Introduction
Immunoglobulin G4 (IgG4) related disease is an inflammatory condition characterised by the presence of ill-defined fibrotic lesions which have an IgG4-positive plasma cell infiltrate. Hamano et al. first described patients with fibrotic pseudotumours in the pancreas and elevated serum concentrations of IgG4.Reference Hamano, Kawa, Horiuchi, Unno, Furuya and Akamatsu1 Immunoglobulin G4 related disease is increasingly being recognised, although it remains a rare disease entity.
Immunoglobulin G4 related disease often affects the head and neck, with reported cases involving the orbital adnexa, paranasal sinuses, major salivary glands, thyroid, mastoid and lymph nodes.Reference Zen and Nakanuma2–Reference Bhatti and Stelow4 Mass lesions arising in the pterygopalatine or infratemporal fossae, although uncommon, pose considerable challenges in terms of diagnosis and management. Traditionally, these areas were approached via open techniques which were often associated with significant morbidity. Advances in endoscopic sinus surgery over the past decade have allowed transnasal access to this area with greater precision and with fewer complications.
Case reports
Case one
A 62-year-old Chinese woman presented to an ophthalmologist with a 5-year history of progressively worsening left retro-orbital pain and altered sensation over her left cheek. Visual acuity was normal. Hertel exophthalmometry values were 17 mm (left) and 15 mm (right). Extraocular muscle movements were painless, with restricted abduction of the left eye upon lateral gaze. Fundoscopy was normal bilaterally. Hypoaesthesia was demonstrated over the cutaneous distribution of the left maxillary nerve.
A contrast-enhanced magnetic resonance imaging (MRI) scan demonstrated a homogeneously enhancing mass lesion centred in the left pterygopalatine fossa that extended into the infratemporal fossa and inferolateral extraconal space (Figure 1).

Fig. 1 Axial, T1-weighted, post-contrast magnetic resonance images of case one, showing: (a) a view of the enhancing lesion in the left extraconal space (arrow), and (b) homogeneous, diffuse enhancement in the left pterygopalatine fossa extending into the infratemporal fossa (arrow).
The patient was referred to the otolaryngology service for endoscopic, transnasal biopsy of the lesion. However, she subsequently returned to China to obtain a second opinion. When she returned to New Zealand a month later, there had been an increase in left retro-orbital pain and a new onset of blurred vision in the left eye.
On examination, her visual acuity had deteriorated to 6/30 in the left eye and was normal in the right eye. Hertel measurements were 20 mm in the left eye and unchanged in the right eye.
Repeat orbital computed tomography (CT) showed increasing involvement of the left orbital apex and the inferior aspect of the left orbit. The patient was commenced on oral prednisone (60 mg daily).
The patient subsequently underwent an image-guided, endoscopic, transnasal biopsy of the pterygopalatine and infratemporal fossae mass. The posterior maxillary wall was opened and the infratemporal fossa was exposed, revealing a densely fibrotic lesion. Multiple biopsies were taken of the fibrotic tissue within the infratemporal fossa.
Histological examination revealed densely sclerotic fibrous tissue with lymphoplasmacytic infiltration and obliterative phlebitis (Figure 2). Subsequent immunostaining was positive for IgG4. A diagnosis of IgG4-related disease was made. Serum IgG4 concentration was within the normal limit (0.05 g/l; normal range 0.04–0.90 g/l).

Fig. 2 Histopathological slides for: (a) case one, showing lymphoplasmacytic infiltrate, obliterative phlebitis and storiform fibrosis (H&E; ×50), and (b) case two, showing immunoglobulin G4 (IgG4) positive plasma cell infiltrate (immunostaining with anti-IgG4 antibodies; ×400).
At three months' follow up, the patient's visual acuity had improved to 6/9 bilaterally. The prednisone dose was reduced by 10 mg every fortnight with no recurrence of visual loss.
Case two
A 69-year-old man presented with the symptoms of nasal congestion, right ocular pain and altered sensation over the right cheek, which had gradually worsened over several years. Endoscopic examination revealed polyps in the right middle meatus. A CT scan showed rarefaction of the right lamina papyracea and right fovea ethmoidalis (Figure 3). An MRI scan subsequently showed soft tissue changes within the right medial orbit, the dura adjacent to the fovea and the infratemporal fossa (Figure 4).

Fig. 3 Coronal (non-contrast) computed tomography image of case two, showing dehiscence of the right lamina papyracea and ethmoid roof (arrows).

Fig. 4 T1-weighted, post-contrast magnetic resonance images of case two, showing: (a) diffuse enhancement in the right infratemporal fossa (arrow) (axial view), and (b) the enhancing lesion in right medial orbit and dura adjacent to the ethmoid roof (coronal view).
Endoscopic sinus surgery was performed but the initial biopsy specimen was reported as showing typical inflammatory polyps. The autoimmune antibody screen was normal, as were serum immunoglobulin and C-reactive protein levels.
