Wang, Lin, Li, Shi, Guo and Lin2020; Lin et al., Reference Lin, Yu, Wu, Li, Zhang, Luo, Li, Li and Li2022). Ecosystems impacted by HABs show resilience in most cases, eventually being able to return to original or similar status. However, the frequency and scale of HAB events in coastal waters have shown steady increases in the past decades. That has raised the major concern whether the coastal ecosystem will cross the threshold and come to the irreversible tipping point, which means a drastic change in ecosystem services.
Coastal deoxygenation and hypoxia
Besides HABs, another notorious consequence of coastal eutrophication is the worldwide occurrence of hypoxic, or even anoxic, areas (Figure 1d). Though hypoxia and “dead zones” have been widely used in the literature and by the general public. It should be pointed out that hypoxia is very often arbitrarily defined by a DO level of <2 mg L−1, the unit of which is dependent upon temperature, salinity and pressure. Rather, concentration units such as μmol O2 kg−1 are independent of temperature, salinity and pressure (Hofmann et al., Reference Hofmann, Peltzer, Walz and Brewer2011). However, this review uses mg L−1 for consistency with the literature reports. Nevertheless, the expansion of oxygen-depleted coastal environments has been accelerating since the 1950s, most notably over the past two decades (Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Zhang et al., Reference Zhang, Gilbert, Gooday, Levin, Naqvi, Middelburg, Scranton, Ekau, Peña, Dewitte, Oguz, Monteiro, Urban, Rabalais, Ittekkot, Kemp, Ulloa, Elmgren, Escobar-Briones and Van der Plas2010). The number of reported hypoxic sites has increased exponentially at a rate of ~5.5% yr−1 over the past six decades, approximately doubling each decade and increasing at more than 500 sites (Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Vaquer-Sunyer and Duarte, Reference Vaquer-Sunyer and Duarte2008; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018). Hypoxic conditions in coastal waters are increasing in occurrence, frequency, intensity, and duration, and affect a total area of more than 0.25 × 106 km2 in the coastal ocean (Diaz and Rosenberg, Reference Diaz and Rosenberg2008; Breitburg et al., Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley, Garçon, Gilbert, Gutiérrez, Isensee, Jacinto, Limburg, Montes, Naqvi, Pitcher, Rabalais, Roman, Rose, Seibel, Telszewski, Yasuhara and Zhang2018).
Coastal hypoxia seems to follow a predictable pattern (Diaz and Rosenberg, Reference Diaz and Rosenberg2008). First, episodic oxygen depletion implies a critical point of eutrophication that, in combination with a stratified water column, tips the system into hypoxia. About 17% of the hypoxic systems report episodic hypoxia (Diaz and Rosenberg, Reference Diaz and Rosenberg2008), experiencing infrequent oxygen depletion with less than one event per year. Over time, with the build-up of nutrients and OM in the near-bottom waters and sediments, hypoxia becomes seasonal or periodic, generally occurring after spring blooms in summer and lasting until autumn (Rabalais et al., Reference Rabalais, Díaz, Levin, Turner, Gilbert and Zhang2010; Murphy et al., Reference Murphy, Kemp and Ball2011; Wang et al., Reference Wang, Wei, Chen and Xie2012). Owing to local weather events, spring-neap tidal cycles, production-respiration diel cycles or winds, periodic hypoxia lasts from days to weeks and tends to be less severe than seasonal hypoxia (Breitburg, Reference Breitburg2002; Tyler and Targett, Reference Tyler and Targett2007). Together, episodic and seasonal hypoxia are responsible for about three quarters of the known hypoxic zones. Another 8% of the hypoxic systems experience persistent oxygen depletion on the order of years, during which the hypoxic zone expands, and the oxygen concentration continues to decline until anoxia is established. Despite the dominant control of physical dynamics on the water residence time, which allows for hypoxic conditions, this progression from episodic to seasonal hypoxia has been documented in the Chesapeake Bay (Kemp et al., Reference Kemp, Boynton, Adolf, Boesch, Boicourt, Brush, Cornwell, Fisher, Glibert, Hagy, Harding, Houde, Kimmel, Miller, Newell, Roman, Smith and Stevenson2005), the northern Gulf of Mexico (Turner et al., Reference Turner, Rabalais and Justic2008), off the Changjiang Estuary (Chen et al., Reference Chen, Li, Jin, Jiang, Wang, Wu, Hao, Sun, Chen and Guo2020) and the Pearl River Estuary (Qian et al., Reference Qian, Gan, Liu, He, Lu, Guo, Wang, Guo, Huang and Dai2018), where increasing microbial oxygen consumption rates enhanced by eutrophication facilitate hypoxia formation and maintenance.
Most of the reported coastal hypoxia is confined to shallow brackish tidal rivers and upper estuaries, where heavy loads of labile organic waste and ammonia directly stimulate microbial respiration and nitrification leading to oxygen depletion (Figure 2) (Dai et al., Reference Dai, Guo, Zhai, Yuan, Wang, Wang, Cai, Tang and Cai2006; He et al., Reference He, Dai, Zhai, Guo and Wang2014). The drivers of these hypoxic systems are well studied and oxygen conditions can be improved rapidly and linearly (Kemp et al., Reference Kemp, Testa, Conley, Gilbert and Hagy2009). This review will focus on the hypoxia occurring in lower estuary and inner shelf regimes, where the spatiotemporal coupling/decoupling of eutrophication and hypoxia, sources of OM fueling oxygen consumption, and nutrient legacies causing hypoxia recurrence remain a subject of contention (Figure 2).
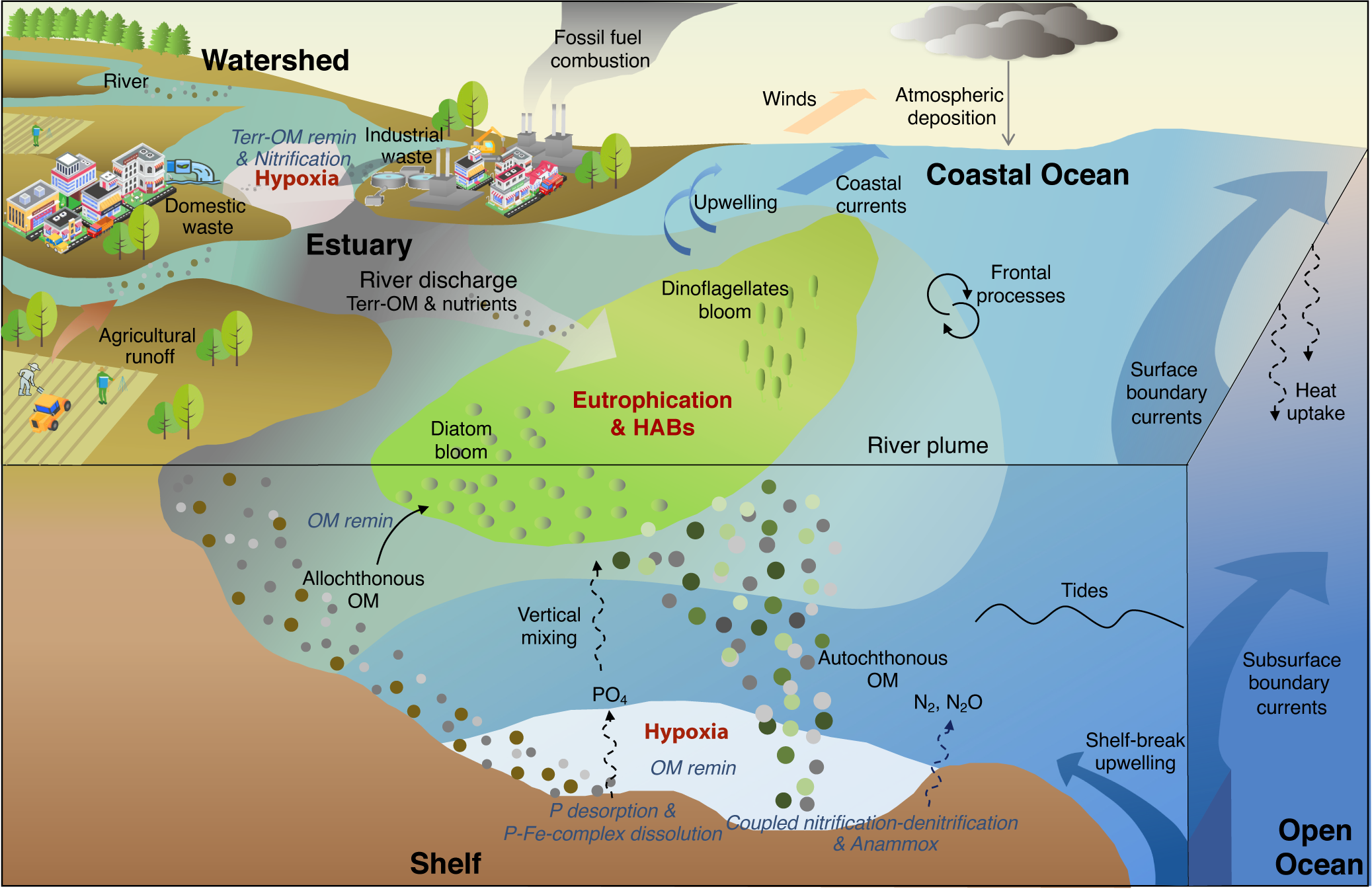
Figure 2. Conceptual diagram of the drivers of and interactions between eutrophication and hypoxia in the coastal ocean. Increasing amounts of anthropogenic nutrients sourced from agricultural and aquacultural runoffs, and domestic and industrial waste discharge are exported into the coastal ecosystems. The terrestrial organic matter (Terr-OM) and inorganic nutrients directly stimulate microbial respiration (Terr-OM remin) and nitrification, leading to oxygen depletion in the tidal rivers and upper estuary. Excess riverine nutrients with nitrogen to phosphorus (N:P) molar ratios above the Redfield ratio of 16 are further transported to the lower estuary and inner shelf regions, causing eutrophication and outbreaks of harmful algal blooms (HABs). The abundant autochthonous OM induced by eutrophication eventually sinks to the bottom and is remineralized, contributing to bottom hypoxia in most coastal regions. In addition, allochthonous OM from land and marine-sourced OM induced by wind-driven upwelling can, to some extent, enhance the formation of hypoxia. With the various sources of OM and complicated physical and topographical features in coastal regions, spatiotemporal heterogeneity between surface eutrophication and bottom hypoxia is often observed (see text). Coupled nitrification–denitrification and anammox in hypoxic waters and sediments remove bioavailable nitrogen from the system. On the other hand, the hypoxic/anoxic environment facilitates the desorption of phosphorus (P) and the dissolution of P–Fe complexes in sediments. Release of bioavailable phosphate (PO4) into the overlying waters and subsequent mixing into the euphotic layer further stimulates primary production.
Mechanisms and interactions
Mechanistic coupling of hypoxia to eutrophication
In most coastal systems, eutrophication is considered the primary cause of the development and intensification of bottom water hypoxia (Diaz, Reference Diaz2001; Rabalais and Gilbert, Reference Rabalais, Gilbert, Urban, Sundby, Malanotte-Rizzoli and Melillo2009; Rabalais et al., Reference Rabalais, Díaz, Levin, Turner, Gilbert and Zhang2010; Rabalais et al., Reference Rabalais, Cai, Carstensen, Conley, Fry, Hu, QuiÑOnes-Rivera, Rosenberg, Slomp, Turner, Voss, Wissel and Zhang2014; Wang et al., Reference Wang, Dai, Liu, Kao, Zhang, Cai, Wang, Qian, Zhao and Sun2016). Excessive anthropogenic inputs of inorganic N and P raise nutrient concentrations and elevate the production of autochthonous OM in coastal regions, leading to an increase in the rate of OM supply to the bottom water and sediment, which enhances microbial respiration and oxygen consumption therein (Figure 2). Under strong vertical stratification and restricted lateral advection or diffusivity of oxygenated waters, once the oxygen consumption rate exceeds that of replenishment, hypoxia forms (e.g., Rabouille et al., Reference Rabouille, Conley, Dai, Cai, Chen, Lansard, Green, Yin, Harrison, Dagg and McKee2008). Remineralization of autochthonous OM thus dominates oxygen consumption in the hypoxic zone (Rabalais et al., Reference Rabalais, Smith, Harper and Justic2001; Rabalais et al., Reference Rabalais, Díaz, Levin, Turner, Gilbert and Zhang2010; Wang et al., Reference Wang, Dai, Liu, Kao, Zhang, Cai, Wang, Qian, Zhao and Sun2016). Indeed, tracing the origin of oxygen-consuming OM reveals that oxygen depletion in the hypoxic zones off the Changjiang Estuary, in the Chesapeake Bay and northern Gulf of Mexico is almost all attributed to respiration of autochthonous OM formed by eutrophication-induced primary production (Wang et al., Reference Wang, Dai, Liu, Kao, Zhang, Cai, Wang, Qian, Zhao and Sun2016; Wang et al., Reference Wang, Hu, Wetz and Hayes2018b; Su et al., Reference Su, Cai, Brodeur, Hussain, Chen, Testa, Scaboo, Jaisi, Li, Dai and Cornwell2020b). In the hypoxic zone off the Pearl River Estuary, about two-thirds of oxygen-consuming OM is originated from the surface eutrophication (Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020), with the rest being allochthonous OM sourced from riverine and wetland inputs which contribute to oxygen removal directly via remineralization (Bianchi et al., Reference Bianchi, DiMarco, Cowan, Hetland, Chapman, Day and Allison2010; Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020) and/or indirectly via the nutrients released during remineralization (Yu et al., Reference Yu, Gan, Dai, Hui, Lu and Li2021; Zhang, Reference Zhang2022) (Figure 2). High primary production driven by nutrient over-enrichment thus dominates hypoxia formation in many coastal systems, especially in the river-dominated open coastal systems.
