Introduction
Antisociality puts adolescents and young adults at high risk of developing a wide range of problems (Brazil, van Dongen, Maes, Mars, & Baskin-Sommers, Reference Brazil, van Dongen, Maes, Mars and Baskin-Sommers2018). However, there is large heterogeneity in the severity and type of antisociality across adolescence and young adulthood (Moffitt, Reference Moffitt2018). To better understand these individual differences, researchers related neurobiological measures such as autonomic nervous system (ANS) and neuroendocrinological functioning to antisociality (e.g. Alink et al., Reference Alink, van IJzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008; Dekkers et al., Reference Dekkers, van Rentergem, Meijer, Popma, Wagemaker and Huizenga2019; Portnoy & Farrington, Reference Portnoy and Farrington2015). However, diverse findings and limitations in study designs hinder firm conclusions on these biobehavioral associations across age. Therefore, we examined the continuous relation between multiple neurobiological measures and distinct dimensions of antisociality across the entire adolescent and young adult age range (9–27 years) by harmonizing six heterogeneous study samples (N = 1489).
A heightened tendency for antisociality has been associated with low arousal (sensation seeking hypothesis; Zuckerman, Reference Zuckerman1990) and a lack of fear (fearlessness hypothesis; Raine & Liu, Reference Raine and Liu1998). Lower resting heart rate (HR), a marker of low ANS activity, is often found to be associated with higher levels of aggression, delinquency, (violent) offending, and psychopathic traits, irrespective of age or sex (Portnoy & Farrington, Reference Portnoy and Farrington2015). In contrast, a higher respiration rate (RR) – a general measure of ANS functioning – was related to antisociality characterized by heightened emotionality and was more prominent in girls than in boys (and was higher in girls than in boys; Oldenhof et al., Reference Oldenhof, Prätzlich, Ackermann, Baker, Batchelor, Baumann and Popma2019). This fits well with models stating that callous-unemotional (CU) aggressive behavior is related to low arousal, while anxiety- or frustration-based aggression is related to high arousal (Blair, Reference Blair2013; Fanti, Reference Fanti2018).
In addition, studies on the parasympathetic branch of the ANS (promoting resting conditions) show that respiratory sinus arrhythmia (RSA) an index of PNS activity and a marker of self-regulation is lowered in antisocial youths (Beauchaine & Thayer, Reference Beauchaine and Thayer2015). This seems related to dysfunctions in emotion-regulation abilities. However, findings diverge between sexes, or studies included only boys, and non-significant findings have also been reported (Beauchaine, Hong, & Marsh, Reference Beauchaine, Hong and Marsh2008; Bimmel, Van IJzendoorn, Bakermans-Kranenburg, Juffer, & De Geus, Reference Bimmel, Van IJzendoorn, Bakermans-Kranenburg, Juffer and De Geus2008; De Vries-Bouw et al., Reference De Vries-Bouw, Popma, Vermeiren, Doreleijers, Van De Ven and Jansen2011; De Wied, Boxtel, Posthumus, Goudena, & Matthys, Reference De Wied, Boxtel, Posthumus, Goudena and Matthys2009; de Wied, van Boxtel, Matthys, & Meeus, Reference de Wied, van Boxtel, Matthys and Meeus2012; Marsh, Beauchaine, & Williams, Reference Marsh, Beauchaine and Williams2008; Oldenhof et al., Reference Oldenhof, Prätzlich, Ackermann, Baker, Batchelor, Baumann and Popma2019).
Findings on the sympathetic branch of the ANS (promoting fight or flight reactions) are also mixed depending on the type of antisociality studied. Lowered SNS (longer pre-ejection period, PEP) has been associated with conduct disorder, while heightened SNS (higher skin conductance levels, SCLs) with more grandiose-manipulative traits, with effects primarily found in adolescence, and less in childhood (Beauchaine, Gatzke-Kopp, & Mead, Reference Beauchaine, Gatzke-Kopp and Mead2007; Beauchaine, Katkin, Strassberg, & Snarr, Reference Beauchaine, Katkin, Strassberg and Snarr2001; MacDougall, Salekin, & Gillen, Reference MacDougall, Salekin and Gillen2019; Marsh et al., Reference Marsh, Beauchaine and Williams2008). However, non-significant findings have also been reported (van Zonneveld, Platje, de Sonneville, van Goozen, & Swaab, Reference van Zonneveld, Platje, de Sonneville, van Goozen and Swaab2017). Thus, findings on ANS functioning suggest specific associations with different aspects of antisociality, yet importantly, findings are inconclusive, mostly limited to males, and the role of age remains unclear.
Findings on neuroendocrinological measures and antisociality in youth also depend on the specific behavior examined. First, testosterone has been associated with aggression (although modestly), social dominance, impulsivity, and approach-related behaviors (Archer, Reference Archer2006; Carré & Archer, Reference Carré and Archer2018; Geniole et al., Reference Geniole, Bird, McVittie, Purcell, Archer and Carré2020; Peper, Braams, Blankenstein, Bos, & Crone, Reference Peper, Braams, Blankenstein, Bos and Crone2018; Rowe, Maughan, Worthman, Costello, & Angold, Reference Rowe, Maughan, Worthman, Costello and Angold2004), but there is limited evidence for associations with conduct disorder- or CU-like traits (e.g. Loney, Butler, Lima, Counts, & Eckel, Reference Loney, Butler, Lima, Counts and Eckel2006; but see Pajer et al., Reference Pajer, Tabbah, Gardner, Rubin, Czambel and Wang2006). Second, lower levels of basal cortisol or a lower cortisol awakening response (CAR) have been related to higher levels of externalizing behavior in children (but not adolescents, Alink et al., Reference Alink, van IJzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008). In adolescents, associations were found with psychopathic/CU-traits (Jambroes et al., Reference Jambroes, Jansen, Oostermeijer, Ven, Doreleijers, Vermeiren and Popma2019; Loney et al., Reference Loney, Butler, Lima, Counts and Eckel2006; but see Feilhauer, Cima, Korebrits, & Nicolson, Reference Feilhauer, Cima, Korebrits and Nicolson2013), persistent aggression (McBurnett, Lahey, Rathouz, & Loeber, Reference McBurnett, Lahey, Rathouz and Loeber2000; Platje et al. Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw and Meeus2013a; Yi-Zhen & Jun-Xia, Reference Yi-Zhen and Jun-Xia2009), and impulsivity (Feilhauer et al., Reference Feilhauer, Cima, Korebrits and Nicolson2013). However, these testosterone and cortisol studies too included mostly males, and findings diverge across age ranges. Finally, it has been suggested that high testosterone combined with low cortisol levels relate to status-seeking behaviors such as dominance (Mehta & Josephs, Reference Mehta and Josephs2010). A recent meta-analysis that focused on these behaviors, and in addition on risk taking, aggression, and psychopathy, only moderately supports this ‘dual-hormone hypothesis’ (Dekkers et al., Reference Dekkers, van Rentergem, Meijer, Popma, Wagemaker and Huizenga2019). It is currently unclear if this dual-hormone hypothesis applies to specific dimensions of antisociality across adolescence and young adulthood. This warrants a robust study on neuroendocrinological factors and antisociality across the entire adolescent age range in the context of multiple neurobiological measures.
