Introduction
Seabirds are increasingly threatened and some seabird families (such as albatrosses Diomedidae) are amongst those with a higher proportion of threatened species among avian taxa (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). For this reason, but also because they are easy to study in comparison to most other marine life, seabirds are increasingly taken into account in spatially explicit marine planning, management and monitoring (e.g. Ronconi et al. Reference Ronconi, Lascelles, Langhamc, Reid and Oro2012).
In recent years, tracking studies of seabirds have proliferated, to a large extent with the justification of their relevance to identify marine areas of importance for birds and potentially for other organisms too. Most of these studies use satellite tracking or GPS technology, and infer the types of use of different marine areas from spatial patterns of movement (for example, using Area Restricted Search analyses), often focusing on identifying areas of likely intense foraging (e.g. Fauchald & Tveraa Reference Fauchald and Tveraa2003, Lascelles et al. Reference Lascelles, Taylor, Miller, Dias, Oppel, Torres, Hedd, Le Corre, Phillips, Shaffer, Weimerskirch and Small2016). The nature of the tracking devices used and the focus on identifying areas where energy and biomass transfer may be larger, means that other types of usage of the marine environment often are overlooked. Some studies even exclude tracking data in defined areas (e.g. close to the breeding colony) to improve the performance of models focused on identifying foraging hotspots (e.g. Catry et al. Reference Catry, Lemos, Brickle, Phillips, Matias and Granadeiro2013, Lascelles et al. Reference Lascelles, Taylor, Miller, Dias, Oppel, Torres, Hedd, Le Corre, Phillips, Shaffer, Weimerskirch and Small2016). In the particular case of flying seabirds, this can be further justified by the belief that when commuting, these birds overfly marine sectors where they do not forage, and hence are less susceptible to interact with local threats compared to species, such as penguins or pinnipeds, which make a more intimate use of the sea surface or the water column.
Several flying seabirds regularly gather in significant numbers in the vicinity of the colonies (e.g. Weimerskirch et al. Reference Weimerskirch, Bertrand, Silva, Marques and Goya2010). This is better known and more typical of some petrels, particularly shearwaters, which gather offshore of nesting sites in dense flocks, often designated as “rafts”, while waiting for darkness to allow nocturnal landing at the colonies (Warham Reference Warham1996, Rubolini et al. Reference Rubolini, Maggini, Ambrosini, Imperio, Paiva, Gaibani, Saino and Cecere2014).Such aggregations can also act as a mechanism of information sharing, presumably providing cues to departing birds in relation to the location of good quality foraging patches (Weimerskirch et al. Reference Weimerskirch, Bertrand, Silva, Marques and Goya2010). Recently, breeding Northern Gannets Morus bassanus were also demonstrated to engage in rafting behaviour, with no apparent relationship with subsequent foraging parameters (Carter et al. Reference Carter, Cox, Scales, Bicknell, Nicholson, Atkins, Morgan, Morgan, Grecian, Patrick and Votier2016). Such behaviour was hypothesized to be related with maintenance activities, such as bathing. Regardless of its ecological significance (protection from predators, maintenance, information centres), this behaviour has conservation implications because it means that in the immediate vicinity of the colony birds are potentially highly vulnerable to threats such as disturbance, pollutants (including hydrocarbons), collision with artificial structures and light attraction (Le Corre Reference Le Corre, Ollivier, Ribes and Jouventin2002, Ronconi, Allard &Taylor Reference Ronconi, Allard and Taylor2015). On the other hand, for flying species with little or no bathing or rafting activity, it could be argued that the sea in vicinity of the colony is of marginal relevance.
In the present paper, we document an unexplored aspect of seabird behaviour that has similar implications as near-colony rafting in terms of usage of the marine environment. By combining precise GPS-tracking and activity recorders, plus direct observations, we show that albatrosses intensively use the sea surface in immediate vicinity of the colony for bathing purposes before departing to a foraging trip, and that this usage is much more prevalent than rafting and involves virtually entire populations.