The patient's symptoms improved after surgery and a course of prednisone. However, the symptoms returned and worsened. On two further occasions, the polyps were biopsied but the histology was again consistent with inflammatory polyps. The symptoms worsened, particularly the headache and eye pain. Treatment with a longer course of prednisone was commenced, with some improvement but not resolution.
A transnasal endoscopic biopsy of the abnormal tissue within the right infratemporal fossa was performed. The tissue was fibrous, and immunohistochemical staining of IgG4 confirmed the diagnosis of IgG4-related disease (Figure 2).
Discussion
Immunoglobulin G4 related disease is an increasingly recognised disorder characterised by the development of pseudoneoplastic lesions in affected organs. Histopathological findings of lymphoplasmacytic infiltrate by IgG4-positive plasma cells on a background of fibrosis are pathognomonic of this disorder.Reference Cheuk and Chan5 Mikulicz disease, Küttner tumours and Riedel thyroiditis are well-described conditions known to ENT professionals. Previously regarded as distinct entities, these conditions are now widely accepted to be members of IgG4-related disease based on similarities in histopathological appearance. Reference Stone, Zen and Deshpande6
The pathogenesis of IgG4-related disease is incompletely understood. Hypotheses range from autoimmunity to chronic allergic reaction based on observed cytokines and T-helper type 2 dominant responses, and the increased prevalence of allergic conditions in patients with IgG4-related disease.Reference Zen and Nakanuma7 It is not entirely clear whether or not IgG4 has pathological potential.Reference Cheuk and Chan5–Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8
An international panel has recently proposed a set of guidelines for the diagnosis of IgG4-related disease.Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8 The crucial histopathological features are a dense lymphoplasmacytic infiltrate, a storiform pattern of fibrosis and obliterative phlebitis. Tissue IgG4 counts and tissue IgG4:IgG ratios on immunohistochemical staining are widely regarded as less important, although various cut-offs have been proposed.Reference Cheuk and Chan5, Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8
Elevated serum concentration of IgG4 is often, but not always, observed. Serum IgG4 is the least common subclass, accounting for only 6 per cent of IgG in normal subjects.Reference Sato, Ohshima, Ichimura, Sato, Yamadori and Tanaka9 Interestingly, one-third of patients with histology-proven IgG4-related disease have normal serum levels of IgG4,Reference Deheragoda, Church, Rodriguez-Justo, Munson, Sandanayake and Seward10 as did our patients (but both were on prednisone at the time of sampling). In our cases, the long histories and presence of extensive fibrosis suggests that the disease process had entered its ‘burnt out’ phase, which may explain the normal serum IgG4 level.
Immunoglobulin G4 related disease in ENT
Salivary gland
Küttner tumour (chronic sclerosing sialadenitis) was first described in 1896. It classically presents as a swelling in the submandibular gland.Reference Geyer, Ferry, Harris, Stone, Zukerberg and Lauwers11 Kitagawa et al. first demonstrated a link between Küttner tumour and IgG4-related disease by observing extensive IgG4-positive plasma cells in biopsy specimens.Reference Kitagawa, Zen, Harada, Sasaki, Sato and Minato12 In a case–control series, Geyer et al. examined 13 patients with Küttner tumour and 15 patients with chronic sialadenitis not otherwise specified, and found that the former group had a significantly higher number of IgG4-positive plasma cells.Reference Geyer, Ferry, Harris, Stone, Zukerberg and Lauwers11
Mikulicz disease was first described in 1888, and consists of painless swelling of the lacrimal, parotid and submandibular glands. It has historically been regarded as a subtype of Sjögren's syndrome. Yamamoto et al. challenged this concept by pointing out several discrepancies in the original thesis that first documented this notion.Reference Yamamoto, Harada, Ohara, Suzuki, Naishiro and Yamamoto13 They argued that the histological specimens in the original paper were dominated by lymphoepithelial lesions and would not meet the modern diagnostic criteria of Mikulicz disease. The lack of anti-SS-A or anti-SS-B antibodies in Mikulicz disease further distances it from Sjögren's syndrome. The histological appearance of Mikulicz disease is similar to its counterpart, Küttner tumour, in that it also shows dense aggregates of IgG4-positive plasmacytes in affected organs.Reference Yamamoto, Harada, Ohara, Suzuki, Naishiro and Yamamoto13, Reference Yamamoto, Takahashi, Ohara, Suzuki, Naishiro and Yamamoto14 Finally, unlike Sjögren's syndrome, recovery in salivary function and a decrease in swelling are often seen in Mikulicz disease after treatment with corticosteroids. Because of the significant overlap in histological appearance, some authors have used the term ‘IgG4-related sialadenitis’ to describe these two conditions and have postulated that they may in fact be analogous conditions arising in different organs.Reference Abe, Sato, Tomaru, Sakata, Kokabu and Hori15, Reference Geyer and Deshpande16
Thyroid gland
Hashimoto's thyroiditis is a well-recognised form of autoimmune thyroid disease. Recent evidence has suggested a distinct subtype of Hashimoto's thyroiditis which displays elevated tissue levels of IgG4-plasma cells and more pronounced fibrosis. The term ‘IgG4 thyroiditis’ has been proposed to reflect this variant of Hashimoto's thyroidits.Reference Kakudo, Li, Hirokawa and Ozaki17, Reference Li, Bai, Liu, Ozaki, Taniguchi and Mori18 Li et al. examined the clinical and histopathological characteristics of 70 patients with Hashimoto's thyroiditis.Reference Li, Nishihara, Hirokawa, Taniguchi, Miyauchi and Kakudo19 Using 20 IgG4-positive plasma cells per high power field and a tissue IgG4 or IgG ratio of 0.3 as cut-offs for classification of IgG4 thyroiditis, they identified 19 cases that fell into that category. A higher grade of stromal fibrosis was seen in this subgroup of patients. Clinically, patients with IgG4 thyroiditis had higher concentrations of thyroid autoantibodies, diffuse low echogenicity on sonographic examination, and a more rapid onset and shorter course, supporting the hypothesis that IgG4 thyroiditis is a distinct clinicopathological entity and not merely a late or burnt out phase of Hashimoto's thyroiditis.