In sum, although prior studies have laid the groundwork for biological underpinnings of antisociality in youth, many used case-control designs, narrow age ranges, and modest sample sizes. As a result, little is known about the relation between the severity of distinct dimensions of antisociality and neurobiological functioning throughout adolescence and young adulthood, ranging from typically developing individuals to individuals with severe antisociality. Indeed, recent frameworks call for a more dimensional approach to understand mental dysfunctions (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), which may eventually aid in the assessment of specific forms of antisocial behavior in practice (Glenn, Reference Glenn2019). Studying a large heterogeneous sample may therefore provide a deeper, fundamental insight into these dynamics.
The current study
We studied (1) effects of multiple neurobiological measures in unison (i.e. conjointly), (2) in a large heterogeneous sample, (3) across a broad age range, and (4) on multiple dimensions of antisociality. We combined six heterogeneous samples (1489 participants, combined age range = 9–27 years, 67% male) to examine associations between ANS and neuroendocrinological measures and antisociality across the entire adolescent and young adult age range. Because we set out to examine heterogeneity in antisociality throughout adolescence and young adulthood, we included individuals from populations with varying backgrounds in antisociality severity: adolescents from the general population, adolescents referred to a diversion program (i.e. due to minor delinquent acts), adolescents with a conduct disorder diagnosis, adolescents and young adults currently in closed youth care or detained in juvenile detention center due to severe antisocial behavior, and young adults characterized by a multitude of problems. The inclusion of these varied samples thus enables to study this heterogeneity. In this study, a prominent focus was thus to harmonize and find the optimal statistical approach for analyzing multiple datasets in order to robustly answer our research questions.
Our main goal was to uncover specific dimensions of antisociality and subsequently aimed to relate these to ANS measures [HR, RR, PEP (SNS), RSA (PNS), SCL (SNS)], testosterone, cortisol, and the CAR. We focused on resting measures, which give an index of the body's neurophysiological and neuroendocrinological attunement of the stress system. We included testosterone for its role in aggression and social-dominance-related behaviors. We expected that ANS measures would differentially relate to specific dimensions of antisociality (Portnoy & Farrington, Reference Portnoy and Farrington2015), whereas testosterone would relate to dimensions such as aggression and social dominance (Archer, Reference Archer2006; Dekkers et al., Reference Dekkers, van Rentergem, Meijer, Popma, Wagemaker and Huizenga2019). Finally, we expected that heightened cortisol functioning related to reduced psychopathic/CU-like traits (Loney et al., Reference Loney, Butler, Lima, Counts and Eckel2006) and heightened aggression (McBurnett et al., Reference McBurnett, Lahey, Rathouz and Loeber2000; Yi-Zhen & Jun-Xia, Reference Yi-Zhen and Jun-Xia2009). For each dimension of antisociality we tested associations with all neurobiological measures in unison, and tested the role of age and sex.
Methods and materials
Participants
Participants came from six samples of cross-sectional and longitudinal studies, collected in the Netherlands between 2002 and 2016. All studies were approved by their respective ethical committees and all participants and caregivers gave written informed consent. The complete sample included 1489 participants with a total of 2443 observations, due to the longitudinal nature of two of the samples (see below). Participants were between 9.00 and 27.18 years old (M age = 16.54, s.d.age = 2.39), and 67% were male (note that the age range of girls was limited to 18 years, warranting a cautious interpretation of related effects). Table 1 provides an overview of each sample's participants’ characteristics and descriptive statistics of the behavioral outcome variables and the neurobiological independent variables. Below we give a brief description of each sample (see also online Supplementary Appendix A and Table S1). Papers that include data that were used in the current study are indicated with an asterisk in the reference list. All samples included at least five neurobiological measures, and at least one self-report measure on antisociality.
Table 1. Descriptive statistics of the total sample and of each subsample

CU, callous-unemotional; HR, heart rate; PEP, pre-ejection period; RSA, respiratory sinus arrhythmia; RR, respiration rate; SCL, skin conductance level; Testos, testosterone; CAR AUCg, cortisol awakening response: area under the curve with respect to the ground; CAR AUCi, cortisol awakening response: area under the curve with respect to the increase.
a Sample includes two or more measurement waves. Descriptive statistics depicted here are collapsed across all measurement waves for parsimony.