Methods
We studied black-browed albatrosses (BBA) Thalassarche melanophris from 4 colonies in the Falkland Islands, Southwest Atlantic Ocean, including the two largest BBA colonies in the world (Steeple Jason and Beauchene), with a combined population of ca 350.000 breeding pairs. Tracking was carried out on New Island West (2008, 2009, 2012, 2013), New Island North (2010), Steeple Jason (2009, 2011, 2012), while direct observations were performed on New Island and on Beauchene. Breeding birds were tracked with GPS loggers (E&Ocean Technologies, Germany and i-gotU GT-120, Mobile Action Technology, Taiwan), with a mass ranging from 20g to 30g, which were attached to back or tail feathers with Tesa tape. GPS devices were programmed to collect positional data at intervals ranging from 1 to 60 minutes varying with year and stage of breeding cycle, with the longer intervals used only for long incubation trips (242 out of 315 trips set at <=14 min). Birds were also equipped with British Antarctic Survey geolocator-immersion loggers (GLS: Mk 7 and MK3005, weighing 2.0-3.5g) deployed on the metal leg band, to record the timings of all changes of state (from wet to dry, and vice versa), at 3s resolution. These high-resolution data concerning the timing of landings (wet) and take-offs (dry) were used to determine the period of flight and landing at sea and were then combined with GPS locations to estimate the location of birds at 3s resolution (see Granadeiro et al. Reference Granadeiro, Phillips, Brickle and Catry2011 for further details of this analysis). When a GLS recorded a “wet” episode between two GPS points, we assumed that the bird was static during this wet period i.e., did not suffer any wind or current drift while in the water. Large (>12 minutes) intervals between consecutive GPS points overestimated the estimates of distance of first landing, and so we calculated a correction factor to apply to each value according to its specific sampling interval (see supplementary material) For the purposes of this study, we selected a single trip per individual to avoid any potential problem of pseudoreplication.
Overall, we obtained 314 foraging trips during incubation (n = 79) and during early chick-rearing, while chicks were being brood by an adult (n = 235). In order to examine any relationship between the likelihood of sea-landing and some (highly correlated) characteristics of the foraging trips (trip duration, trip length and maximum distance from the colony), we derived a composite variable, calculated as the trip scores on the first axis of a Principal Component Analysis, after centring and reducing the data to unit variance. This variable (“trip characteristics”) was included in the generalised linear models when appropriate.
Observation of individuals leaving the colony after changing over with their partners at the nest were made on New Island and on Beauchene to visually assess the behaviour of birds when they landed in the vicinity of the colonies. For this, we visually tracked birds departing from the colony until they landed at sea or were lost of sight with binocular (10x). We could not establish the maximum distance we could observe bird with great accuracy, but since observations were carried out in good visibility conditions (clear sky) and from vantage points, we roughly estimate that we could clearly watch the behaviour of birds for up to ca. 1 km. For birds that landed, and during a maximum of ca. 5 min we tried to record whether they washed their plumage (“bathing”), whether they simply rested amongst other individuals or showed any signs of foraging.
Results
Foraging trips carried out during incubation and brooding lasted for an average of 5.2 ± 3.0 days (range: 0.3-11.3 days, n = 79) and 1.9 ± 1.22 days (range: 0.1-6.3 days, n = 235) respectively. Regardless of some differences among colonies in trip characteristics (Table 1), the vast majority of the study birds landed on the sea surface very soon after departing from their nests (Fig. 1). In fact, 97.8% (n = 314) of all birds landed within 5 km from the deployment site (median distance = 0.66 km), where they spent an average of 49.5 ± 40.8 (SD) min (Table 1). Birds were less likely to engage in a bath during incubation than during brooding, with no significant differences observed among study sites (binomial GLM, effect of phase: χ21,312 = 6.76, P < 0.005; effect of site: χ22, 305 = 0.68, P = 0.71). The amount of time spent in this initial sea-landing was significantly shorter during incubation (19.2 ± 14.4 min, n = 67) in comparison to brooding (31.8 ± 43.2 min, n = 233) and did not differ between islands (33.0 ± 51.6 min, n = 111 vs 26.4 ± 29.4 min, n = 189 for New Island). Furthermore, it was not related to the spatial or temporal extent of the trip or to the island (ANCOVA on log(time+1); effect of phase: F1, 295 = 9.3, P < 0.001; effect of trip characteristics (PCA scores): F1, 310 = 0.4, P = 0.82; effect of site: F1, 295 = 3.7, P = 0.24).
Table 1. Characteristics (means ± SD, sample sizes in parenthesis) of foraging trips of black-browed albatrosses in study colonies during incubation and early chick-rearing (brooding) and behaviour of individuals immediately after departure (start of trip) and before arrival (end of trip) and within 5km of tagging site. Estimates of distance to first landing were corrected according to GPS sampling interval (see methods and supplementary material for details).


Figure 1. Cumulative proportion of birds landing at sea at the start of the foraging trip in relation to distance in the three study sites, during incubation (left) and brooding (right, data grouped in 250 m classes). Estimates of distance to first landing were corrected according to GPS sampling interval (see methods and supplementary material for details).