Through these mechanisms, increased anthropogenic nutrient inputs due to enhanced human socioeconomic activities are hypothesized to be responsible for the expansion of hypoxia throughout the coastal ocean. A global-scale model of coastal oxygen and nutrient dynamics suggests that coastal systems with more densely populated watersheds and/or more intensive agricultural activities exhibit higher sensitivity to changes in nutrient loading and are more prone to develop hypoxia (Reed and Harrison, Reference Reed and Harrison2016). By comparing major coastal hypoxic systems worldwide, Fennel and Testa (Reference Fennel and Testa2019) pointed out that larger hypoxic areas are statistically related to higher nutrient loads with freshwater inputs. Model experiments with different nutrient loadings further reveal a non-linear response of hypoxia to nutrient input, and a higher sensitivity of oxygen levels to N than P loads in N-limited coastal ecosystems, such as in Chesapeake Bay (Testa et al., Reference Testa, Li, Lee, Li, Brady, Di Toro, Kemp and Fitzpatrick2014). However, N loading alone cannot fully explain the interannual variability of the hypoxic area in many open coastal ecosystems (e.g., only 40–50% in the northern Gulf of Mexico (Scavia et al., Reference Scavia, Rabalais, Turner, Justić and Wiseman2003; Bianchi et al., Reference Bianchi, DiMarco, Cowan, Hetland, Chapman, Day and Allison2010; Obenour et al., Reference Obenour, Michalak, Zhou and Scavia2012), because the formation and maintenance of bottom-water hypoxia also depends on stratification of the water column by freshwater inputs (Bianchi et al., Reference Bianchi, DiMarco, Cowan, Hetland, Chapman, Day and Allison2010; Obenour et al., Reference Obenour, Michalak, Zhou and Scavia2012) and nutrient supply by upwelling of nutrient-rich subsurface oceanic waters offshore. Recent modeling studies estimated that the respiration of oceanic N-supporting OM accounts for about 16% and 22–40% of oxygen consumption in hypoxic systems in the northern Gulf of Mexico and off the Changjiang Estuary, respectively (Große et al., Reference Große, Fennel and Laurent2019; Große et al., Reference Große, Fennel, Zhang and Laurent2020). Furthermore, geomorphological characteristics and filtration power of watershed and coastal zone may create marked variations in the ecological effects of nutrient loading (Cloern, Reference Cloern2001). It is also noted that eutrophication often results in deviation of the N:P ratios from the Redfield ratio of 16, which drives ‘unusual’ or harmful algal species to thrive, thus promoting HABs (e.g., Glibert et al., Reference Glibert, Al-Azri, Icarus Allen, Bouwman, Beusen, Burford, Harrison, Zhou, Glibert, Berdalet, Burford, Pitcher and Zhou2018). The unusual or harmful species cannot be grazed efficiently by zooplankton and other herbivores, shunting the biomass to respiration by bacteria. Hypoxia in bottom water ensues.
In addition, OM decomposition and subsequent nitrification in marine sediments also contribute to oxygen consumption at the sediment–water interface (Zhang et al., Reference Zhang, Zhao, Sun, Wang and Wei2017b) though its relative contribution to the water column oxygen consumption is debatable. Estimated rates of sedimentary oxygen consumption indeed vary widely, ranging from 5–168 mmol O2 m−2 day−1 in the estuarine and coastal systems (Boynton et al., Reference Boynton, Ceballos, Bailey, Hodgkins, Humphrey and Testa2018). In hypoxic zones with a large thickness of the bottom water, sediment oxygen demand typically makes a minor contribution to total oxygen loss (Kemp et al., Reference Kemp, Boynton, Adolf, Boesch, Boicourt, Brush, Cornwell, Fisher, Glibert, Hagy, Harding, Houde, Kimmel, Miller, Newell, Roman, Smith and Stevenson2005; Zhang et al., Reference Zhang, Zhao, Sun, Wang and Wei2017b). Water column respiration contributes to ~70% of the total biological oxygen consumption under hypoxic conditions in Chesapeake Bay, northern Gulf of Mexico and off the Changjiang Estuary (McCarthy et al., Reference McCarthy, Carini, Liu, Ostrom and Gardner2013; Li et al., Reference Li, Li and Kemp2015; Zhou et al., Reference Zhou, Zhu, Hu, Zhang and Wang2021). Even in very shallow (1 ~ 2 m), semi-enclosed lagoons with a long water residence time (~ 1 yr), integrated oxygen consumption in the water column also accounts for 67–73% of total DO consumption (Wang et al., Reference Wang, Hu, Wetz and Hayes2018b).
In more enclosed systems, such as in the eastern Gotland Basin of the Baltic Sea, simulated oxygen consumption in the sediment is much larger than that in the water column, despite oxygen consumption increased more in the water column than in the sediment after 1970 (Meier et al., Reference Meier, Väli, Naumann, Eilola and Frauen2018). In these systems, the nutrient legacy could play a very important role in the persistency of eutrophication and should be taken into consideration for their management and mitigations. Nutrient legacy could also be related to the fact that a greater amount of labile OM can escape from microbial degradation and reach the sediments in hypoxic waters compared to in oxic waters (Middelburg and Levin, Reference Middelburg and Levin2009; Jessen et al., Reference Jessen, Lichtschlag, Ramette, Pantoja, Rossel, Schubert, Struck and Boetius2017; Yang et al., Reference Yang, Zhang, Kang, Zhao and Tang2020; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020), and thus the accumulation of OM over 1 year or longer might make the hypoxic systems more sensitive to N loading over time (Turner et al., Reference Turner, Rabalais and Justic2008).
Spatial–temporal decoupling of hypoxia and algal blooms
Despite the mechanistically biogeochemical coupling of human-driven eutrophication to hypoxic zone expansion in the global coastal ocean, the occurrence of hypoxia and eutrophication-induced algal bloom may mismatch in both space and time (Figure 2) because of the distinct physical and geographical constraints on hypoxia formation. In addition to microbial oxygen removal due to abundant OM supplies, the formation of hypoxia is also affected by the stability of water column that is influenced by vertical stratification and lateral advection, and they are all greatly governed by highly variable wind and terrestrial discharge in the coastal zone over the synoptical and even longer time scale. In well-ventilated energetic coastal systems, hypoxia might not form despite strong eutrophication. This can be explained by the concept of hypoxia timescale, which is the ratio between the oxygen amount needed to be drawn down to reach hypoxia and the net oxygen consumption rate (i.e., rate of oxygen consumption minus supply); such timescale must be shorter than the water residence time in order to develop hypoxia (Fennel and Testa, Reference Fennel and Testa2019). As such, the formation of hypoxia does not synchronize with phytoplankton blooms, but experiences a time lag ranging from several days to months (Fennel and Testa, Reference Fennel and Testa2019). It has been widely observed that the intense spring algal production is followed by late-spring and summer heterotrophic remineralization of OM and initiation of bottom-water hypoxia, such as in Chesapeake Bay (Hagy et al., Reference Hagy, Boynton, Keefe and Wood2004), Long Island Sound (Lee and Lwiza, Reference Lee and Lwiza2008), the northern Gulf of Mexico (Rabalais and Turner, Reference Rabalais and Turner2019), and off the Changjiang Estuary (Zhou et al., Reference Zhou, Chai, Huang, Wells, Ma, Meng, Xue, Xuan, Wang, Ni, Zhao, Liu, Su and Li2020). A recent study in the Chesapeake Bay suggests that bottom-water oxygen levels are significantly correlated with surface algal biomass during the preceding weeks (Zheng and DiGiacomo, Reference Zheng and DiGiacomo2020). Off the Changjiang Estuary, a time lag of 1 to 8 weeks is estimated between the occurrence of surface diatom production and bottom hypoxia based on a coupled physical-biogeochemical model (Zhou et al., Reference Zhou, Chai, Huang, Wells, Ma, Meng, Xue, Xuan, Wang, Ni, Zhao, Liu, Su and Li2020). Furthermore, the accumulation of OM in bottom waters and sediment can sustain hypoxic conditions even after the bloom terminates in the surface waters. The feedback of hypoxia on surface algal blooms will be elaborated in the following section.
It is increasingly recognized that the locations of hypoxic zones and surface algal blooms might be separated (Figure 2) as jointly modulated by the confluence of river discharge, coastal circulation (Caballero-Alfonso et al., Reference Caballero-Alfonso, Carstensen and Conley2015), tides and atmospheric forcing such as air-sea momentum (wind), heat and buoyancy fluxes (Li et al., Reference Li, Gan, Hui, Yu, Liu, Lu, Kao and Dai2021) as well as their interactions with topography (Liu et al., Reference Liu, Zu and Gan2020; Lu et al., Reference Lu, Yu and Gan2022). Specifically, the multiscale variability of coastal currents can profoundly regulate coastal water residence times (Lu and Gan, Reference Lu and Gan2015; Wang and Yang, Reference Wang and Yang2015; Du and Shen, Reference Du and Shen2016) and re-distribute biogenic materials such as nutrients, OM, and DO (Li et al., Reference Li, Gan, Hui, Liu, Yu, Lu and Dai2020; Li et al., Reference Li, Gan, Hui, Yu, Liu, Lu, Kao and Dai2021; Liu et al., Reference Liu, Gan, Wu, Hu, Cai and Deng2021b), leading to spatial heterogeneity in phytoplankton production and bottom-water hypoxia. In river-dominated open coastal systems such as off the Changjiang, Pearl River and Mississippi River estuaries, wind-driven currents largely constrain the spread of nutrient-enriched, buoyant river waters (i.e., river plume) from the river/estuary mouth, where phytoplankton blooms occur in surface waters riding over the oceanic waters causing strong stratification and inhibiting the aeration of the subsurface (Bianchi et al., Reference Bianchi, DiMarco, Cowan, Hetland, Chapman, Day and Allison2010; Zhang et al., Reference Zhang, Wu, Hetland and Zhu2019a; Li et al., Reference Li, Gan, Hui, Yu, Liu, Lu, Kao and Dai2021) (Figure 2). The faster-flowing surface plume waters, with relatively short water residence times, can spread the phytoplankton extensively offshore, whilst bottom current circulation patterns are usually affected by variable shelf topography and experience longer water residence times (Rabouille et al., Reference Rabouille, Conley, Dai, Cai, Chen, Lansard, Green, Yin, Harrison, Dagg and McKee2008). Indeed, the bottom-water residence time off the Pearl River Estuary is estimated to be more than 15 days, nearly five times that of the surface-spreading plume (Lu and Gan, Reference Lu and Gan2015; Li et al., Reference Li, Gan, Hui, Liu, Yu, Lu and Dai2020). The trapping of particulate OM by fronts at the seaward flank of the river plume (Hetland and DiMarco, Reference Hetland and DiMarco2008; Zhou et al., Reference Zhou, Chai, Huang, Wells, Ma, Meng, Xue, Xuan, Wang, Ni, Zhao, Liu, Su and Li2020) and dissolved OM by convergent currents (Lu et al., Reference Lu, Gan, Dai, Liu and Zhao2018b) thus constrains the subsequent development of hypoxia to specific locations, which differ from that of extensive surface blooms. This kind of dislocation between phytoplankton blooms and bottom-water hypoxia has also been observed in semi-enclosed estuaries (e.g., Chesapeake Bay; Zhou et al. (Reference Zhou, Chai, Huang, Wells, Ma, Meng, Xue, Xuan, Wang, Ni, Zhao, Liu, Su and Li2020)) and after the passage of a typhoon as in the Pearl River Estuary (Zhao et al., Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021). In addition, the upslope transport of low-oxygen subsurface oceanic waters can act as a remote driver to lower the initial oxygen conditions (Qian et al., Reference Qian, Dai, Xu, Kao, Du, Liu, Wang, Guo and Wang2017), facilitating the formation of hypoxia that is less dependent on the OM supply from the phytoplankton blooms. Such spatial–temporal decoupling of hypoxia to algal bloom along with its multiple underlying mechanisms further complicates the cause–effect relationship between eutrophication and hypoxia and weakens the mitigation efficiency by reducing external nutrient supply alone, which should be taken into account in designing and evaluating nutrient management schemes.
Feedbacks of hypoxia on algal blooms
The relationship between eutrophication and hypoxia is not causally unidirectional. Bottom-water oxygen depletion changes the benthic redox environment and modulates the biogeochemical cycling of nutrients over varying timescales, in turn providing an effective feedback on surface algal blooms (Figure 2) (Howarth et al., Reference Howarth, Chan, Conley, Garnier, Doney, Marino and Billen2011). Indeed, enhanced phytoplankton growth following lower bottom-water oxygen has been observed in seasonally hypoxic systems, such as in Chesapeake Bay (Zheng and DiGiacomo, Reference Zheng and DiGiacomo2020) and off the Pearl River Estuary (Zhao et al., Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021).
In hypoxic or anoxic benthic environment, denitrification and anammox (i.e., anaerobic ammonium oxidation) compete with dissimilatory nitrate reduction to ammonium (DNRA) for nitrate and nitrite (Song et al., Reference Song, Liu, Zhang, Zhu, Zhang, Marchant, Kuypers and Lavik2021; Roberts et al., Reference Roberts, Wong, Shimeta, Kessler and Cook2022), the former of which result in N removal from the system. This pathway of N loss is estimated to be responsible for about 30–50% of N-loss from the world’s oceans, or 16–27% from land and oceans combined (Codispoti et al., Reference Codispoti, Brandes, Christensen, Devol, Naqvi, Paerl and Yoshinari2001). McCarthy et al. (Reference McCarthy, Newell, Carini and Gardner2015) reported that denitrification and anammox on the hypoxic Louisiana–Texas continental shelf removed up to 68% of the total N loaded from the Mississippi River watershed. In this regard, hypoxic events may mitigate the N:P ratio imbalance resulting from the riverine N overloading over the long term. Moreover, under anoxic conditions, P-containing deposits such as Fe-P minerals, one of the main P burial phases in strongly reducing sediment, are thermodynamically unfavorable, resulting in the release of P (Figure 2) (Middelburg and Levin, Reference Middelburg and Levin2009). Therefore, hypoxia can potentially enhance P supply for phytoplankton in the overlying euphotic zone (Rozan et al., Reference Rozan, Taillefert, Trouwborst, Glazer, Ma, Herszage, Valdes, Price and Luther2002; Sulu-Gambari et al., Reference Sulu-Gambari, Seitaj, Meysman, Schauer, Polerecky and Slomp2016). Thus, from a long-term perspective, terrestrially-derived P can be accumulated in the anoxic areas where the majority of riverine N is removed, especially in semi-enclosed coastal systems (Dalsgaard et al., Reference Dalsgaard, De Brabandere and Hall2013). This enhanced internal bioavailable P supply, together with the N leakage through denitrification and anammox, can fuel the phytoplankton growth with a potential shift of the dominant species of HABs and the timing of HABs outbreaks due to the delayed response (Gustafsson et al., Reference Gustafsson, Schenk, Blenckner, Eilola, Meier, Müller-Karulis, Neumann, Ruoho-Airola, Savchuk and Zorita2012). This indeed occurs in the Baltic Sea, where a trend towards intensifying summer N-fixing cyanobacterial blooms is mainly attributed to the mixing-induced replenishment of inorganic P from deep water, and is correlated with increases in the extent of hypoxia (Funkey et al., Reference Funkey, Conley, Reuss, Humborg, Jilbert and Slomp2014). The increased rates of N2 fixation in the Baltic Sea are found to counteract the reduced N loading in recent decades (Savchuk, Reference Savchuk2018). The legacy nutrients, and HABs that may result, are particularly notable in semi-enclosed coastal regions. This can also be a crucial factor responsible for cyanobacterial HAB outbreaks, because the external loading of P has been reduced following the regulatory measures to curb eutrophication.