Sample descriptions
Sample 1 included three annual waves of data of the longitudinal population sample RADAR-Y (Branje & Meeus, Reference Branje and Meeus2018). Sample 2 included two waves of data of male adolescents who were referred to a delinquency diversion program after having committed a minor offense, and non-delinquent controls (Popma et al., Reference Popma, Jansen, Vermeiren, Steiner, Raine, Van Goozen and Doreleijers2006, Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol and Doreleijers2007). Sample 3 consisted of adolescent girls and boys with conduct disorder and controls from the Dutch portion of a European multi-center study (Freitag, Reference Freitag2014; Oldenhof et al., Reference Oldenhof, Prätzlich, Ackermann, Baker, Batchelor, Baumann and Popma2019). Sample 4 consisted of adolescents (girls and boys) in a closed treatment facility for compulsory treatment due to severe antisocial behavior (Jambroes et al., Reference Jambroes, Jansen, Oostermeijer, Ven, Doreleijers, Vermeiren and Popma2019). Sample 5 included adolescent boys in juvenile justice institutions, referred because of severe behavioral problems or criminal offenses (de Ruigh, Jansen, Vermeiren, & Popma, Reference de Ruigh, Jansen, Vermeiren and Popma2019). Finally, sample 6 included multi-problem adolescent and young adult males who struggle with a variety of psychosocial problems and have a history of juvenile justice problems (Zijlmans et al., Reference Zijlmans, Marhe, Bevaart, Luijks, van Duin, Tiemeier and Popma2018, Reference Zijlmans, Bevaart, van Duin, Luijks, Popma and Marhe2019).
Self-report measures
To derive dimensions of antisociality we used the following self-report measures: the Reactive and Proactive Aggression questionnaire (RPQ; Raine et al., Reference Raine, Dodge, Loeber, Gatzke-Kopp, Lynam, Reynolds and Liu2006), the Youth Self-Report (YSR; Achenbach & Rescorla, Reference Achenbach and Rescorla2001), the Adult Self-Report (ASR; Rescorla & Achenbach, Reference Rescorla and Achenbach2004), and the Youth Psychopathic Index-short version (YPI-sv; van Baardewijk et al., Reference van Baardewijk, Andershed, Stegge, Nilsson, Scholte and Vermeiren2010). The RPQ assesses 23 proactive and reactive aggressive actions on a three-point Likert scale (0: never, 1: sometimes, 2: often; for example: ‘Got angry when I did not get my way’). For the YSR and ASR we included 31 and 29 items of aggressive and rule-breaking behaviors, respectively, which are scored on a three-point Likert scale (0: never, 1: sometimes, 2: often; for example: ‘I don't keep by the rules at school/work or somewhere else’). Finally, the YPI-sv assessed 18 psychopathic traits, on a four-point Likert scale (ranging from 0: Does not apply at all, to 3: Applies very well; for example: ‘It's easy for me to manipulate people’).
Importantly, these questionnaires were not administered in each sample, but there was overlap in used items from the questionnaires across samples (see online Supplementary Table S1), and our goal was to study associations between broad dimensions of antisociality and neurobiology. Therefore, we entered all raw item scores – of all samples together – in a principal component analyses (PCA)-like method [coupled matrix factorization (CMF)] that took the overlap in questionnaires (items) between samples into account. As such, broad dimensions of antisociality were derived in a data-driven way rather than including pre-defined subscales of these questionnaires in only a portion of the samples. Importantly, this technique derived antisociality measures representing the same broad dimensions across the different samples (see section ‘Data analyses’).
Neurobiological measures
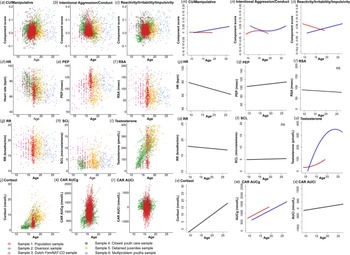
Neurobiological measures included resting HR, PEP, RSA, RR, SCLs, testosterone levels, cortisol, and the CAR. Specific data collection procedures and analysis methods for each sample can be found in the original respective papers (indicated with an asterisk in the reference list). In the online Supplementary materials (Appendix B), we briefly describe assessment protocols across samples. Online Supplementary Table S1 depicts data availability of the samples, and Fig. 1(d–l) depicts raw observed data of the neurobiological measures across age, showing considerable between-subjects heterogeneity.

Fig. 1. (a)–(l) Observed data of the behavioral dimensions of antisociality across age, before multiple imputations, for CU/manipulative traits, intentional aggression/conduct, and reactivity/irritability/impulsivity, and the neurobiological measures. The different colors indicate the different samples. The data show considerable heterogeneity. (m)–(x) Results of the general linear models with clustered bootstraps for the models testing age (linear and quadratic) and sex effects across 100 imputed datasets, for CU-traits/manipulative aggression, intentional aggression/conduct, and reactivity/irritability/impulsivity and the neurobiological measures. The blue line represents males and the red line represents females. Note that the age overlap for girls and boys is limited to 13–18 years, therefore the developmental patterns can only be compared with caution. HR, heart rate; PEP, pre-ejection period; RSA, log-transformed respiratory sinus arrhythmia RR, respiration rate; SCL, skin conductance level; CAR AUCg, cortisol awakening response area under the curve with respect to the ground; CAR AUCi, cortisol awakening response, area under the curve with respect to the increase.
Data analyses
We used three sets of analyses, which we briefly summarize here and are further described in Appendix C of the online Supplementary materials. Code is available via https://osf.io/qn95r/. It is important to note that in these analyses – in which we combined and analyzed the datasets – we did not control for sample. This was done to not remove any interesting variance between the samples, i.e. by controlling for sample, one removes heterogeneity. First, to derive broad general dimensions of antisociality from the – partially overlapping – questionnaires assessed across the samples, we used coupled matrix factorization (CMF) (Sorber, Van Barel, & De Lathauwer, Reference Sorber, Van Barel and De Lathauwer2015; Van Deun, Smilde, Van Der Werf, Kiers, & Van Mechelen, Reference Van Deun, Smilde, Van Der Werf, Kiers and Van Mechelen2009). CMF reduces the items across all questionnaires from all samples – even when not all questionnaires (items) are administered in each sample – to their underlying dimensions. We tested for a one-, two-, three-, and four-component solution and retained the solution that optimally balances model fit (i.e. sum of squared residuals) and model complexity (i.e. the number of components). The items' content and the component loadings of the chosen solution were used to label each component and to link each component to an underlying dimension of antisociality. We used a varimax rotation to ensure a clear structure of the loadings of each item per component (i.e. simple structure), thus facilitating interpretation of the components. Components were allowed to correlate, as they all reflect dimensions of antisociality.