In their return flights, 28.7% (n = 314) of the birds also landed within 5 km of the colony prior to relieving their partners at the nests, remaining there for an average of 28.6 ± 43.7 min (n = 90). The probability of landing in the water did not vary between incubation and brooding, was significantly higher at Steeple Jason than in the two study sites located at New Island and did not relate with the trip characteristics (binomial GLM, effect of phase: χ21,312 = 0.15, P = 0.70; effect of site: χ22,310 = 12.3, P = 0.002, effect of trip characteristics: F1,309 = 0.004, P = 0.95). The high prevalence of the bathing and rafting behaviour implied that the areas close to the colonies were by far the most used by albatrosses, in terms of the proportion of individuals landing in water (Fig. 2).
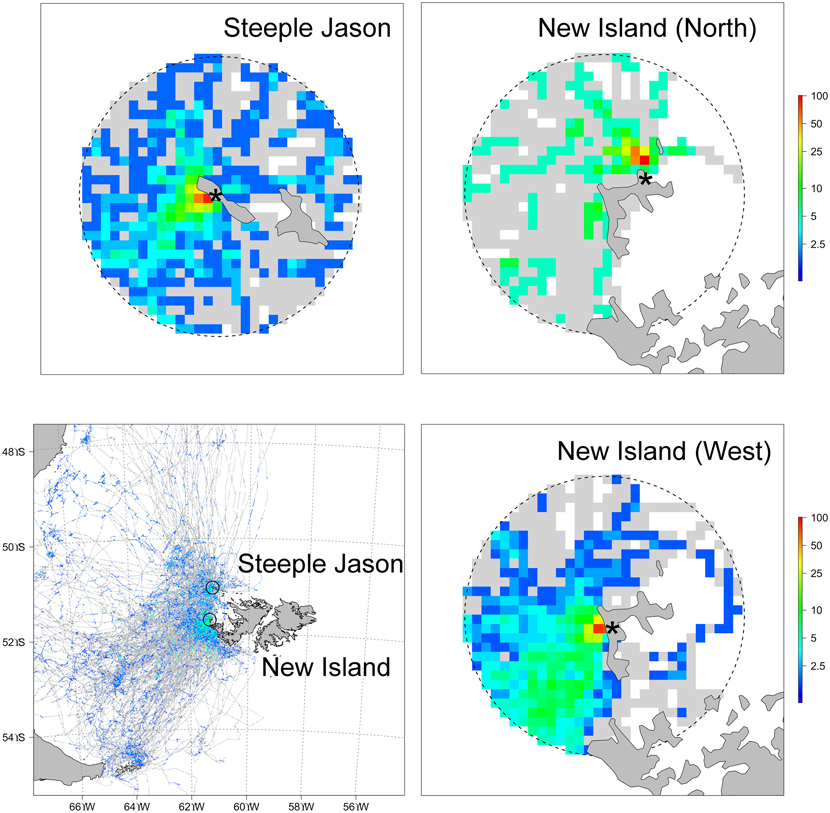
Figure 2. Percentage of birds landing in the water during incubation and chick rearing periods calculated separately for each study sites (bottom left, 5 km cell size) and distribution in a 15 km radius around each study site (top row and bottom right, 1 km cell size; note the logarithmic scale). Colours represent the maximum value recorded at any colony (Steeple Jason, New Island North or New Island West) and grey cells represent areas overflown by albatrosses (i.e. with no landings). Note that on Steeple Jason, birds were all tracked from one specific location in the colony and therefore the near-shore spatial marine use of those birds may not be representative of the entire colony.
The proportion of individuals landing in the water decreased with increasing distance (and area) from the colony, and appeared to level off between 5 and 25 km distance bands, where ca. 60% of the study birds landed to feed or rest (Fig. 3).

Figure 3. Variation in the overall proportion of birds landing in the water during the whole trip calculated over 1 km distance bands from each study site. Note the logarithmic scale of the x-axis, which results in a compressed representation of 1km bands at the larger distances.
We watched 16 departing birds on New Island, of which 13 landed at the sea surface and 3 were lost from sight before landing. Another 30 departing individuals were visually tracked from Beauchene, and all were seen landing in close proximity of the colony. All of the 43 individuals seen landing on the sea surface made preening and washing movements immediately after landing. For those which landed closer and were more readily visible, their movements suggested the ingestion of water in the first few seconds after landing, but not of foraging behaviour.
Discussion
By using activity recorders coupled with GPS tracking and direct observations, we have shown that virtually all black-browed albatrosses land on the water close to the colony when departing for a foraging trip. This behaviour, which is linked to important maintenance activities such as bathing and perhaps drinking, implies that albatross populations are potentially highly sensitive to threats present at the sea surface within less than 5 km of the nesting areas. Such a finding is very relevant in providing cues to define the spatial extent for Marine Protected Areas around breeding colonies of BBA and potentially several other seabird species (Thaxter et al. Reference Thaxter, Lascelles, Sugar, Cook, Roos, Bolton, Langston and Burton2012, Carter et al. Reference Carter, Cox, Scales, Bicknell, Nicholson, Atkins, Morgan, Morgan, Grecian, Patrick and Votier2016).