Long-term trends: Socioeconomic-ecological drivers and lessons
Despite the global persistence of coastal eutrophication, HABs and hypoxia, the complex and non-linear response of hypoxia to eutrophication is site-specific and closely related to local geographical features, physical dynamics, climatic variability and trajectory of regional socioeconomic development. By re-examining the long-term trends in the expansion of eutrophication and its interactions with economic development and societal management in two model systems in Europe (Baltic Sea) and North America (Chesapeake Bay) and four coastal ecosystems in East Asia (i.e., Changjiang Estuary, Pearl River Estuary, Upper Gulf of Thailand and Xiamen Bay) (Figure 3), which are at different stages of development and/or management, we assess and compare key fundamental variables to summarize past experiences and lessons related to this crucial environmental issue.
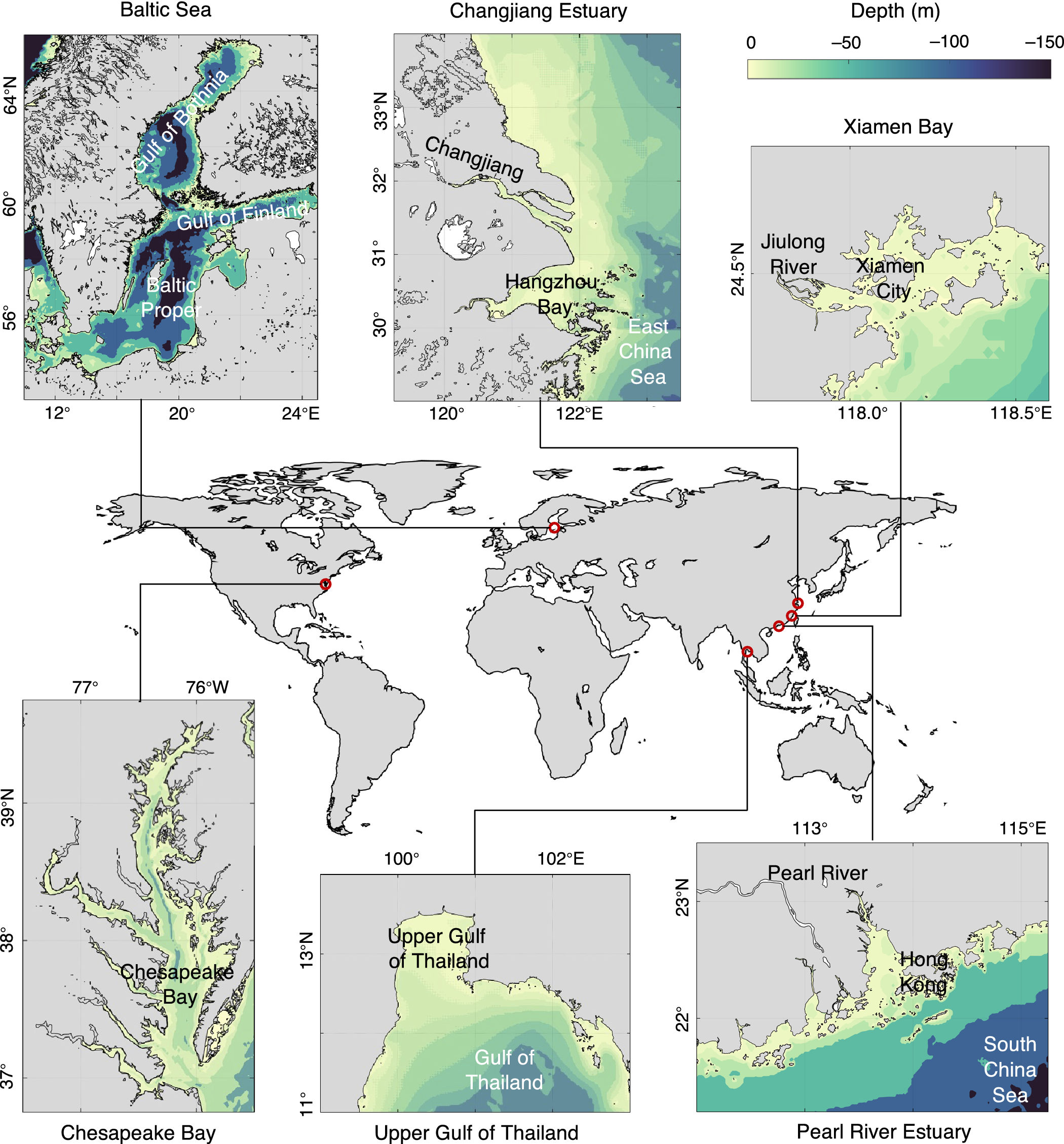
Figure 3. Bathymetry of selected coastal systems: Baltic Sea, Chesapeake Bay, Changjiang Estuary, Pearl River Estuary, Upper Gulf of Thailand, and Xiamen Bay. The color shows depth (m).
Europe – Baltic Sea
The Baltic Sea is a semi-enclosed water body in northern Europe surrounded by nine industrialized countries and five more within the catchment area, representing 85 million inhabitants. As the largest anthropogenically induced hypoxic area in the world (Conley et al., Reference Conley, Carstensen, Aigars, Axe, Bonsdorff, Eremina, Haahti, Humborg, Jonsson, Kotta, Lännegren, Larsson, Maximov, Medina, Lysiak-Pastuszak, Remeikaitė-Nikienė, Walve, Wilhelms and Zillén2011; Carstensen et al., Reference Carstensen, Andersen, Gustafsson and Conley2014; Carstensen and Conley, Reference Carstensen and Conley2019), it is one of the most intensively studied coastal areas, and often serves as a model study of eutrophication, deoxygenation, and ecosystem response to perturbations by nutrient pollution and warming (Carstensen et al., Reference Carstensen, Andersen, Gustafsson and Conley2014; Saraiva et al., Reference Saraiva, Markus Meier, Andersson, Höglund, Dieterich, Gröger, Hordoir and Eilola2019).
Eutrophication and deoxygenation have degraded Baltic Sea’s marine ecosystems over the past ~200 years. Signs of ecosystem harm emerged in the 19th century and accelerated in the 20th century, especially after the post-World War II developments (Yasuhara et al., Reference Yasuhara, Hunt, Breitburg, Tsujimoto and Katsuki2012; Yasuhara et al., Reference Yasuhara, Rabalais, Conley, Gutierrez, Laffoley and Baxter2019). Prior to the 1990s, the total population and GDP of the countries surrounding the Baltic Sea rapidly increased (Figure S2a in the Supplementary Material). Since then, the population has remained stable, although the fast-growing total GDP continued and reached its maximum after the 2010s (Figure S2a in the Supplementary Material). Social drivers of the long-term changes in total nutrient loads to the Baltic Sea outweigh the impacts of climate change, with land use and agricultural activities being the most important drivers (Pihlainen et al., Reference Pihlainen, Zandersen, Hyytiäinen, Andersen, Bartosova, Gustafsson, Jabloun, McCrackin, Meier, Olesen, Saraiva, Swaney and Thodsen2020). Rivers account for about 70% and 90% of total N and P inputs, respectively, to the Baltic Sea with diffuse sources mainly from agricultural activities making up ~70% and ~53% of their anthropogenic N and P loads, respectively (Sonesten et al., Reference Sonesten, Svendsen, Tornbjerg, Gustafsson, Frank-Kamenetsky and Haapaniemi2018). As such, strong correlations are found between total fertilizer use and nutrient loads to the Baltic Sea (R ≥ 0.65, p < 0.001; Figure 4a). Management approaches to reduce nutrients have been promoted since the 1970s (Backer et al., Reference Backer, Leppänen, Brusendorff, Forsius, Stankiewicz, Mehtonen, Pyhälä, Laamanen, Paulomäki, Vlasov and Haaranen2010). The total fertilizer use showed a three-phase temporal transition pattern, which increased through the early 1970s, stayed at high levels of ~5 × 106 tons yr−1 for approximately 20 years, and then dropped abruptly to a relatively constant value of ~3 × 106 tons yr−1 in the following 30 years (Figure S2b in the Supplementary Material). Tangible improvements abating eutrophication have been achieved in some basins since the late 1990s (Backer et al., Reference Backer, Leppänen, Brusendorff, Forsius, Stankiewicz, Mehtonen, Pyhälä, Laamanen, Paulomäki, Vlasov and Haaranen2010; Lønborg and Markager, Reference Lønborg and Markager2021; Murray et al., Reference Murray, Müller-Karulis, Carstensen, Conley, Gustafsson and Andersen2019; Reusch et al. (Reference Reusch, Dierking, Andersson, Bonsdorff, Carstensen, Casini, Czajkowski, Hasler, Hinsby, Hyytiäinen, Johannesson, Jomaa, Jormalainen, Kuosa, Kurland, Laikre, MacKenzie, Margonski, Melzner, Oesterwind, Ojaveer, Refsgaard, Sandström, Schwarz, Tonderski, Winder and Zandersen2018)).
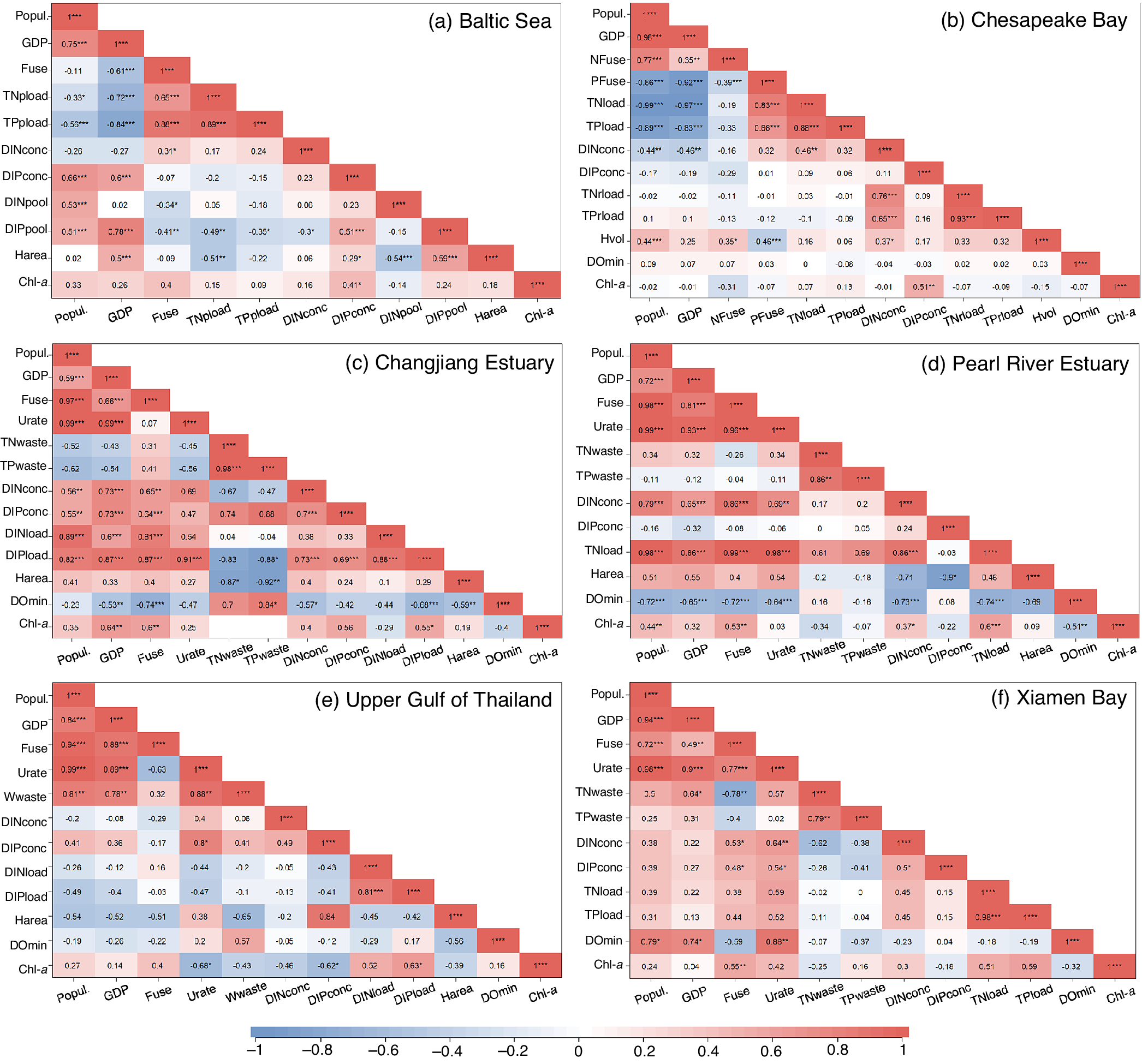
Figure 4. The heat map of relationships between eutrophication/hypoxia and their socioeconomic drivers. (a) Changjiang Estuary, (b) Pearl River Estuary, (c) Baltic Sea, (d) Upper Gulf of Thailand, (e) Chesapeake Bay and (f) Xiamen Bay. The numbers in each cell indicate the coefficient of correlation (R). The subscripts denote the results of significance test, i.e., * denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001. Only the observed data of Chl-a concentration (not included the extremely high values in 2003) are used for CJE. Abbreviation: Popul., total population; GDP, gross domestic product; Fuse, total fertilizer use; NFuse, N fertilizer use; PFuse, P fertilizer use; Urate, urbanization rate; TNwaste, total N loads in wastewater discharge; TPwaste, total P loads in wastewater discharge; Wwaste, wastewater discharge; DINconc, concentration of dissolved inorganic nitrogen (DIN); DIPconc, concentration of dissolved inorganic phosphorus (DIP); DINload, DIN loads; DIPload, DiP loads; TNload, total N loads; TPload, total P loads; TNrload, riverine total N loads; TPrload, riverine total P loads; DINpool, DIN pool; DIPpool, DIP pool; Harea, hypoxic area; Hvol, hypoxic volume; DOmin, minimum DO concentration; Chl-a, chlorophyll-a concentration.