Next, we applied multiple imputations to deal with missing neurobiological data (Rubin, Reference Rubin2004; Van Buuren & Groothuis-Oudshoorn, Reference Van Buuren and Groothuis-Oudshoorn2010). The data structure and availability of the variables across the samples are depicted in online Supplementary Table S1, and details of the multiple imputation procedure are outlined in online Supplementary Appendix C. The data were imputed 100 times. Each of the 100 imputed datasets has a rather complicated structure where some parts have a nested structure (i.e. multiple waves within persons; samples 1 and 2) and other parts do not have such a nested structure (samples 3–6). To deal with this complexity, we applied a general linear model with clustered bootstrap that takes into account dependency in parts of the data (GLMCB; Deen & de Rooij, Reference Deen and de Rooij2019). This technique is better able to deal with this partial dependency than multilevel/linear mixed effects models, a common technique applied to nested data. An additional advantage of the clustered bootstrap approach is that almost no distributional assumptions need to be satisfied for the data (and the model residuals).
Model specification
We calculated quasi-likelihood information criteria (QIC) values (Pan, Reference Pan2001) for different models for each of the 100 imputed datasets. QIC values indicate model fit and lower values indicate a better fitting model. We compared the resulting 100 QIC values between the models via box plots. The model with the lowest QIC values across the 100 imputed datasets was retained as the best-fitting model. In the case of equal model fits, the simplest model was chosen. The best-fitting model was subsequently entered in a GLMCB, using 100 bootstrap samples for each imputed dataset. To obtain the estimated parameter value, we took the average of the 100 estimates from the 100 imputed datasets (Rubin, Reference Rubin2004). To obtain a confidence interval we used the percentile approach on the 100 × 100 estimated values so that both the variance within the imputed datasets as well as the variance between the imputed datasets was taken into account (van Ginkel & Kiers, Reference van Ginkel and Kiers2011). If zero was not included in the confidence interval, the effect was considered significant (at α = 0.05).
The models that were compared to describe age and sex patterns of all measures are specified in online Supplementary Table S2 (upper half). Using QIC values we compared models with age as a linear effect, age as a quadratic polynomial, and models including an interaction between sex and these age effects. Our main hypotheses concerned the associations of the neurobiological measures with the dimensions of antisociality, and the role of age and sex. Using QIC values, for each dimension we compared a model with the main effects of neurobiological measures only, with models additionally including age as a linear effect, age as a quadratic polynomial, and models including an interaction between sex and these age effects. These model specifications are depicted in online Supplementary Table S2 (lower half). When there was an age effect, we additionally compared different models in which age interacted with the neurobiological measures.
Results
Disclosing dimensions of antisociality through CMF
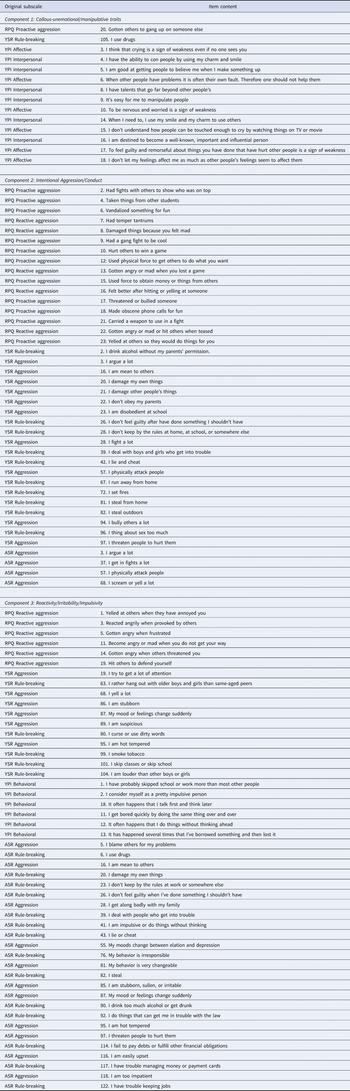
The CMF analysis showed that the three-component solution was most appropriate as it optimally balanced model fit and model complexity. In particular, although being more complex, the three-component solution (loss = 31 920, explained variance of 56.9%) fitted the data clearly better than the one- (loss = 36 459, 50.8%) and two-component (loss = 33 965, 54.2%) solutions. The four-component solution fitted the data better (loss = 30 394, 58.9%) but not so much better to warrant the added complexity. Moreover, the three-component solution was preferred over the four-component solution as the items loaded relatively unambiguously on the three components, whereas none of the items had a highest loading on the fourth component, which explained only 5.74% of the variance. Online Supplementary Fig. S1A shows, for each pair of the three components, the loadings (after varimax rotation) of each item on their respective components and illustrates the separation of the items based on the component loadings. The three-component solution explained 56.9% of the variance in the data, with all three components being more or less of equal importance (i.e. each component explains more or less the same amount of variance in the data). Online Supplementary Table S2 gives an overview of explained variances per solution, Table 2 provides a complete list of the items loading substantially on each component for the chosen three-component solution, and depicts the items' original subscales, and online Supplementary Table S4 additionally shows the item loadings for the one-, two-, and four-component solutions.
Table 2. Raw items per component and original subscales

RPQ, Reactive Proactive Aggression Questionnaire; YPI, Youth Psychopathic Trait Index (short version); YSR, Youth Self Report; ASR, Adult Self Report.
After inspection of the loadings of each item on the components and the item content, we labeled the components:
Callous-unemotional/Manipulative traits: included items such as ‘To be nervous or worried is a sign of weakness’, ‘I have talents that go far beyond other people's’, ‘I don't let my feelings affect me as much as other people's feelings seem to affect them’.
Intentional aggression/conduct: included items such as ‘Had fights with others to show who was on top’, ‘I physically attack people’, ‘I bully others a lot’.
Reactivity/irritability/impulsivity: included items such as ‘Gotten angry when frustrated’, It often happens that I do something without thinking about it’, ‘I am stubborn, sullen, or irritable’.