BBA make long incubation or brooding stints, typically of several days at the colony. During this period, their plumage gets dirty due to defecation from other albatrosses or other birds (a frequent occurrence in dense nesting seabird colonies, including in the Falklands where the space is shared with cormorants and penguins), but also from nest building activities (albatross keep building their nests with mud even during incubation and brooding), from dust and rain and perhaps from body secretions. Furthermore, incubating and brooding albatrosses have no access to drinking water and presumably get somewhat dehydrated after several days exposed to the sun and wind. Given the importance of keeping a clean plumage for waterproofing and efficient flying, as well as the maintenance of an adequate water-balance , it is perhaps not surprising to find that when relieved at the nest by the partner, BBA immediately go to the sea surface to wash and potentially also to drink. This is further supported by the fact that almost all birds bath on departure, but do not land on water every time on return. A role of bathing in thermoregulation is also possible (Oswald et al. Reference Oswald, Bearhop, Furness, Huntley and Hamer2008), but we recorded albatrosses bathing in all kinds of weather (including on very windy and cold days), and the very high incidence of bathing after departure recorded by tracked birds indicates that this is currently not happening only in a defined set of conditions (e.g. on warm sunny days).
This consistent bathing behaviour had not been described before in highly pelagic seabirds (but see Carter et al. Reference Carter, Cox, Scales, Bicknell, Nicholson, Atkins, Morgan, Morgan, Grecian, Patrick and Votier2016), and its prevalence is much higher compared to more conspicuous and better known and documented behaviours such as rafting (Rubolini et al. Reference Rubolini, Maggini, Ambrosini, Imperio, Paiva, Gaibani, Saino and Cecere2014). In fact, the incidence of bathing was much higher than the incidence of landing close to the colony at the end of the foraging trip, a pattern similar to that observed in Northern gannets (Carter et al. Reference Carter, Cox, Scales, Bicknell, Nicholson, Atkins, Morgan, Morgan, Grecian, Patrick and Votier2016). It is interesting to note significant differences in the rafting behaviour between New Island South and Steeple Jason, which may be linked to the size of the colonies. In fact, the Steeple Jason colony is nearly 15 times larger than the New Island colony, and while on Steeple Jason rafts can often be observed, on New Island they are hardly ever present. It is possible that where more albatrosses are present, an attraction effects contributes to recruit birds into these gatherings. Irrespective of these differences, and the low level of rafting in at least some colonies, virtually all BBA make use of the near shore environment for bathing. This makes the birds potentially highly vulnerable to risks in this particular area. For example, if a large oil spill would surround a colony during brooding, considering that each BBA foraging trip at this stage takes on average 1.9 days (see also Granadeiro et al. Reference Granadeiro, Phillips, Brickle and Catry2011), in the absence of any avoidance behaviour it would take only a few days for the entire breeding population to be affected.
In conclusion, our study demonstrates that the sea surface area in the immediate vicinity of black-browed albatross colonies, despite probably not being used for foraging, is visited by a proportion of the population that is higher than in any other marine area. As such, the close neighbourhood of albatross colonies are potentially highly sensitive areas, and this needs to be taken into account when carrying out risk assessments or during marine spatial planning exercises. It seems plausible that this applies to many other seabirds too, but more research is needed to confirm the generality of these findings, particularly focusing in the specific behaviour of birds when landed at sea.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000459
Acknowledgements
This study was supported by Fundação para a Ciência e a Tecnologia (FCT) through the strategic project UID/MAR/04292/2013 granted to MARE, UID/AMB/50017/2013 granted to CESAM, the project PEst-OE/MAR/UI0331/2011 and the European Regional Development Fund, through PTDC/MAR/121071/2010 and the FCT grant awarded to L.C. (SFRH/BPD/89904/2012). Logistical and partial financial support to undertake the observations at Beauchene Island was provided by the South Atlantic Environmental Research Institute’s (SAERI) GAP project, funded by the Falkland Islands Government (FIG) and Falkland Islands Petroleum Licensees Association (FIPLA). Formal permits to carry out this research were received from the Falkland Islands Government. Many people helped with albatross tracking, particularly Miguel Lecoq, Deborah Pardo, Rafael Matias, Maria Dias, and Ana Almeida. New Island Conservation Trust and Wildlife Conservation Society allowed work to be carried out at their reserves in the Falklands.