However, the total fertilizer use is not significantly positively correlated to variations in either the concentrations or pools of DIN and dissolved inorganic phosphorus (DIP, mainly phosphate) in the central Baltic Proper (Figure 4a). The reduction of nutrient use and loading since the 1970s has not lessened eutrophication and the nutrient pools in the Baltic Proper (Figure S2c in the Supplementary Material), suggesting alternative sources or drivers that regulate the nutrient distributions in the largest open basin of the Baltic Sea. The expansion of hypoxia in the Baltic Sea removes 42–96% of riverine N annually through denitrification (Dalsgaard et al., Reference Dalsgaard, De Brabandere and Hall2013) and promotes P release from sediments into the water column, resulting in inverse changes in DIN and DIP pools (Figure S2c in the Supplementary Material) and excess P accumulation in the Baltic Proper (Hieronymus et al., Reference Hieronymus, Eilola, Hieronymus, Meier, Saraiva and Karlson2018). As such, strong N limitation has shifted algal blooms from a diatom- and flagellates-dominated regime in spring to a N-fixing cyanobacteria-dominated regime in late summer since the 2000s (Conley et al., Reference Conley, Humborg, Rahm, Savchuk and Wulff2002; Hieronymus et al., Reference Hieronymus, Eilola, Hieronymus, Meier, Saraiva and Karlson2018; Stigebrandt and Andersson, Reference Stigebrandt and Andersson2020). The estimated magnitude of external N supplies from the atmosphere (including N2 fixation and atmospheric deposition) is comparable to those from riverine inputs (Olofsson et al., Reference Olofsson, Klawonn and Karlson2021). Such biogenic N addition into the system further stimulates sedimentary oxygen consumption and P release, which was termed the “vicious circle of the Baltic Sea” (Conley et al., Reference Conley, Humborg, Rahm, Savchuk and Wulff2002; Vahtera et al., Reference Vahtera, Conley, Gustafsson, Kuosa, Pitkänen, Savchuk, Tamminen, Viitasalo, Voss, Wasmund and Wulff2007). The internal P source has been considered as the major driver of the Baltic Sea eutrophication since the late 1990s (Stigebrandt and Andersson, Reference Stigebrandt and Andersson2020).
Oxygenation deteriorated in the Baltic Sea since the early 20th century with the hypoxic area increasing by 10-fold (Conley et al., Reference Conley, Carstensen, Aigars, Axe, Bonsdorff, Eremina, Haahti, Humborg, Jonsson, Kotta, Lännegren, Larsson, Maximov, Medina, Lysiak-Pastuszak, Remeikaitė-Nikienė, Walve, Wilhelms and Zillén2011; Carstensen et al., Reference Carstensen, Andersen, Gustafsson and Conley2014). The hypoxic area in the Baltic Proper, Gulf of Finland, and Gulf of Riga seemed to decrease gradually during the 1970s–1990s, but increased since the early 1990s (Figure S2d in the Supplementary Material). Due to the long residence time (~10 years; Table 2) of bottom water in the Baltic Proper, neither nutrient loads nor seawater nutrient concentrations are strongly correlated with the hypoxic area. Instead, the hypoxia area is highly dependent on the DIP pools (R = 0.59, p < 0.001; Figure 4a), suggesting the magnitude of the DIP pool plays important role in regulating the hypoxic extent in this region (Savchuk, Reference Savchuk2018). In addition, it was perennial almost anoxic in the central Baltic Proper throughout recent decades (Figure S2d in the Supplementary Material), despite the fluctuating nutrient levels and pools. These findings suggest that external nutrient loading is not the sole driver controlling the formation and maintenance of hypoxia in this region. The episodic inflow of well-oxygenated bottom water creates only short-term relief from hypoxia and can even worsen oxygen conditions over longer time scales, because it enhances stratification and reduces the vertical mixing of oxygen to the bottom waters (Carstensen and Conley, Reference Carstensen and Conley2019). Furthermore, the water-column oxygen consumption has been reported to increase after the 1980s despite the decline in nutrient supply, counteracting the effect of natural ventilation on alleviating the anoxic situation in deep waters (Meier et al., Reference Meier, Väli, Naumann, Eilola and Frauen2018). Hence, the internal nutrient recycling in the Baltic Sea that affects oxygen consumption should be paid more attention. The overall objective to restore the Baltic Sea has not been reached (Elmgren et al., Reference Elmgren, Blenckner and Andersson2015) and an updated Baltic Sea Action Plan in 2021 calls for more efficient measures (e.g., eliminating internal P sources; Conley et al. (Reference Conley, Humborg, Rahm, Savchuk and Wulff2002); Stigebrandt and Andersson (Reference Stigebrandt and Andersson2020)) to combat eutrophication, which is also suggested by model predictions (Murray et al., Reference Murray, Müller-Karulis, Carstensen, Conley, Gustafsson and Andersen2019; Lønborg and Markager, Reference Lønborg and Markager2021).
Table 2. Characteristics of the six selected coastal systems (Baltic Sea, Chesapeake Bay, Changjiang Estuary, Pearl River Estuary, Upper Gulf of Thailand, and Xiamen Bay) in terms of GDP, total fertilizer use, N load, regional characteristics, water residence time, annual average surface DIN concentration, summer surface Chl-a concentration, seasons of hypoxia occurrences, maximum hypoxic area/volume from the historical records and average frequency of HABs in the past 5 years
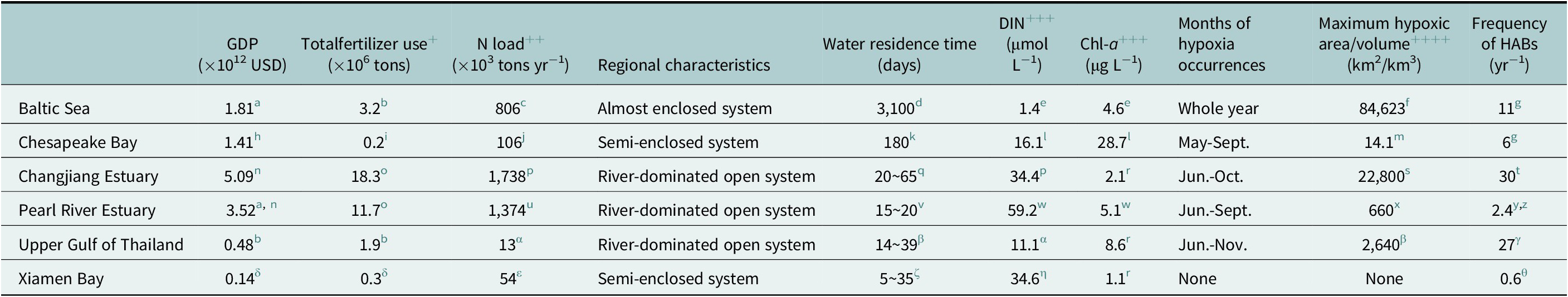
+ Total fertilizer use of Chesapeake Bay is the sum of N fertilizer and P fertilizer.
++ DIN loading of Changjiang Estuary and Upper Gulf of Thailand, TN loading of Pearl River Estuary, Xiamen Bay, Baltic Sea and Chesapeake Bay.
+++ DIN and Chl-a concentration are wet season (May–October) surface average value at Upper Gulf of Thailand.
++++ Max hypoxic volume of Chesapeake Bay (km3).
The characteristics of each system are compiled from the literature sources cited in the footnotes below.
a The World Bank (https://databank.worldbank.org/).
b Food and Agriculture Organization of the United Nations (http://www.fao.org/faostat/en/#data).
c Savchuk (Reference Savchuk2018).
d Fennel and Testa (Reference Fennel and Testa2019).
e SMHI SHARK web (https://sharkweb.smhi.se/hamta-data/).
f Hansson and Viktorsson (Reference Hansson and Viktorsson2021).
g Harmful Algal Event Database (http://haedat.iode.org/).
h Bureau of Economic Analysis U.S. Department of Commerce.
i Sabo et al. (Reference Sabo, Sullivan, Wu, Sobota, Cooter, Dobrowolski, Houlton, Rea, Schwede, Morford and Compton2022).
j Murphy et al. (Reference Murphy, Keisman, Harcum, Karrh, Lane, Perry and Zhang2022).
k Du and Shen (Reference Du and Shen2016).
l Eyes on the Bay (https://eyesonthebay.dnr.maryland.gov/).
m Scavia et al. (Reference Scavia, Bertani, Testa, Bever, Blomquist, Friedrichs, Linker, Michael, Murphy and Shenk2021).
n National Bureau of Statistics.
o China Statistical Yearbook, 2022.
p Wang et al. (Reference Wang, Xin, Wei and Xie2018a).
q Zhang et al. (Reference Zhang, Moriarty, Wu and Feng2021).
r Climate Change Initiative-European Space Agency project (http://www.esa-oceancolour-cci.org/).
s Chen et al. (Reference Chen, Li, Jin, Jiang, Wang, Wu, Hao, Sun, Chen and Guo2020).
t Bulletin of marine disaster of China (https://www.mnr.gov.cn/sj/sjfw/hy/gbgg/zghyzhgb/).
u Cui et al. (Reference Cui, Huang, Wu, Liu and Hong2020).
v Li et al. (Reference Li, Gan, Hui, Liu, Yu, Lu and Dai2020).
w Environmental Protection Department (EPD) of Hong Kong (https://cd.epic.epd.gov.hk/EPICRIVER/marine/).
x Zhao et al. (Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021).
y Li et al. (Reference Li, Lu and Cen2018).
z Bulletin of marine disaster of the South China Sea.
α Marine and Coastal Resources Research and Development Institute (2020).
β Buranapratheprat et al. (Reference Buranapratheprat, Morimoto, Phromkot, Mino, Gunbua and Jintasaeranee2021).
γ Marine and Coastal Resources Research and Development Institute (2021).
δ Statistics Bureau of Fujian Province.
ε Report on the Second National Pollution Census.
ζ Cheng et al. (Reference Cheng, Wang, Wu, Lin, Chen and Chen2021).
η Unpublished data from Fujian Provincial Environmental Monitor Center Station.
θ Chen et al. (Reference Chen, Wang, Dong and Lin2021a).
North America – Chesapeake Bay
Nourishing a fast-growing population and GDP (Figure S3a in the Supplementary Material), the drainage basin of the Chesapeake Bay extends over an area of 1.6 × 105 km2. The large nutrient inputs and a long residence time, i.e., about 180 days (Table 2), greatly impact the ecosystem in the Chesapeake Bay (Kemp et al., Reference Kemp, Boynton, Adolf, Boesch, Boicourt, Brush, Cornwell, Fisher, Glibert, Hagy, Harding, Houde, Kimmel, Miller, Newell, Roman, Smith and Stevenson2005). Early signs of eutrophication appeared in the 1700s (Kemp et al., Reference Kemp, Boynton, Adolf, Boesch, Boicourt, Brush, Cornwell, Fisher, Glibert, Hagy, Harding, Houde, Kimmel, Miller, Newell, Roman, Smith and Stevenson2005; Willard and Cronin, Reference Willard and Cronin2007; Yasuhara et al., Reference Yasuhara, Hunt, Breitburg, Tsujimoto and Katsuki2012; Yasuhara et al., Reference Yasuhara, Rabalais, Conley, Gutierrez, Laffoley and Baxter2019). After ~50% of land clearance in the mid-19th century and initial fertilizer use in the 1900s with growing human population and intense fertilizer use after the 1950s, the coastal environments were heavily influenced by ever-expanding nutrient loading. Nearly 60% of total nutrient inputs to the Chesapeake Bay are diffuse sources, of which agriculture accounts for more than a half (Boesch et al., Reference Boesch, Brinsfield and Magnien2001). Increased nutrient inputs led to an unprecedented level of hypoxia in the estuary since 1960–1980s (Willard and Cronin, Reference Willard and Cronin2007; Yasuhara et al., Reference Yasuhara, Rabalais, Conley, Gutierrez, Laffoley and Baxter2019).
The volume of hypoxia in the Chesapeake Bay increased two-fold from the 1950s to the 1980s, following a similar increase in the fertilizer use (Figures S3b and S3d in the Supplementary Material) (Orth et al., Reference Orth, Batiuk, Bergstrom and Moore2002). During the last three decades, the implementation of policies to protect and restore the ecosystem as well as upgrades in city wastewater treatment have decreased agricultural surplus and point source export, together contributing to the decrease in nutrient loading after 2000 (Figure S3b in the Supplementary Material) (Sabo et al., Reference Sabo, Sullivan, Wu, Sobota, Cooter, Dobrowolski, Houlton, Rea, Schwede, Morford and Compton2022). The concentrations of DIN in the estuary also showed a decreasing trend but fluctuated in a range similar to levels recorded in the 1990s (Figure S3c in the Supplementary Material), while the Chl-a concentrations still kept at a high level of around 25 μmol L−1 with substantial interannual variability (Figure S3d in the Supplementary Material). This undiminished eutrophication might be attributed to unexpected increases in agricultural surpluses in certain parts of the watersheds from 2009 to 2019, which exerted further pressure on the coordinated management efforts (Sabo et al., Reference Sabo, Sullivan, Wu, Sobota, Cooter, Dobrowolski, Houlton, Rea, Schwede, Morford and Compton2022). Nevertheless, without the nitrogen regulation and reduction over the past 35 years, the hypoxic conditions in the bay would likely have been much larger in volume and longer in duration (Frankel et al., Reference Frankel, Friedrichs, St-Laurent, Bever, Lipcius, Bhatt and Shenk2022). Similar to the temporal pattern of nutrient concentrations, the long-term trend in the volume of hypoxia also exhibits large annual and interannual variability since 1990 (Figure S3d in the Supplementary Material). Results from multiple modeling studies suggest that several factors co-regulate the variability of hypoxia over a range of time scales, including climatic effects (e.g., irregular dry and we periods), weather (e.g., precipitation), nutrient loading, physical conditions (e.g., river discharge, water temperature, wind speed and wind direction), and biological activities (e.g., respiration) (Du et al., Reference Du, Shen, Park, Wang and Yu2018; Harding et al., Reference Harding, Mallonee, Perry, Miller, Adolf, Gallegos and Paerl2019).