The varimax-rotated participant scores on these components were used in the subsequent analyses. The components were only moderately and negatively interrelated. To give some indication on how the components relate to subscales the raw items were originally derived from, online Supplementary Fig. S1B shows a Pearson correlation plot. However, note that these correlations are biased, because items of these subscales partly make up the components, and correlations are limited to participants who completed these questionnaires in the original studies. Finally, Fig. 1(a–c) shows the raw data of the component scores across age and samples, showing considerable heterogeneity in these behavioral measures. This set the stage for our main analyses.
Development of behavioral outcome measures
Here, we describe the developmental patterns of our behavioral outcome measures, i.e. the components derived from the CMF. Test statistics are summarized in Table 3 and effects are visualized in Fig. 1(m–o). Recall that we evaluated model fits by comparing the QIC values per multiply imputed dataset via box plots (online Supplementary Fig. S2). The model with the lowest QIC values across the 100 imputed datasets was retained as the best-fitting model. It should be noted that the age overlap for girls and boys is limited, therefore these developmental patterns should be compared with caution.
Table 3. Results of the best age models of each variable
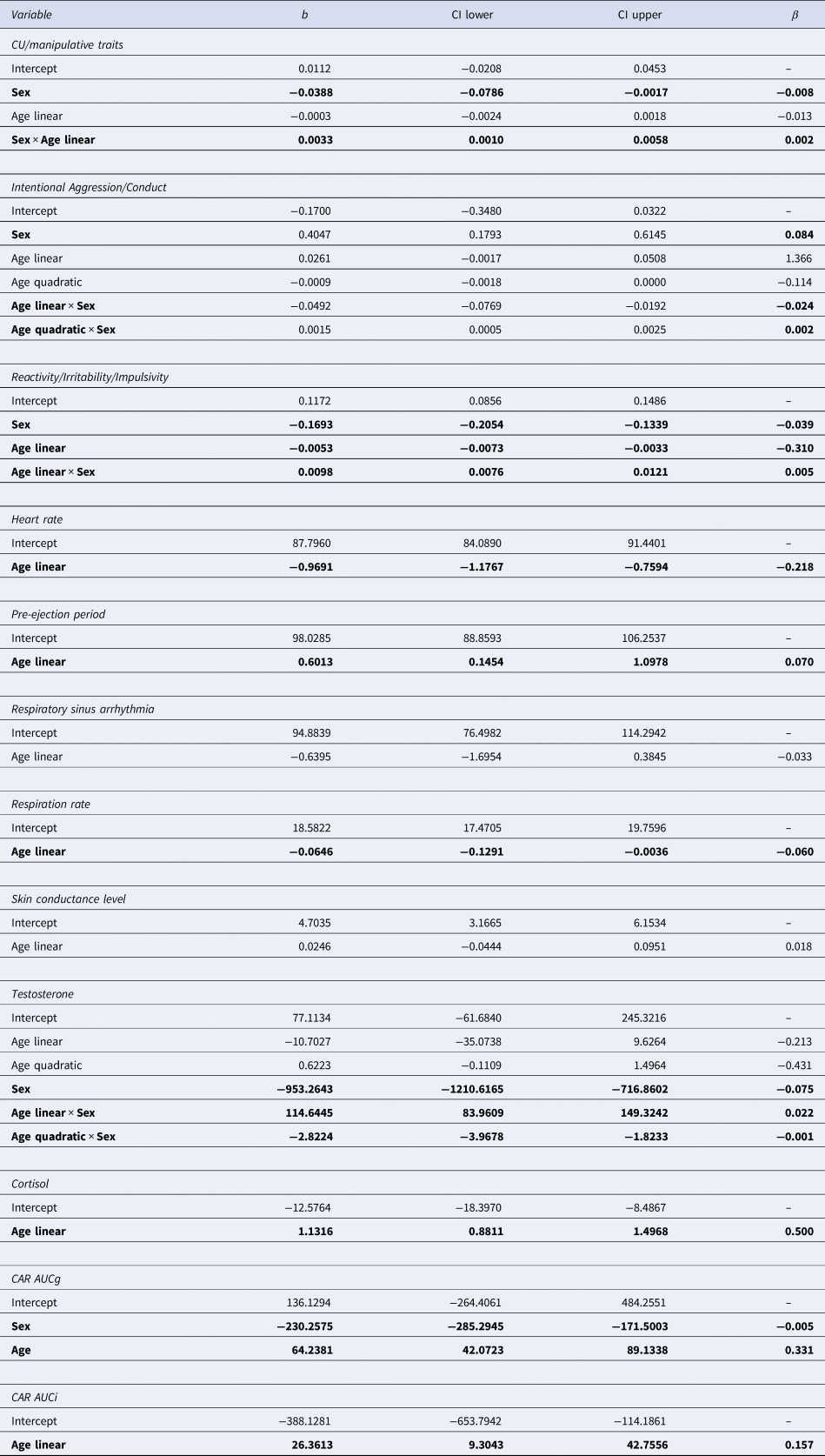
CI, confidence interval; CAR AUCg, cortisol awakening response, area under the curve with respect to the ground; CAR AUCi, cortisol awakening response, area under the curve with respect to the increase.
Variables in the first column indicate the included independent variables based on the model selection via QICs. Significant effects (α = 0.05) are in bold.
First, CU/manipulative traits was best described by a model including the interaction between (linear) age and sex (i.e. CU/manipulative = Age × Sex). For girls, this dimension appeared stable (but note the limited age range until 18 years), while for boys an increase from until 27 years was observed. Intentional aggression/conduct was best described by a quadratic age by sex interaction effect (i.e. intentional aggression/conduct = Age2 × Sex). Here, reactive aggression peaked around 14 years for girls, while for boys, a quadratic dip was observed around 18–19 years. Finally, reactivity/irritability/impulsivity showed a linear age by sex interaction effect (i.e. reactivity/irritability/impulsivity = Age × Sex). Across age this measure decreased for girls while it increased for boys.