Ocean warming is thought to be a dominant control on the long-term decline in the DO concentration, overwhelming the effects of sea level rise and modest oxygen increases associated with nutrient reduction (Ni et al., Reference Ni, Li and Testa2020). Climate change, mainly global warming and the associated decrease in oxygen solubility in water, is predicted to increase the summer hypoxic and anoxic volumes in the bay up to 30% by the mid-21st century (Ni et al., Reference Ni, Li, Ross and Najjar2019). The combined effects of anthropogenic and biological CO2 accelerate coastal acidification (Cai et al., Reference Cai, Huang, Luther, Pierrot, Li, Testa, Xue, Joesoef, Mann, Brodeur, Xu, Chen, Hussain, Waldbusser, Cornwell and Kemp2017), further reducing buffering capacity and resilience of the regional marine ecosystem (Su et al., Reference Su, Cai, Brodeur, Chen, Hussain, Yao, Ni, Testa, Li, Xie, Ni, Scaboo, Xu, Cornwell, Gurbisz, Owens, Waldbusser, Dai and Kemp2020a).
South East Asia
Changjiang Estuary
Stretching for 6,200 km and draining an area of 1.8 × 106 km2 with a human population of over 400 million, the Changjiang (Yangtze River) is the third largest river in the world in terms of water discharge. The population in the Changjiang watershed has doubled from 1960 to 2020 (Figure S4a in the Supplementary Material). Meanwhile, the urbanization rate and total GDP have been accelerating over the last two decades (Figures S4a in the Supplementary Material). The ever-expanding population and economic aggregation have greatly impacted the Changjiang Estuary coastal system, and rendered this region one of the world’s largest hypoxic zones (Chen et al., Reference Chen, Li, Jin, Jiang, Wang, Wu, Hao, Sun, Chen and Guo2020).
Tributaries including Dongting Lake account for about 50% of total riverine N loads, followed by non-point sources (36%), while discharges from non-point sources to the Changjiang main stream contribute about 63% of total riverine P loads (Tong et al., Reference Tong, Bu, Chen, Zhou, Chen, Liu, Tan, Yu, Zhang, Mi, Ma, Wang and Ni2017). The nutrient loading is significantly correlated with total fertilizer use (R > 0.8, p < 0.001; Figure 4c) (Fu et al., Reference Fu, Kang, Li, Liu and Xu2021), and increases by a factor of eight from 1960–2010 (Figures S4b and S4c in the Supplementary Material) (Liang and Xian, Reference Liang and Xian2018). Following the national regulation of fertilizer usage, the total fertilizer use peaked in the early 2010s and declined afterward (Figures S4b in the Supplementary Material). A modeling study indicates that synthetic fertilizer and manure together are the largest contributor, accounting for more than half of DIN yields from the Changjiang River since 1985 (Yan et al., Reference Yan, Mayorga, Li, Seitzinger and Bouwman2010).
Changes in the riverine nutrient inputs have significantly increased DIN and DIP concentrations in coastal surface waters since the mid-1990s (Figure S4c in the Supplementary Material) (Wang et al., Reference Wang, Xin, Wei and Xie2018a). In addition to nutrient enrichment, the N:P ratio increased at a rate of 0.35 yr−1, while the Si:N decreased at a rate of 0.04 yr−1 from 1960–2018 (Dai et al., Reference Dai, Du, Zhang, Su and Li2011; Shi et al., Reference Shi, Xu, Li and Ge2022). The altered nutrient stoichiometry in the Changjiang, superimposed over the regional warming at a fastest global warming rate in the world (Liu et al., Reference Liu, Xiao, Landry, Chiang, Wang and Huang2016; Xiao et al., Reference Xiao, Liu, Irwin, Laws, Wang, Chen, Zeng and Huang2018), has clearly induced the algal species shift with an increasing percentage of dinoflagellates abundance up to over 25% at the cost of a decline in diatoms since the 2000s (Chen et al., Reference Chen, Li, Jin, Jiang, Wang, Wu, Hao, Sun, Chen and Guo2020; Chen et al., Reference Chen, Hong and Gao2021b; Shi et al., Reference Shi, Xu, Li and Ge2022) and increased HABs (e.g., dinoflagellates Prorocentrum donghaiense and Noctiluca scintillans, and diatoms Skeletonema costatum) both in frequency and impacted area (Li et al., Reference Li, Tang, Shi, Zhang and Wang2014).
Hypoxia off the Changjiang Estuary has become a new norm during summertime in recent decades, persisting from June to October (Table 2) (Wang et al., Reference Wang, Wei, Chen and Xie2012). It was first detected in 1959 (~1,800 km2) and developed into an extensive area exceeding 2 × 104 km2 by 2016 (Figure S4d in the Supplementary Material) (Zhu et al., Reference Zhu, Wu, Liu, Wu, Huang, Zhang and Zhang2017). The minimum DO concentration off the Changjiang Estuary shows a stepwise reduction at a rate of 0.05 mg L−1 yr−1 since the 1980s, which has probably led to a meiobenthic faunal shift (Cheung et al., Reference Cheung, Yasuhara, Iwatani, Wei and Dong2019). A weak but statistically significant negative correlation is found between the DO minima and hypoxic areas (R = 0.66, p < 0.01; Figure 4c). The low DO conditions and extensive hypoxia off the Changjiang Estuary have not receded, although both the nutrient inputs and the phytoplankton biomass have show declining trends recently due to a reduction in human inputs and the increasing damming upstream (Figure S4d in the Supplementary Material) (Shi et al., Reference Shi, Xu, Li and Ge2022).
The decoupling of hypoxia and eutrophication trends suggests that processes other than human-induced eutrophication might play an increasing role in the formation and maintenance of hypoxia, such as the intrusion of the Kuroshio Current (Zhu et al., Reference Zhu, Wu, Liu, Wu, Huang, Zhang and Zhang2017; Zhang et al., Reference Zhang, Wu, Hetland and Zhu2019a). Kuroshio subsurface water can act as a remote driver contributing to the development of summer hypoxia by lowering the initial DO levels off the Changjiang Estuary when it intrudes into the East China Sea (Qian et al., Reference Qian, Dai, Xu, Kao, Du, Liu, Wang, Guo and Wang2017). The nutrients carried by the Kuroshio intrusion also fuel the surface blooms through upwelling, leading to subsequent bottom oxygen depletion off the Changjiang Estuary (Große et al., Reference Große, Fennel, Zhang and Laurent2020). In this regard, the hypoxic status off the Changjiang Estuary is sensitive to climate change at both local and regional scales. The hypoxic area off the Changjiang Estuary is projected to increase by 50% due to the open-ocean deoxygenation as global warming continues, but the reduced N load from the Changjiang River can considerably mitigate the increasing extent of hypoxia (Große et al., Reference Große, Fennel, Zhang and Laurent2020). These results highlight the importance of an integrated, efficient regulation strategy for nutrient reduction to abate eutrophication and counteract the potential future decline in remote oxygen supply to this region.
Pearl River Estuary
The Pearl River Estuary is situated in the world’s most populated bay area, the Guangdong-Hong Kong-Macao Greater Bay Area, on the northern shelf of the South China Sea. Similar to the Changjiang watershed regions, the GDP has grown quickly at a rate of ~11% per year on average over the last four decades (Figure S5a in the Supplementary Material). Concomitant rapid industrial and agricultural development has increased total synthetic fertilizer use in the watershed by four-fold in order to satisfy the needs of an almost doubled population, which corresponds to the four-fold increase in urbanization rates (Figures S5a and S5b in the Supplementary Material). Domestic sewage, industrial wastewater, agricultural fertilizer and marine culture are the main nutrient sources to the Pearl River Estuary (Huang et al., Reference Huang, Huang and Yue2003). The fertilizer use in this region is significantly positively correlated with the population and total GDP (R = 0.81, p < 0.001), and urbanization rate (R = 0.96, p < 0.001) and significantly contributes to the N loading in the watershed (Figure 4d). As a result, the DIN concentration in the lower estuary increased dramatically at a rate of ~1.4 ± 0.3 μmol N L−1 yr−1 along with elevated N:P ratios (~30–300) and OM contents from the 1990s to the 2010s (Huang et al., Reference Huang, Huang and Yue2003; Ye et al., Reference Ye, Huang, Zhang, Zhang, Zeng and Tian2012), and only started to decline since 2015 due to decreasing total fertilizer use and wastewater discharge with the implementation of soil testing and formulated fertilization technology, transformation of fertilization methods, utilization of organic fertilizers and improvement of cultivated land quality (Figures S5b and S5c in the Supplementary Material).
Eutrophication and hypoxia in the lower estuary are primarily caused by the ecosystem’s responses to the increasing nutrient discharge from the Pearl River and local sewage effluent and an increasing discharge of organic pollutants (Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Li et al., Reference Li, Gan, Hui, Liu, Yu, Lu and Dai2020). Excessive nutrient inputs have exerted great pressure on the estuary and the shelf beyond, triggering outbreaks of HABs (e.g., dinoflagellates N. scintillans and Gymnodinium sp., and diatoms S. costatum, Thalassiosira sp., and Chaetoceros sp.) in the estuary impacting an area of about 2,850 km2 (Huang et al., Reference Huang, Huang and Yue2003; Callahan et al., Reference Callahan, Dai, Chen, Li, Lu and Huang2004; Harrison et al., Reference Harrison, Yin, Lee, Gan and Liu2008). The low oxygen conditions have also remarkably deteriorated in the bottom waters of the lower estuary (Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Qian et al., Reference Qian, Gan, Liu, He, Lu, Guo, Wang, Guo, Huang and Dai2018; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020), even though its shallow topography (averaged depth of ~10 m) and relatively strong mixing (induced by tides and wind) physically make the estuary less prone to develop large-scale hypoxic events (Harrison et al., Reference Harrison, Yin, Lee, Gan and Liu2008). A significant decline in bottom-water oxygenation, at a rate of ~2 ± 0.9 μmol kg−1 yr−1, has been observed in the lower estuary over the past three decades (Qian et al., Reference Qian, Gan, Liu, He, Lu, Guo, Wang, Guo, Huang and Dai2018; Qian et al., Reference Qian, Zhang, Tong, Sardans, Peñuelas and Li2022), with an expansion of the hypoxic area over the last two decades (Figure S5d in the Supplementary Material). Two prominent hypoxic centers have been found with a total area of ~700 km2 in the coastal transition zone off the estuary (Yin et al., Reference Yin, Lin and Ke2004; Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020; Hu et al., Reference Hu, Zhang, Wang and Huang2021; Zhao et al., Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021). Over the last decade, the estuary seems to be undergoing a transition from having episodic, small-scale hypoxic events to seasonal, estuary-wide hypoxia occurring during summer (Hu et al., Reference Hu, Zhang, Wang and Huang2021; Zhao et al., Reference Zhao, Uthaipan, Lu, Li, Liu, Liu, Gan, Meng and Dai2021).
In the lower estuary, the mean Chl-a concentration during summer showed a generally increasing trend before 2010 and peaked in 2011, then dropped and kept relatively stable afterward (Figure S5d in the Supplementary Material), suggesting a possible decoupling of surface phytoplankton biomass accumulation and bottom water hypoxia. Such decoupling may partially be attributed to the warming and altered hydrodynamics that make the system physically more prone to hypoxia. A recent modeling study reveals that cyclonic vortices and center of convergence in the coastal transition zone between the Pearl River Estuary and the adjacent continental shelf cause the accumulation of nutrients and OM to promote the development of hypoxia (Li et al., Reference Li, Gan, Hui, Liu, Yu, Lu and Dai2020).
Regional efforts to improve sewage treatment (e.g., the Harbor Area Treatment Scheme in Hong Kong since 2001) have been invested to abate the deteriorating water quality of the Pearl River Estuary, with the sewage treatment rate increasing from 32.9% in 2004 to 97.8% in 2020. The sewage treatment has been especially effective in reducing P, enabling the DIP concentration in the lower estuary to remain as low as around 1 μmol L−1 and leading to a negative correlation between the DIP concentration and the hypoxic area (p < 0.05; Figure 4d) that indicates P-limitation in the estuary. Yet, more rigorous nutrient reduction strategies at the river basin scale, building upon the quantitative knowledge of the relative contribution of different sources of organic matter (Su et al., Reference Su, Dai, He, Wang, Gan, Guo, Zhao and Yu2017; Zhao et al., Reference Zhao, Liu, Uthaipan, Song, Xu, He, Liu, Gan and Dai2020; Yu et al., Reference Yu, Gan, Dai, Hui, Lu and Li2021) and nutrients (Yu and Gan, Reference Yu and Gan2022), are required to reverse the trend of worsening eutrophication and hypoxia in the region.
Upper Gulf of Thailand
The Upper Gulf of Thailand is a shallow tropical gulf with extensive coastline, heavily populated urban and agricultural areas. Compared to the Changjiang and Pearl River estuaries, the Upper Gulf of Thailand is influenced more substantially by heavy rainfall and storm tides due to cyclones, putting the coastal ecosystem directly in the face of the dual impacts of anthropogenic activities and climate change pressures. HABs were first reported in 1952, and since then HAB events have been reported at least 450 times in Thai waters, mainly concentrated in the Upper Gulf of Thailand with the rate of incidences growing by roughly 6.5% each decade (Lirdwitayaprasit, Reference Lirdwitayaprasit2003; Paphavasit et al., Reference Paphavasit, Siriboon, Piumsomboon, Sivaiparam and Saramul2006). HABs in the Upper Gulf of Thailand are dominated by non-toxic species (e.g., blue-green algae Trichodesmium erythraem, and Noctilluca sp.) (Cheevaporn and Menasveta, Reference Cheevaporn and Menasveta2003). Hypoxia was first recorded in the mid-1970s, and minimum DO concentrations lower than 2 mg L−1 were reported frequently after 2010 (Figure S6d in the Supplementary Material). Hypoxic waters were mostly detected in river-estuary zones, and among all detected areas, the Upper Gulf of Thailand emerged as the largest hypoxic zone in Thai waters (Buranapratheprat et al., Reference Buranapratheprat, Morimoto, Phromkot, Mino, Gunbua and Jintasaeranee2021).