Development of neurobiological measures
Observed data of neurobiological measures across age, before multiple imputations, are shown in Fig. 1d–l. Results of the GLMCBs on the imputed datasets are visualized in Fig. 1p–x and summarized in Table 3. ANS measures were best described by models including age linear only (see online Supplementary Fig. S2 for QIC values across multiply imputed datasets, lower values indicate a better fit). While HR and RR decreased linearly with age, PEP increased with age, and RSA and SCL remained stable across age. Testosterone was best described as a model including a quadratic age by sex interaction effect, but note the limited age range in girls. Testosterone levels increased steeply for boys and leveled off into young adulthood. For girls, testosterone levels increased moderately during puberty. Basal cortisol and cortisol awakening response, area under the curve with respect to the increase (CAR AUCi) were best described by models including a linear age term only and increased across age, while cortisol awakening response, area under the curve with respect to the ground (CAR AUCg) showed an additional main effect of sex (girls > boys). These analyses across the imputed dataset show that the development of these neurobiological measures were in the expected direction and support the use of these imputed measures for our main analyses.
Relation between neurobiological measures and dimensions of antisociality
Next, we tested associations between neurobiological measures and the antisociality dimensions. Online Supplementary Fig. S3 shows a correlation plot of all measures across imputed datasets. These include zero-order correlations and partial correlations controlling for age and sex. These exploratory correlations indeed suggest meaningful associations between neurobiological measures and our dimensions of antisociality, which we formally tested using GLMCB. Results of the GLMCB models are summarized in Table 4 and significant effects are visualized in Fig. 2. Recall that we inspect QIC values across multiple imputed datasets to assess which model best fitted the data. QIC values are visualized in online Supplementary Fig. S4, and lower values indicate a better fit.

Fig. 2. Results of the general linear models with clustered bootstraps for the biobehavioral models. Displayed are significant associations between dimensions of antisociality and neurobiological measures. PEP, pre-ejection period; RR, respiration rate; CAR AUCi, cortisol awakening response, area under the curve with respect to the increase (i.e. cortisol awakening reactivity).
Table 4. Results of the bootstrapped models relating the antisociality dimensions to the neurobiological measures

CI, confidence interval; HR, heart rate; PEP, pre-ejection period; log_RSA0, log-transformed respiratory sinus arrhythmia; RR, respiration rate; SCL, skin conductance level; CAR AUCg, cortisol awakening response, area under the curve with respect to the ground; CAR AUCi, cortisol awakening response, area under the curve with respect to the increase.
Variables in the first column indicate the included independent variables based on the model selection via QICs. Significant effects (α = 0.05) are in bold.
First, QIC values showed that CU/manipulative traits were best described by a model with neurobiological main effects only (i.e. the model: CU/manipulative = HR + PEP + log_RSA0 + RR + SCL + Testosterone + Cortisol + AUCg + AUCi; see online Supplementary Fig. S4A, left panel). After bootstrapping this model, we observed significant effects of PEP, testosterone, and CAR AUCi. Here, shorter PEP, more testosterone, and greater cortisol awakening reactivity response were related to higher levels of CU/manipulative traits (see Fig. 2a–c).
Second, intentional aggression/conduct was also best described by a model with neurobiological main effects only (i.e. intentional aggression/conduct = HR + PEP + log_RSA0 + RR + SCL + Testosterone + Cortisol + AUCg + AUCi, online Supplementary Fig. S4B, left panel). The bootstrapped model showed that a higher AUCi (cortisol awakening reactivity) was significantly related to higher levels of reactive/frustration-based aggression (see Fig. 2d). QIC values in online Supplementary Fig. S4B suggest that the model including an Age2 × Sex interaction may marginally outperform the model with neurobiological main effects only. Bootstrapping this model (HR + PEP + log_RSA0 + RR + SCL + Testosterone + Cortisol + AUCg + AUCi + Age2 × Sex) showed that the effect of CAR AUCi remained significant (with β = 0.141). Thus, the effect of CAR AUCi on intentional aggression/conduct remained robust when controlling for the Age2 × Sex interaction effect.
Third, reactivity/irritability/impulsivity was best described by a model including the main effects of the neurobiological measures, as well as a (linear) age by sex interaction effect (i.e. reactivity/irritability/impulsivity = HR + PEP + log_RSA0 + RR + SCL + Testosterone + Cortisol + AUCg + AUCi + Age1 × Sex). Models including interactions between neurobiological measures and age and sex did not indicate a better fit. After bootstrapping the best model, we observed that only effects of sex, age, and their interaction were significant. Here, reactivity/irritability/impulsivity values increased for boys and decreased for girls.
Finally, to add to existing literature on the dual-hormone hypothesis, we also explicitly examined whether models including testosterone-by-cortisol interactions improved model fits. This was not the case: for none of the antisociality components did QIC values indicate a better fit when including any of the possible testosterone-by-cortisol interactions (i.e. testosterone × cortisol, testosterone × CAR AUCg, testosterone × CAR AUCi; see online Supplementary Fig. S4, right panels for QIC box plots).
Discussion
This multi-sample study is unique in that we were able to examine associations between multiple neurobiological measures conjointly in 1489 participants ranging from none to severe antisocial behavior problems across the entire adolescent age range (9–27 years, 67% male). Three dimensions of antisociality emerged: CU/manipulative traits, intentional aggression/conduct, and reactivity/irritability/impulsivity, which showed considerable heterogeneity. Our main analyses revealed that (1) more CU/manipulative traits related to shorter PEPs, higher levels of testosterone, and higher cortisol awakening reactivity, independent of age and sex; (2) higher intentional aggression/conduct related to higher cortisol awakening reactivity, independent of age and sex; and (3) reactivity/irritability/impulsivity was explained by age and sex only, in which a decrease across age was found for girls and an increase across age for boys.
To derive broad general dimensions of antisociality that are consistent across the different samples, we used CMF. This is a PCA-like method that can deal with multiple samples by assuming overlap in items between samples. A solution with three dimensions was retained which reflected CU and manipulative, intentional aggressive, and reactive and irritable behaviors. These dimensions resonate with existing models on (adolescent) antisocial behavior, which also consider similarly differentiated aspects of antisociality (e.g. Blair, Reference Blair2013) and prior factor analyses (although on aggression specifically) in adolescents (Smeets et al., Reference Smeets, Oostermeijer, Lappenschaar, Cohn, Van der Meer, Popma and Buitelaar2017), and adults (Van Donkelaar et al., Reference Van Donkelaar, Hoogman, Shumskaya, Buitelaar, Bralten and Franke2020). This indicates that CMF results in meaningful components which could be used in subsequent analyses.