Similar to the case of most coastal ecosystems, eutrophication in the Upper Gulf of Thailand is driven largely by the increased anthropogenic nutrient input. However, the attribution of nutrient loads to social economic sectors remains unclear due to the influence of diverse sources and a lack of comprehensive budget assessments. The rapidly growing population and GDP (Figure S6a in the Supplementary Material) and associated growing demands for food production and energy have resulted in expanding agricultural, livestock, aquacultural, urban, and industrial land at the expense of the forests during the last three decades (Royal Forest Department, 2017) (Figure S6e in the Supplementary Material). Closely related to the increasing population and GDP has been total fertilizer use and wastewater discharge (R > 0.78; Figure 4e), which increased by 20-fold from 0.11 × 106 tons in early 1970s to 2.0 × 106 tons in the 2010s and by nearly 50% in the 2010s, respectively (Figure S6b in the Supplementary Material). Interestingly, fertilizer use per unit of agricultural land in the Upper Gulf of Thailand is only 6–10% of that in the Changjiang and Pearl River watersheds, unlikely to explain the high DIN concentrations in the Upper Gulf of Thailand with levels approximating those of the other two systems (Figure S6c in the Supplementary Material) as suggested by the poor correlations between nutrient loads and fertilizer use (Figure 4e). In addition to the agriculture-derived inputs, discharge of untreated domestic waste (e.g., with a percentage of 60–70% in the Chao Phraya Basin; Cheevaporn and Menasveta (Reference Cheevaporn and Menasveta2003)), release of untreated wastewater and sewage sludge from livestock, fish and shrimp farms (Schaffner et al., Reference Schaffner, Bader and Scheidegger2009; Kupkanchanakul et al., Reference Kupkanchanakul, Kwonpongsagoon, Bader and Scheidegger2015) and groundwater discharge (Burnett et al., Reference Burnett, Wattayakorn, Taniguchi, Dulaiova, Sojisuporn, Rungsupa and Ishitobi2007) might also contribute substantially to the eutrophication in the coastal waters in the Upper Gulf of Thailand. The N loads from the livestock and aquaculture account for ~60% of that into Mea Kong, Tha Chin, and Bang Pakong Rivers that flow into the Upper Gulf of Thailand (Schaffner et al., Reference Schaffner, Bader and Scheidegger2009; Kupkanchanakul et al., Reference Kupkanchanakul, Kwonpongsagoon, Bader and Scheidegger2015; Pharino et al., Reference Pharino, Sailamai and Kamphaengthong2016). Estimated fluxes of DIN and DIP supplied via groundwater discharge are 40–50% and 60–70% of those delivered by the Chao Phraya River (Burnett et al., Reference Burnett, Wattayakorn, Taniguchi, Dulaiova, Sojisuporn, Rungsupa and Ishitobi2007), respectively. As such, in addition to reducing the point sources of nutrients, controlling nutrient inputs via groundwater discharge, such as modifying farming methods to use fertilizers more effectively and efficiently, creating and restoring wetlands and riparian zones and increasing nutrient controls on wastewater treatment plants (McCoy and Corbett, Reference McCoy and Corbett2009), also warrants close attention.
On the other hand, superimposed on the rising trend of fertilizer demand and wastewater discharge (Figure S6b in the Supplementary Material) are the well-noted extreme fluctuations of DIN and minimum DO concentrations in coastal waters (Figures S6c and S6d in the Supplementary Material), collectively indicating the profound impact of local weather, current circulation, and climate on this system. During the rainy, warm southwest monsoon season (from June to August), strong stratification favors rapid oxygen depletion (10–40 μmol kg−1 d−1; Sukigara et al. (Reference Sukigara, Mino, Yasuda, Morimoto, Buranapratheprat and Ishizaka2021)) in bottom waters within a relatively long residence time (14–39 days; Table 2) (Buranapratheprat et al., Reference Buranapratheprat, Morimoto, Phromkot, Mino, Gunbua and Jintasaeranee2021), contributing to the occurrence of hypoxia in the northeast part of the Upper Gulf of Thailand (Morimoto et al., Reference Morimoto, Mino, Buranapratheprat, Kaneda, Tong-U-Dom, Sunthawanic, Yu and Guo2021). The hypoxic zone moves westward along with the reversed direction of surface circulation and westward spread of low-salinity water during the monsoon transition period (from September to November) when river discharge usually peaks (Morimoto et al., Reference Morimoto, Mino, Buranapratheprat, Kaneda, Tong-U-Dom, Sunthawanic, Yu and Guo2021; Park et al., Reference Park, Lim, Ho, Herrin and Chitwatkulsiri2021). Short-term variations in weather and long-term shifts in climate also have a strong influence on certain cultivation practices, rice in particular (Kiguchi et al., Reference Kiguchi, Takata, Hanasaki, Archevarahuprok, Champathong, Ikoma, Jaikaeo, Kaewrueng, Kanae, Kazama, Kuraji, Matsumoto, Nakamura, Nguyen-Le, Noda, Piamsa-Nga, Raksapatcharawong, Rangsiwanichpong, Ritphring, Shirakawa, Somphong, Srisutham, Suanburi, Suanpaga, Tebakari, Trisurat, Udo, Wongsa, Yamada, Yoshida, Kiatiwat and Oki2021), which alters the demand for fertilizer use. In addition, regional warming may also amplify the impact of land-use changes on runoff, exceeding the effect of rainfall (Kiguchi et al., Reference Kiguchi, Takata, Hanasaki, Archevarahuprok, Champathong, Ikoma, Jaikaeo, Kaewrueng, Kanae, Kazama, Kuraji, Matsumoto, Nakamura, Nguyen-Le, Noda, Piamsa-Nga, Raksapatcharawong, Rangsiwanichpong, Ritphring, Shirakawa, Somphong, Srisutham, Suanburi, Suanpaga, Tebakari, Trisurat, Udo, Wongsa, Yamada, Yoshida, Kiatiwat and Oki2021). These changes modulate the eutrophication-driven oxygen depletion in coastal waters. In addition, wind-induced circulation and local flood events may also greatly impact the location and volume of the hypoxic waters (Morimoto et al., Reference Morimoto, Mino, Buranapratheprat, Kaneda, Tong-U-Dom, Sunthawanic, Yu and Guo2021). Under the dual pressures of anthropogenic activity and weather, it is projected that hypoxia in Thailand will be exacerbated by the intensified ocean warming and more frequent extreme precipitation events over the next century (Limsakul et al., Reference Limsakul, Kachenchart, Singhruck, Saramul, Santisirisomboon and Apipattanavis2019).
Xiamen Bay
Xiamen Bay is located in southeast China in a region of rapid urbanization that benefits from implementing integrated coastal management (Winther et al., Reference Winther, Dai, Rist, Hoel, Li, Trice, Morrissey, Juinio-Meñez, Fernandes, Unger, Scarano, Halpin and Whitehouse2020). It receives discharges from the Jiulong River from the northwest. Xiamen City was selected as one of the five special economic zones in China in 1980, which boosted population growth and the GDP, especially after 2000 (Figure S7a in the Supplementary Material). Similar to rapidly developing areas elsewhere, heavy nutrient loading, wastewater and pollutants associated with urbanization and industrialization have affected Xiamen Bay (Chen et al., Reference Chen, Peng, Hong, Turyaheebwa, Cui and Mo2013). Riverine N loads from agricultural land dominate the total N inputs to Xiamen Bay, while riverine P loads and domestic/industrial wastewater discharge share similar contributions to the total P inputs. The total fertilizer use in the Xiamen Bay watershed increased from 5.5 × 104 tons in the 1980s to around 35 × 104 tons in the mid-2000s, then steadily decreased until around 2017 when a relatively sharp decline began (Figure S7b in the Supplementary Material). Concurrently, the total N and P loads in Xiamen Bay increased by four and nine folds, respectively, from 2000 to 2016, following the increasing patterns in the population and GDP (Figures S7a and S7b in the Supplementary Material). However, starting from 2017 the total N and P loads dropped sharply to about one-third of their peaks in 2020, a trend similar to that of the fertilizer use. As a result, the DIN concentration in Xiamen Bay increased rapidly from about 19 μmol L−1 in the late 1980s to more than 60 μmol L−1 in 2013, then started to drop sharply in general to 18 μmol L−1 in 2020 and the DIP concentrations experienced a similar increase and reached a peak of 1.5 μmol L−1 in 2013, but fluctuated around 1.2 ± 0.5 μmol L−1 afterward (Figure S7c in the Supplementary Material). Closely related to the changes in the DIN and DIP concentrations, HABs were first noted in the mid-1980s. The summer Chl-a concentration in Xiamen Bay peaked in 2008, after which a general decreasing trend was observed (Figure S7d in the Supplementary Material).
The fertilizer use in this region is positively correlated with both the population, urbanization rate (p < 0.001), and total GDP (p < 0.01) (Figure 4f). The wastewater N loading is also positively correlated with the total GDP (p < 0.05). The concentrations of DIN and DIP in the bay are both positively correlated with the urbanization rate significantly (p < 0.01 for DIN and p < 0.05 for DIP). In addition, the DIN and DIP concentrations are related with the total fertilizer use in this region with a positive correlation (p < 0.05), indicating remarkable socioeconomic controls on the coastal eutrophication. So far, observations of extremely low oxygen concentrations in the bottom water of Xiamen Bay are rare, and hypoxia has not been observed in the open area of the bay (Figure S7d in the Supplementary Material). This may be due to well flushing as inferred from the relatively short residence time in Xiamen Bay (Table 2). An unusual trend is present in that the minimum DO concentration in the bay is positively correlated with both the population and GDP in this region (p < 0.05). The Chl-a concentration in the bay is positively correlated with the fertilizer use in this region (p < 0.01).
The improvement of the environmental quality of Xiamen Bay is attributed to integrated coastal management actions. In 1994, Xiamen City was selected as a demonstration site for Integrated Coastal Management (ICM) practices, which involve a series of coastal management laws, policies, and actions to support marine ecosystem rehabilitation and ensure a balance between protection and production (Winther et al., Reference Winther, Dai, Rist, Hoel, Li, Trice, Morrissey, Juinio-Meñez, Fernandes, Unger, Scarano, Halpin and Whitehouse2020). A number of policies and actions have been at work, including new construction or upgrades of wastewater treatment plants since 1997, banning of P-based detergents since 1999, banning of aquaculture activities, and dredging of channels since 2002 (Cai et al., Reference Cai, Liu, Chen, Huang and Yang2016). Xiamen Bay could be a potential model that exemplifies the efficacy of integrating science and governance to environmental management.
Comparisons and lessons learned
Anthropogenic nutrient inputs are known to be one of the factors driving coastal eutrophication and hypoxia. Comparisons of the long-term trends in economic development, societal management and the development of eutrophication and low-oxygen conditions among selected model coastal systems show the evolution of the interaction between human socioeconomic activities and environmental degradation/restoration through changes in anthropogenic nutrient loads and mitigation efforts (Figure 5). Based on the development of socioeconomics, the duration of human forces on the system, low-oxygen conditions, the potential shift of community structure and implementation of measures, such evolution could be summarized as three stages:
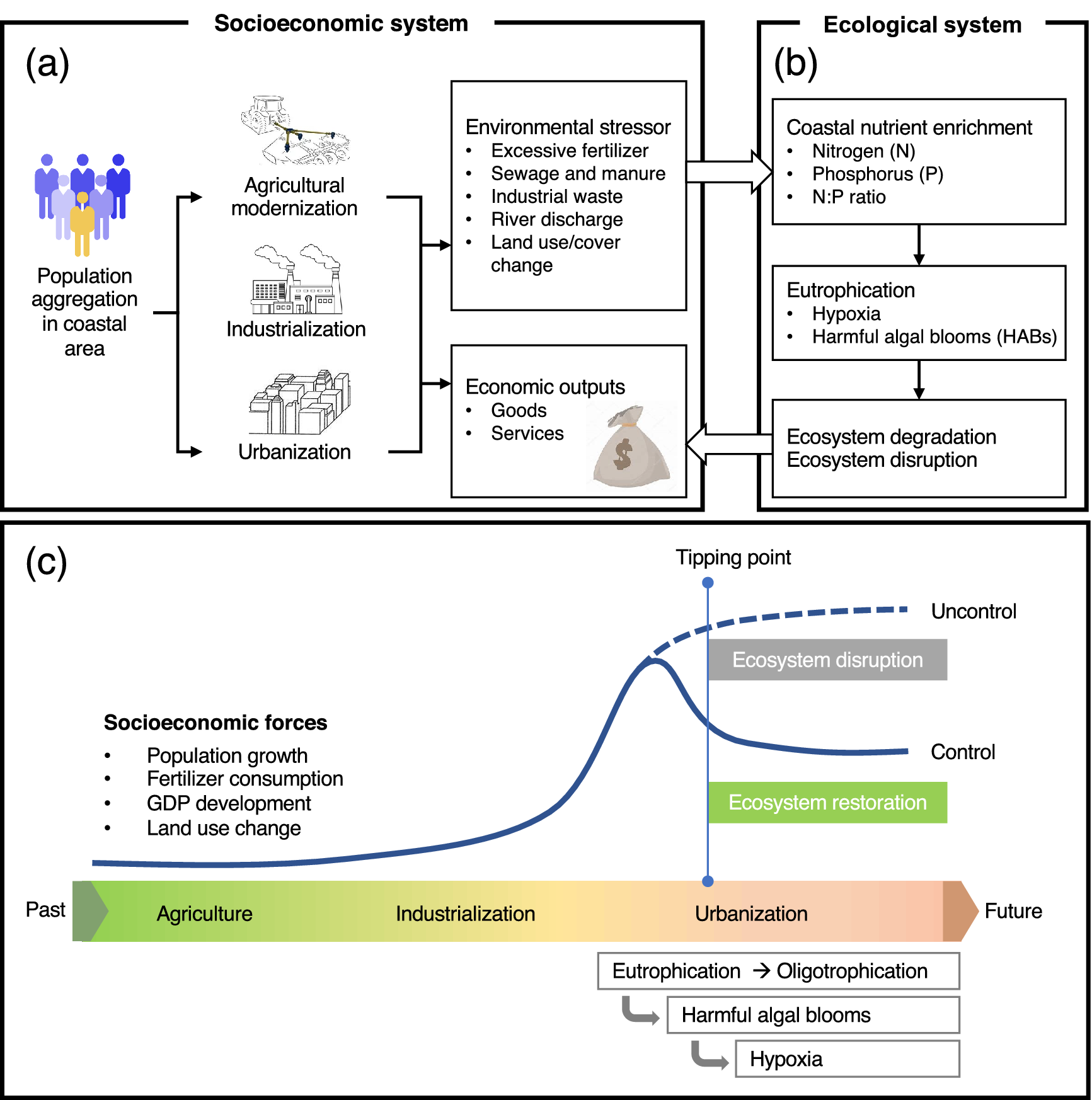
Figure 5. Schematic of socioeconomic forcing and ecological response in coastal ecosystems. (a) Driven by the population aggregation in coastal areas, the development of agricultural modernization, industrialization and urbanization brings about plenty of goods and services as well as environmental forces, such as excessive fertilizer, sewage and manure, river discharges and land use change. These socioeconomic forces stress the health of the ecological system (b), causing enrichment of nitrogen (N) and phosphorus (P) and changes in N:P ratios in coastal waters, which subsequently enhances eutrophication and subsequent harmful algal blooms and hypoxia. The degradation and disruption of the coastal ecosystem also restrict the economic outputs of the socioeconomic system. (c) Through the development of agriculture, industrialization and urbanization with intensifying socioeconomic forces, ecosystem disruption occurs after passing a tipping point unless great efforts are taken and control management strategies are applied for oligotrophication.