Our developmental analyses showed that CU/manipulative traits increased moderately with age for boys, and was stable for girls. An important caveat is the limited overlap in age ranges between boys (10–27) and girls (9–18 years), therefore these comparisons have to be interpreted with caution. Prior research indicates that CU-traits are relatively stable from early childhood on, but primarily for those individuals with elevated CU-traits (Frick, Ray, Thornton, & Kahn, Reference Frick, Ray, Thornton and Kahn2014). Our sample was more heterogeneous as it included a wider range of individuals, ranging from typically-developing participants to participants with moderate to severe problem behavior. The developmental pattern for intentional aggression/conduct fits well with prior study on the development of physical aggression and violence (Tremblay, Reference Tremblay2010), although overrepresentation of youths with problem behavior in late adolescence/young adulthood may in part explain the increase for males in late adolescence. Finally, reactivity/irritability/impulsivity decreased for girls and increased for boys. Prior research in typical development (including both sexes) have shown both decreases (Harden & Tucker-Drob, Reference Harden and Tucker-Drob2011), increases, and adolescent peaks (Peper et al., Reference Peper, Braams, Blankenstein, Bos and Crone2018) in impulsivity measures, while irritability remains stable across adolescence (Brotman, Kircanski, & Leibenluft, Reference Brotman, Kircanski and Leibenluft2017; Caprara, Paciello, Gerbino, & Cugini, Reference Caprara, Paciello, Gerbino and Cugini2007). Our dimension covers both impulsivity as well as irritability. Therefore, future research should confirm the developmental pattern of this construct.
Most neurobiological measures showed expected developmental patterns. HR, PEP, and RR all decreased with age, confirming prior research. Although RSA also decreased linearly with age, in line with prior research, this effect did not attain significance. SCL was stable throughout adolescence and young adulthood, which extends prior research in childhood and early-middle adolescence (El-Sheikh, Reference El-Sheikh2007). Furthermore, basal cortisol levels and the CAR also increased with age (Gunnar, DePasquale, Reid, & Donzella, Reference Gunnar, DePasquale, Reid and Donzella2019; Kiess et al., Reference Kiess, Meidert, Dressendörfer, Schriever, Kessler, Köunig and Strasburger1995; Oskis, Loveday, Hucklebridge, Thorn, & Clow, Reference Oskis, Loveday, Hucklebridge, Thorn and Clow2009; Platje et al., Reference Platje, Vermeiren, Branje, Doreleijers, Meeus, Koot and Jansen2013b). Finally, testosterone showed the expected increase for boys, and to a lesser extent for girls (Hiort, Reference Hiort2002; Peper et al., Reference Peper, Braams, Blankenstein, Bos and Crone2018). In sum, these findings confirm existing knowledge on neurobiological development, and extend these findings by robustly documenting the developmental pathways across the entire adolescent and young adult age range in a heterogeneous sample.
Our main focus was on associations between neurobiological measures and dimensions of antisociality. First, we found increased SNS activity (specifically, shorter PEP) and hypothalamus–pituitary–adrenal (HPA)-axis functioning (specifically, a higher CAR) to be related to increased CU/manipulative traits. Other research reported blunted – rather than heightened – SNS activity with psychopathic traits, although these studies focused on youths with problem behaviors (Fanti, Reference Fanti2018; Fanti et al., Reference Fanti, Eisenbarth, Goble, Demetriou, Kyranides, Goodwin and Cortese2019). Yet, other research does report heightened SNS functioning (although reflected in higher SCL) in adolescent males with – specifically – more grandiose-manipulative traits (MacDougall et al., Reference MacDougall, Salekin and Gillen2019). Our dimension reflected both CU as well as (grandiose-)manipulative aspects. Moreover, our study included a wide range of individuals, ranging from typically developing adolescents to adolescents with more moderate to severe problems. Findings may differ when examining a wide range of individuals rather than focus on specific subgroups. Alternatively, the low arousal theory (e.g. Raine & Liu, Reference Raine and Liu1998; Zuckerman, Reference Zuckerman1990), suggesting blunted SNS and HPA-axis activation, may only be pronounced in subgroups of individuals characterized by severe antisociality. In our exploratory analyses limited to the samples that could be characterized by more severe antisociality we did find negative associations between cortisol and Intentional aggression/conduct and between RR and reactivity/irritability/impulsivity (see online Supplementary materials p. 19), but not with CU/manipulative traits. Because examining biobehavioral associations in such subgroups in particular was not the goal of this study, future studies better equipped to define and select individuals with ‘severe’ antisociality, may formally address this research question. Instead, the current findings add to the literature by yielding a deeper understanding of these fundamental biobehavioral dynamics across a large heterogeneous sample.
Furthermore, a pronounced finding was that testosterone related positively CU/manipulative traits. This is in line with prior research on behaviors related to social dominance (Archer, Reference Archer2006; Carré & Archer, Reference Carré and Archer2018). However, no evidence was found for the dual-hormone hypothesis (Mehta & Josephs, Reference Mehta and Josephs2010) on any of the dimensions of antisociality. The dual-hormone hypotheses suggest that a combination of low levels of cortisol combined with high levels of testosterone is related to higher levels of status-seeking behaviors, such as dominance. A recent meta-analysis (Dekkers et al., Reference Dekkers, van Rentergem, Meijer, Popma, Wagemaker and Huizenga2019) found modest evidence for this hypothesis for status, dominance, risk taking, aggression, and psychopathy. We add to existing research in showing that the dual-hormone effect is absent across adolescent and young adult heterogeneous development for these dimensions of antisociality, in the context of multiple neurobiological measures.