(1) As for the Upper Gulf of Thailand which are in the early stage, the economic growth during the recent two decades or longer goes along with an increasing population and depends on the development of agriculture, livestock and/or aquaculture to a large degree, resulting in land-use changes from forest to croplands and grasslands (Figure S6e in the Supplementary Material), and intense usage of synthetic fertilizer and feeds to meet food and energy demands. Due to low nutrient uptake efficiency by crops and the reduced capacity of nutrient retention when flushed by rainfall, a large fraction of fertilizer nutrients, together with untreated wastewater and sewage sludge from livestock and aquacultural farms, ultimately enter the coastal ocean mainly through the river and underground flows. Despite relatively low DIN concentration in open coastal waters, the Chl-a concentrations in the Upper Gulf of Thailand reach up to nearly 9 μg L−1 (Table 2). Eutrophication thus frequently occurs downstream in the river as well as near the river mouth with no significant shift in phytoplankton community structure. Hypoxia might emerge sporadically at a relatively small spatial scale, but measures to mitigate eutrophication and hypoxia have not been implemented or in the infancy. In this stage, the deterioration of water quality is closely related to intensified socioeconomic activities and anthropogenic nutrient inputs (Figure 4).
(2) As for the Changjiang and Pearl River estuaries which are in the intermediate stage, with continued population growth and increasing food demand for more than three decades, heavy fertilizer usage on croplands leads to higher amounts of nutrient inputs to the coastal ecosystem. Along with rapid industrialization and urbanization especially in the river delta regions, the discharge of sewage and manure greatly worsens nutrient pollution in coastal waters with population aggregation nearby. Nutrient concentrations have thus reached substantially high levels in the coastal surface waters, i.e., on average 35–60 μmol L−1 off these estuaries during summer (Table 2). In this stage due to long-term large inputs of nutrients with increased N:P ratios, the dominant species of phytoplankton tend to shift and outbreaks of algal blooms become more frequent or in a larger spatial extent in the wet seasons. These changes likely tip the coastal region into a seasonal hypoxic system with expanding areas, which is evidenced by tightly negative correlations between the DO minimum and the parameters regarding anthropogenic activities observed in the Changjiang and Pearl River estuaries over the last decade or longer (Figure 4). Increases in the frequency, spatial extent, duration, and severity of HAB outbreaks and hypoxia in turn severely impair economic development and human health, leading to greater investment efforts to abate eutrophication and associated cascading consequences. In this stage, efforts in reducing nutrient loads can be effectively reflected by the declining trend in nutrient and Chl-a concentrations in coastal waters (Figures S4 and S5 in the Supplementary Material). However, whether and how long the nutrient and Chl-a concentration can decrease to the past norms would be a long-term struggle between societal management and environmental degradation, depending on the subtle balance between socioeconomic growth and ecological restoration.
(3) As for the Baltic Sea and the Chesapeake Bay which have gone through the former stages and are now in the late stage, extremely low DO environments (close to 0 mg L−1) have been persistently developed under the influences of human socioeconomic activities for more than half a century. These systems have experienced a non-linear degeneration of the ecosystem by way of a complex system evolution, which leads to limited achievements in curbing eutrophication and mitigating hypoxia through reducing external nutrient input since the 1980s. The experience of controlling coastal eutrophication in developed regions sheds light on the non-linear, complex relationship between socioeconomic growth and environmental degradation. Apart from a long-term reduction in nutrient loads, the hysteresis effect of feedback between sub-systems and irreversible consequences would be minimized if we adopt cost-effective mitigation strategies and take precautional measures (e.g., removal of excess DIP from the Baltic Sea) before the coastal ecosystems stressed by eutrophication reach a tipping point. Also, when comparing the Baltic Sea and Chesapeake Bay with the Changjiang and Pearl River estuaries, it should be noted that the longer time the anthropogenic pressures exert influence on the ecosystems, the greater reduction in pressures ecosystem recovery is needed compared to that initiated degradation and the more difficult ecosystems recover to past norms. In this regard, controls on nutrient loadings and reducing pressures during the early stages of eutrophication-hypoxia development appear to be more effective and efficient in reducing the nutrient levels and the frequency of HAB outbreaks and mitigating hypoxia in the coastal regions (Figure 5).
Exception is the Xiamen Bay in the stage between the early and intermediate levels of eutrophication-hypoxia development. Despite the dominant riverine N inputs to the coastal waters from agricultural activities, rapid industrialization and urbanization also contribute substantial P inputs from the domestic sewage and industrial wastewater. The nutrient levels thus reach as high as the Changjiang and Pearl River estuaries (Table 2). However, neither hypoxia nor shift in dominant HAB species has been reported in the Xiamen Bay. In this sense, the evolution of the interaction between human socioeconomic activities and environmental degradation/restoration might be not progressive, dependent on the trajectory of socioeconomic development and investment, strategy and timing of mitigation efforts put into force.
Besides the understanding of developing stages for coastal eutrophication and hypoxia related to economic development and societal management, comparisons between multiple systems (Table 2) also reveal that the strength and synergistic effect of multiple driving forces of coastal eutrophication and hypoxia also depend on regional geographic features. The river-dominated open coastal systems (e.g., Changjiang and Pearl River estuaries) have emerged as recent hotspots of HAB outbreaks and hypoxia primarily driven by the increased anthropogenic nutrient inputs from the land. In this sense, the fertilizer usage and nutrient emissions for food production remain, and will be, a major contributor to the total nutrient loads in the future, as the human population increases and food preferences change to include more meat and dairy (Beusen et al., Reference Beusen, Doelman, Van Beek, Van Puijenbroek, Mogollón, Van Grinsven, Stehfest, Van Vuuren and Bouwman2022). However, larger hypoxic areas and higher frequency of HABs are reported off the Changjiang Estuary compared to the Pearl River Estuary (Figures S3 and S4 in the Supplementary Material), which are likely due to longer residence times of bottom waters and deeper water depths off the Changjiang Estuary.
In addition, the impacts of the human dimension on the coastal ecosystem, especially in semi-enclosed systems, can be accumulative over time and trigger regime shifts eventually passing tipping points, and rendering the coastal ecosystem more sensitive to external disturbances. One example is the impact of nutrient legacy on shifts in the dominant species of HABs. In contrast to a shifting trend of dominant HAB species from diatoms to dinoflagellates off the Changjiang Estuary (Chen et al., Reference Chen, Li, Jin, Jiang, Wang, Wu, Hao, Sun, Chen and Guo2020, Reference Chen, Hong and Gao2021b; Shi et al., Reference Shi, Xu, Li and Ge2022), in the Baltic Sea the phytoplankton community has shifted from diatom- and dinoflagellate-dominant in spring to cyanobacterial-dominant in late summer, driven by enhanced internal P supply (Funkey et al., Reference Funkey, Conley, Reuss, Humborg, Jilbert and Slomp2014; Hieronymus et al., Reference Hieronymus, Eilola, Hieronymus, Meier, Saraiva and Karlson2018). Nitrogen fixation by the cyanobacterial provides extra N inputs into this system, resulting in less effective remediation of eutrophication by reducing terrestrial nutrient loads.
However, mitigating hypoxia seems to be more difficult than simply abating eutrophication in semi-enclosed systems such as the Baltic Sea and the Chesapeake Bay, not only because of the nutrient legacy problem, but also due to the amplification effect by the changing climate, and potentially phytoplankton adaptation to the eutrophic condition. Superimposed on the reduced oxygen solubility, the stronger stratification induced by ocean warming and higher rates of freshwater input will reduce the ventilation of bottom water in coastal oceans and facilitate oxygen depletion (Oschlies, Reference Oschlies, Laffoley and Baxter2019). In contrast, the development of hypoxia in open coastal systems might be more diverse depending on local geographic, physical, and biogeochemical settings even if the nutrient loadings were effectively controlled. The physical replenishment in the coastal ocean is expected to be stronger at the backdrop of climate change with rising sea levels and increasing frequency of extreme climatic events, which might partly offset the warming-induced deoxygenation.
Summary, challenges and perspectives
Summary
Eutrophication and hypoxia have been widely observed and investigated worldwide, and their complex coupled physical-biogeochemical mechanisms have been increasingly recognized. The excessive nutrient inputs with favorable physical conditions that enable the accumulation of inorganic and organic substances and sufficient residence times for biogeochemical processes to drive the occurrence of eutrophication and hypoxia are conceptually understood. However, the physical and biogeochemical processes at work, in particular their quantitative contributions to the formation, sustainment and spatiotemporal variability of eutrophication and hypoxia, remain largely unclear, which greatly hinders our capability for accurately predicting the formation and alleviation of this marine hazard that affects many coastal oceans globally.
Among the anthropogenic N inputs to coastal ecosystems, synthetic fertilizers are the largest source, with Asia representing more than half of the global use. Eutrophication and associated hypoxia and HABs are still increasing in frequency and severity worldwide, and are an emerging concern in the developing countries of Asia associated with the hotspots of human population and economic development. Toxic bloom events occurring in South East Asia account for nearly half of the recorded global HABs, and river-dominated open coastal systems show a trend in regime shifts towards seasonal hypoxia.
In addition to the well-recognized mechanism for eutrophication-driven hypoxia, diverse OM sources with varying contributions to fueling oxygen consumption complicate the non-linear response of hypoxia to nutrient loadings. The combined effects of the interactive physical and biogeochemical processes commonly lead to spatial–temporal decoupling of hypoxia and algal blooms in coastal regions. This non-linear cause–effect relationship of eutrophication and hypoxia is further complicated by the benthic processes under hypoxic conditions, which by promoting N leakage to the atmosphere and P release from sedimentary deposits can pose profound feedback on shifts in communities of algal blooms over different timescales.
The long-term trends of eutrophication and hypoxia are subject to controls by trends in nutrient loadings and physical dynamics under a changing climate. While anthropogenic nutrient inputs are closely related to the complex features of economic development and societal management, accelerating climate change tends to alter, in space and time, precipitation, terrestrial discharge, water temperature, and atmospheric and then oceanic circulation, which concurrently control the severity and trends of eutrophication and hypoxia.
Though the chain reaction of nutrients-eutrophication-hypoxia has been widely demonstrated in most coastal eutrophic systems, our understanding of the mechanisms and interactions between eutrophication and hypoxia and between estuary and adjacent continental shelf in the coastal transition zone remains limited. The coastal transition zone is biogeochemically and physically conducive for occurrences of eutrophication and hypoxia. Yet, the dynamics arising from the interactions are physically and biogeochemically complex, involving the integrations of estuarine and shelf processes in two distinct regimes that are greatly impacted by multiple stressors and the often non-linear cause–effect relationship of both natural and human ecology in different spatial and temporal scales. This is particularly true from a quantitative viewpoint.
Perspectives to closing the knowledge gaps
While the rapid development and spread of eutrophication and its symptoms far beyond the pre-industrial natural baseline are extensively evidenced, current measures to mitigate coastal eutrophication and associated hypoxia and HABs remain unsatisfactory due to the major knowledge gaps in addressing the problem. We advocate for a holistic approach to management and identify the following aspects that are crucial to understand.
Tipping points and resilience
We suggest that ecosystem resilience and tipping points hold keys to a holistic understanding of coastal ecosystems stressed by eutrophication. The Baltic Sea tipping point showed us the often-unpredictable non-linear degeneration of ecosystems in the manner of complex system evolution. As summarized in this review, the strength and synergistic effects of the multiple driving forces of coastal eutrophication are dependent on regional geographic features, economic, scientific and technological development, and societal management. This suggests that a comprehensive understanding of coastal ecosystems from the viewpoint of a multi-scale complex system composed of physical, biogeochemical, ecological, and societal sub-systems is needed to disentangle their interactive pathways and identify the strengths of the key nodes between sub-systems. Coastal marine ecosystems are the first to respond to eutrophication, hypoxia, and climate change, and their resilience lays the foundation for the capacity of sustainable development. Foremost, we need to identify the “early warning” indicators of potential regime shifts in coastal ecosystems, for example, unusual variations in biomass and biodiversity, by upgrading current models to characterize and predict the non-linear changes under multiple stressors, which is of great importance for the guidance of societal risk management.