Furthermore, a higher CAR was also related to more intentional aggression/conduct, and this effect was slightly stronger than for CU/manipulative traits. This fits well with models suggesting that increased threat sensitivity gives rise to this dimension of antisociality (Blair, Reference Blair2013) through hyperactivity of the ANS and HPA-axis (Fanti, Reference Fanti2018; Oldenhof et al., Reference Oldenhof, Prätzlich, Ackermann, Baker, Batchelor, Baumann and Popma2019). Finally, reactivity/irritability/impulsivity was not related to any of the neurobiological measures, although prior research did find associations between impulsivity and testosterone in typically developing adolescents (Peper et al., Reference Peper, Braams, Blankenstein, Bos and Crone2018) and with cortisol in detained adolescents (Feilhauer et al., Reference Feilhauer, Cima, Korebrits and Nicolson2013). The current study adds to this literature by showing absent associations across a heterogeneous sample, and extends prior research by covering aspects of both irritability and impulsivity.
One of our key aims was to test these associations across the entire adolescent age range. We found that these neurobiological associations were stable throughout adolescence and young adulthood and across both sexes. Indeed, prior research shows developmentally stable and sex-independent associations (Portnoy & Farrington, Reference Portnoy and Farrington2015). We show that this holds for multiple SNS and PNS measures. Regarding the CAR, our findings confirm a meta-analysis showing sex-independent associations, but diverge from this meta-analysis showing an absent association in adolescence (Alink et al., Reference Alink, van IJzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008). However, other, longitudinal, research did find associations between the CAR and CU-traits in adolescents (Jambroes et al., Reference Jambroes, Jansen, Oostermeijer, Ven, Doreleijers, Vermeiren and Popma2019; Loney et al., Reference Loney, Butler, Lima, Counts and Eckel2006). Future research should confirm these findings, preferably within a longitudinal study from childhood to early adulthood, which allows us to capture within-person developmental changes.
In contrast to prior research, we found no associations with HR, RSA, SCL, basal cortisol, and CAR AUCg (i.e. total cortisol during wakening). Possibly, the inclusion of multiple other ANS and hormonal measures resulted in these particular measures accounting for little additional variance. Importantly, our findings underscore that multiple neurobiological measures should be included, which yields more specific information about the contribution of indices of ANS and HPA-axis functioning (Alink et al., Reference Alink, van IJzendoorn, Bakermans-Kranenburg, Mesman, Juffer and Koot2008; Oldenhof et al., Reference Oldenhof, Prätzlich, Ackermann, Baker, Batchelor, Baumann and Popma2019), and that specifically PEP, testosterone, and cortisol awakening reactivity play a pivotal role in explaining distinct dimensions of antisociality.
Strengths, limitations, and future directions
This study has a number of strengths, such as a robust, heterogeneous, and large combined sample size spanning a broad age range, and multiple neurobiological measures. Despite sample differences in the items used and data availability, we could combine and analyze these samples using a sophisticated set of analyses: CMF, multiple imputations, and GLMCB. This combination may be promising for future studies wishing to combine different datasets to increase sample size and robust findings. With the transition toward open science, data-harmonization techniques become increasingly important. Moreover, the current data-driven approach yields more insights into underlying components and mechanisms of antisociality. Insight into fundamental concepts and mechanisms underlying antisociality may subsequently inform clinicians in understanding the origins of antisocial behavior. Future studies may focus on relating these mechanisms to (preventive) interventions (e.g. see de Ruigh et al., Reference de Ruigh, Bouwmeester, Popma, Vermeiren, van Domburgh and Jansen2021).
Despite these strengths, this study also had some limitations. First, sex was not equally distributed across age because of the sampling of the original studies. Conclusions about developmental trajectories for girls are thus limited to a less broad age range. Relatedly, in late adolescence/young adulthood, typically developing controls were underrepresented. Future research should strive for an equal distribution of males and females, and participants from various backgrounds, across all developmental phases. Furthermore, although factor loadings differentiated the three dimensions of antisociality, it should be noted that the dimensions were small-to-moderately negatively correlated. This may have had consequences for the interpretation of the three separate regression models (one for each dimension), which we interpreted independently. A solution would be to run a multivariate model, but this may lead to a less conservative analysis and a more complex – and thus less interpretable – model. Finally, for our behavioral measures we included self-report measures only. An opportunity for future research is to include multiple informants (e.g. self-, teacher-, and parent reports), as well as juvenile-justice registrations, psychosocial stress and emotion regulation tasks, and psychosocial factors such as socio-economic status, substance use, the influence of (delinquent) peers, and trauma (Moffitt, Reference Moffitt2018). Such a comprehensive biopsychosocial perspective of multifaceted antisociality may give rise to person-based predictions of an individual's sensitivity to intervention.
Conclusions
This study is the first to examine associations between multiple dimensions of antisociality and multiple neurobiological measures conjointly in such a large, heterogenous sample across the full adolescent and young adult age range. We found that CU/manipulative traits were related to higher arousal (reflected in higher SNS), and higher levels of testosterone, whereas cortisol awakening reactivity was consistently related to both CU/manipulative traits and intentional aggression/conduct. These findings deepen our understanding of – developmentally stable – neurobiological correlates of antisociality components. Finally, this study also highlights the potential of using the current techniques to harmonize existing datasets, to optimize data use of unique populations, and for robust analyses. Together, this study yields fundamental insights into underlying components and mechanisms of antisociality across adolescence and young adulthood.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721003457.
Acknowledgements
We thank all participants for taking part in the individual studies, and all those who assisted in data collection.
Author contributions
NEB: data curation, formal analysis (multiple imputations, CMF and GLMCB), visualization, writing – original draft, writing – review and editing; MdR: formal analysis (GLMCB), writing – original draft (methods and results); writing – review and editing (methods and results); JvG: formal analysis (multiple imputations), writing – original draft (methods and results); writing – review and editing (methods and results); TW: formal analysis (CMF), writing – original draft (methods and results); writing – review and editing (methods and results); EdR, HO, JZ, TJ, EP, and MdVB: investigation; SB, WM, RV, and AP: conceptualization (of original studies), investigation, writing – review and editing; LJ: conceptualization, investigation, writing – review and editing, supervision, funding acquisition. All authors approved the final version of the manuscript.
Financial support
This research was supported by The Netherlands Organization for Scientific Research (NWA Startimpuls 400.17.602).
Conflict of interest
None to declare.