Model development with inclusion of social-economic drivers
Coastal eutrophication and hypoxia involve not only coupled physical-biogeochemical processes in the ocean, but also terrestrial nutrient inputs that are intimately associated with human activities. Land use, agriculture, sewage, and organic and inorganic pollutant discharges from different socioeconomic sectors greatly control nutrient discharge into the ocean. These human activities together with a changing climate alter the land-atmosphere interface and modulate regional atmospheric circulation and thus oceanic circulation. To tackle coastal eutrophication and hypoxia and project their trends, we need to consider regional ocean-land-atmosphere Earth systems, and human activities as an integrated system. It is obvious that such a complex system needs to be configured using a modeling and forecasting platform that fully integrates our understanding of complex coastal ecosystems, coupled physical-ecosystem models, and regional earth system models that incorporate human dimensions. This modeling platform with sufficient spatiotemporal resolution simulates Earth’s surface processes affecting the sources and sinks of freshwater, nutrients and pollutants on land and is closely linked with atmospheric forcing and with the buoyancy effects and the transformation and consumption of biogeochemical substances in the ocean.
The land surface process is strongly linked with the atmosphere through water, and energy fluxes, while the atmospheric deposition of nutrients, wind forcing, and aerosol fluxes are connected with ocean circulation and productivity. In the ocean, the biosphere responds to the ocean transport and mixing of biogeochemical substances and pollutants and feedback to the ocean ecosystem. The impacts and feedback of climate and human activities are embedded within different components in this regional earth system modeling platform. Ideally, physiological parameters (e.g., μmax, Ks) from different phytoplankton functional groups should be integrated to constraint model predictions. As an illustration, Figure 6 shows such a modeling platform that is able to serve cross-scale and interdisciplinary syntheses, diagnose and simulate key processes, and prognose future changes as well as response-feedback cross-examinations between natural ecosystems and the human dimension. The assimilation of spatiotemporal observational data and machine-learning output into the platform can fill information gaps and help to not only make reliable predictions of biogeochemical cycles, ocean primary production, and the dynamics of fishery resources (Subramanian et al., Reference Subramanian, Balmaseda, Centurioni, Chattopadhyay, Cornuelle, DeMott, Flatau, Fujii, Giglio, Gille, Hamill, Hendon, Hoteit, Kumar, Lee, Lucas, Mahadevan, Matsueda, Nam, Paturi, Penny, Rydbeck, Sun, Takaya, Tandon, Todd, Vitart, Yuan and Zhang2019), but also entire regional Earth systems. Establishing such a regional earth system model that couples the human dimension with the natural system is of great value to integrate natural processes and human activities and provide science-based outcomes for use in decision-making process (Sinha et al., Reference Sinha, Michalak and Balaji2017; Hamilton et al., Reference Hamilton, Ivanova, Stadler, Merciai, Schmidt, van Zelm, Moran and Wood2018).
Figure 6. Schematic view of a regional earth system-human activity modeling platform for diagnosing and prognosing eutrophication and hypoxia.
Perspectives on solutions
Barriers and bridges towards abating coastal eutrophication have been previously reviewed (e.g., Boesch, Reference Boesch2019), and solutions have been proposed from the perspective of actionable science, accountable governance, effective reduction in nutrient loads and measurable outcomes and adjustable strategies. Here we offer two crucial approaches to put the closure of knowledge gaps into practice: a more science-based and holistic index system and a life-cycle nutrient footprint.
A more science-based and holistic index system
One of the essential components of abating coastal eutrophication is to adequately monitor and assess its state both prior to and after the remediation action selected under a defined policy (Boesch, Reference Boesch2019). The eutrophication index has thus been developed for making harmonized assessments of marine eutrophication through the assessment of several key criteria which detail the cause and impact of increased nutrient delivery. Well-designed indexes of this type can evaluate eutrophication in the Baltic Sea by considering nutrients, turbidity, algal blooms, benthic communities, and oxygen levels and dividing the trophic state into five levels by using an expert scoring system and the rule of ‘one out, all out’ (Andersen et al., Reference Andersen, Axe, Backer, Carstensen, Claussen, Fleming-Lehtinen, Järvinen, Kaartokallio, Knuuttila, Korpinen, Kubiliute, Laamanen, Lysiak-Pastuszak, Martin, Murray, Møhlenberg, Nausch, Norkko and Villnäs2010). Another index system, OSPAR-CP, gives more consideration to the human dimension and mechanisms of eutrophication, and evaluates nutrient enrichment and the direct and indirect effects of eutrophication to reveal the trophic state. It also shows the relationship between the ecological quality goals of eutrophication abatement and nutrient inputs caused by human activities (Axe et al., Reference Axe, Clausen, Leujak, Malcolm and Harvey2017). Developed by NOAA, ASSETS attempts to take a comprehensive approach that considers management effectiveness and extends its applicability to a number of coastal regions. It adapts the chain reaction of Pressure-State-Response to divide the whole ecosystem into three parts: a heuristic index of pressure (Overall Human Influence), a symptoms-based evaluation of state (Overall Eutrophic Conditions), and an indicator of management response (Definition of Future Outlook) (Bricker et al., Reference Bricker, Ferreira and Simas2003). From these indexing systems, it is clear that a common framework with an adaptive approach to regions and study sites remains lacking. It must be pointed out that in many countries or regions, such as in China, there is a lack of a thorough and independent evaluation approach for the current state of eutrophication in marine systems. In some studies, methods from other countries have been applied to China’s coastal areas without adequate consideration of specific environmental settings (Xiao et al., Reference Xiao, Ferreira, Bricker, Nunes, Zhu and Zhang2007). Current assessments of eutrophication in China, for example, mainly depend on China’s seawater quality standards which may not be fully applicable to estuarine systems and effective to meet the management requirements (Yang et al., Reference Yang, Zhang, Li, Hu, Zhang and He2018).
Life-cycle nutrient footprint accounting
We also advocate for a life cycle nutrient footprint accounting to best track the nutrient flows. Such a system, similar to carbon footprint, devotes to representing the distribution of various nutrient sources and evaluating the impact of socio-economic human activities on the environment. The system allows decision makers and stakeholders to develop targeted, locally tailored strategies regarding nutrient emissions reduction through identifying hot spots of likely point and non-point source pollution across the coastal regions as well as identifying inefficiencies in the use and handling of nitrogen and phosphorus across production sectors and consumption styles. Attempts have as a matter of fact been available towards establishment of such systems; for example for specific production sectors (e.g., food) (Wang et al., Reference Wang, Ma, Strokal, Ma, Liu and Kroeze2018c) and for a specific confined environmental setting (e.g., a submerged-cage offshore aquaculture facility located in the tropical Caribbean) (Welch et al., Reference Welch, Knapp, El Tourky, Daughtery, Hitchcock and Benetti2019). However, there is a lack of nutrient model that encompasses the full lifecycle nutrient emissions of goods and services. Existing nutrient models used to quantify the nutrient pollution in rivers and coastal seas are usually on biophysical scales or non-administrative scales (e.g., basin scales or grid scales), but ignore the important socio-economic drivers (Chen et al., Reference Chen, Strokal, Van Vliet, Stuiver, Wang, Bai, Ma and Kroeze2019). In particular, the impacts of diverse consumption activities on nutrient emissions have not been extensively studied, and the strong link between consumption and nutrient emissions is unclear and vague. Also, existing nutrient footprint inventories are widely used in large-scale modeling (e.g., provincial data) yet lacking finer scales, which induce the challenges of lacking localized parameters and missing localized characteristics (Li et al., Reference Li, Chen, Cai, Fu, Ting, Chen, Folberth and Liu2022).
Calling for a more sustainable use of our planet, the United Nations (UN) 2030 Agenda for Sustainable Development with its 17 Sustainable Development Goals (SDGs) came into action in 2015. SDG 14 (Life below Water) aims to “conserve and sustainably use the oceans, seas and marine resources for sustainable development.” Among the targets are to “by 2025, prevent and significantly reduce marine pollution of all kinds, in particular from land-based activities, including marine debris and nutrient pollution” and more comprehensively, “by 2020, sustainably manage and protect marine and coastal ecosystems to avoid significant adverse impacts, including by strengthening their resilience, and take action for their restoration in order to achieve healthy and productive oceans”. Yet the question of how to operationalize these targets remains open. In order to adequately address the question of how we can assess, secure, and improve ocean health and the associated benefits for human health and well-being, it is essential to construct a sustainable development index that is suited to coastal areas. Several comprehensive tools for evaluating ocean sustainability have been developed over the years, such as Ocean Health Index, Ecosystem Health Assessment, Marine Biodiversity Assessment(Borja et al., Reference Borja, Elliott, Andersen, Berg, Carstensen, Halpern, Heiskanen, Korpinen, Lowndes, Martin and Rodriguez-Ezpeleta2016; Wu et al., Reference Wu, Chen, Meadows and Liu2021). However, current frameworks only partially focus on human society or marine environment. Efforts to examine the complex links between ocean and human health are still rare, and approaches to integrating human aspects beyond livelihoods and jobs (i.e., health and well-being) into ocean-health definitions and assessments are often missing. We need a more encompassing framework that integrates marine ecosystem and human health and well-being, which is able to provide a profound, holistic understanding of marine ecosystems and their complex interdependencies with human societies. Our aim is to provide both a conceptual and an operational framework for evaluating sustainability of human-ocean nexus.
Finally, we argue that harnessing the power of partnerships among stakeholders, the development of science breakthroughs, and knowledge transfers to achieve effective integration of science, governance and society are key elements to tackle the problems raised and respond to the challenges/priorities identified for the United Nations Decade (2021–2030) of Ocean Sciences for Sustainable Development (“The Decade”). A project entitled “Coastal Zones Under Intensifying Human Activities and Changing Climate: A Regional Programme Integrating Science, Management and Society to Support Ocean Sustainability (Coastal-SOS)” (http://coastal-sos.xmu.edu.cn/) is such an initiative in the framework of the Decade actions. The project is partnering with multiple stakeholders, including leading academic institutions, industry enterprises, non-profit foundations, and NGO/IGOs, from Eastern Asian countries to facilitate the advancement of scientific understanding of critical coastal ocean issues centered around eutrophication. This will allow the translation of improved scientific knowledge into practical solutions (including improved and integrated management strategies), and empower industry stakeholders to implement best practices in ocean resource usage.
One of the key components of the project is to establish a multi-disciplinary integrated model between socioeconomic activities and the marine environment (Figure 7), as the ability to capture the feedback between human and natural systems in coastal regions. This system could assess the contribution of socioeconomic drivers to cumulative nutrient pollution growth in coastal areas. The environmental extended multi-regional input–output model will evaluate the impacts of production patterns and lifestyle changes to the marine environment. The economic footprint analytical model is able to quantify the direct economic losses (e.g., fishing stock decline) due to deteriorations of marine ecology in coastal regions and their cascading effects along economic production chains in interior regions. Finally, the Inclusive Wealth Index framework comprehensively measures the performance of sustainability in the coastal sites of interest.
Figure 7. Schematic of the economic-human dimension modeling system for coastal zones. Human demands and activities for food, energy, construction, and transportation have increased nutrient loads to the coastal ocean, causing eutrophication and associated environmental damage. To disentangle the complex links between socioeconomic system and ecosystem, the multi-disciplinary model integrating nutrient inventory method and marine input–output model can accurately quantify the direct and indirect nutrient discharge by sectors and sources, and identify the priority control areas for efficient decisions on eutrophication and hypoxia management. A comprehensive assessment framework based on Inclusive Wealth Index can also make trade-offs between resource use and economic prosperity and support the realization of conserving and sustainably using the oceans, seas, and marine resources for sustainable development. The photos in the right column under "Coastal Ocean" were from image courtesy of NOAA and websites (https://serc.carleton.edu/microbelife/topics/redtide/general.html; http://61.178.55.253/kpbl/stbh/swbh/content_118700), respectively.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2023.7.
Supplementary materials
To view supplementary material for this article, please visit http://doi.org/10.1017/cft.2023.7.
Data availability statement
This review paper does not include new data. The sources and links of all dataset used in the figures and tables in this paper have been described in the captions.
Acknowledgements
The authors would like to thank Dongjian Ci, Yi Huang, You Jiang, Yaohua Luo, Zhijie Tan, and Shuran Yang of Xiamen University, and Yan Bai, Huanhuan Chen, Zhibin Gao, Yue Jiang, Teng Li, and Xueling You of Second Institute of Oceanography, Ministry of Natural Resources for their support and help in preparing the data and figures used in this contribution. This review was partly built upon a proposal associated with the Coastal-SOS project, Coastal Zones Under Intensifying Human Activities and Changing Climate: A Regional Programme Integrating Science, Management and Society to Support Ocean Sustainability (http://coastal-sos.xmu.edu.cn/). Coastal-SOS is an endorsed project by the United Nations Decade of Ocean Science for Sustainable Development (2021–2030) (UN-Decade). The support of Xiamen Ocean Development Bureau and China Oceanic Development Foundation to the international project office of COASTAL-SOS is also gratefully acknowledged. This paper is also contributing to and benefitted from the work done within the IOC Expert working group ‘Global Ocean Oxygen Network’ and the UN Decade of Ocean Science for Sustainable Development Programme ‘Global Ocean Oxygen Decade’. This work is also partially supported by the University of Hong Kong under the Science Distinguished Visiting Scholars Scheme to Minhan Dai.
Author contributions
Conceptualization: M.D., Y.Z. Section Lead authors: in addition to M.D. and Y.Z. who led drafting and reviewing, G.W. and S.L. (Section 2), J.-Y.T.Y. (Section 3), Y.S. (Section 4), J.G. and D.G. (Section 5) are acknowledged as lead authors. Data curation & Visualization: Y.Y., Y.Z., Y.S., G.W., J.-Y.T.Y., Y.W., Z.W., Y.X., N.C., Z.Z. Funding acquisition: M.D. Investigation: Y.W., Z.W., K.U., D.Y., Z.Z., Y.W., Y.Z., Y.C., F.M. Writing – original draft, review & editing: all authors.
Financial support
This work was supported by Theme Based Research Scheme under Research Grant Council of Hong Kong (grant number T21-602/16-R); The Consultation and Evaluation Project of the Academic Divisions of the Chinese Academy of Sciences (grant number 2022-DX01-B-006); Strategic Research and Consultation Project of the Chinese Academy of Engineering (grant number 2022-XBZD-11); and National Natural Science Foundation of China Basic Science Center Program (grant number 42188102).
Competing interest
The authors declare none.












Comments
No accompanying comment.