Riedel's thyroiditis is an extremely rare condition; patients typically present with a painless thyroid mass. Histological findings of invasive fibrosis with obliterative phlebitis and mixed lymphoplasmacytic infiltrate aid diagnosis.Reference Dahlgren, Khosroshahi, Nielsen, Deshpande and Stone20 These features are quite similar to the IgG4 variant of Hashimoto's thyroiditis as described above, and pathologists sometimes have difficulties separating the two conditions.Reference Li, Nishihara and Kakudo21 Because of its rarity, large series examining Riedel thyroiditis for IgG4-positive cells do not exist.
Ocular adnexa
Recent publications have suggested that the orbit can be affected by IgG4-related disease in two ways: orbital pseudotumour and chronic sclerosing dacryoadenitis.Reference Plaza, Garrity, Dogan, Ananthamurthy, Witzig and Salomao22, Reference Cheuk, Yuen and Chan23 Ocular adnexal involvement in IgG4-related disease has not been as well clarified in the literature as salivary and thyroid gland involvement.
The term ‘orbital pseudotumour’ has been used to refer to a non-specific inflammatory process affecting the orbit. When orbital pseudotumours are demonstrated radiologically, the confirmation of diagnosis often necessitates biopsy and this poses significant challenges as it may lead to vision disturbance or cosmetic disfigurement. Recent evidence suggests that some cases diagnosed as orbital pseudotumour may in fact be orbital manifestations of the IgG4-related disease spectrum.
Ocular adnexal involvement with IgG4-positive plasma cells has so far only been reported in small case series.Reference Plaza, Garrity, Dogan, Ananthamurthy, Witzig and Salomao22, Reference Takahira, Kawano, Zen, Minato, Yamada and Sugiyama24 Sato et al. examined 21 patients with immunohistochemically proven IgG4-related disease of the ocular adnexa and found morphological features identical with previous reports of IgG4-related disease in those patients. Eighty-one percent of patients had lacrimal gland involvement. In 77 per cent of patients tested, serum IgG4 was also elevated.Reference Sato, Ohshima, Ichimura, Sato, Yamadori and Tanaka9
The histological appearance in chronic sclerosing dacryoadenitis is reminiscent of autoimmune pancreatitis and Küttner tumour, with features of lymphoplasmacytic infiltration, periductal sclerosis and prominent IgG4-positive plasmacytes. In addition, lacrimal gland disease related to IgG4-related disease is often associated with salivary gland involvement, as seen in Mikulicz disease, although only isolated cases have been reported.Reference Cheuk, Yuen and Chan23 These findings support the concept of IgG4-related ocular adnexal disease as a distinct clinicopathological entity.
Paranasal sinuses
Cases of IgG4-related disease arising from the paranasal sinuses have been reported in the literature.Reference Ikeda, Awataguchi, Shoji and Oshima25–Reference Pace and Ward28 The majority of cases arise from the maxillary sinus, highlighting the importance of considering IgG4-related disease as a differential diagnosis when investigating a mass lesion in the maxillary sinus. Furthermore, patients presented with non-specific clinical and radiological findings, emphasising the importance of biopsy to confirm the diagnosis.
Suzuki et al. observed that the nasal mucosa is commonly affected in IgG4-related disease. The authors examined the nasal mucosa of 23 patients with extra-sinonasal IgG4-related disease and found that almost 60 per cent had prominent IgG4-positive plasma cell infiltration in the nasal mucosa, with 43 per cent having some form of sinonasal symptoms (obstruction or crusting).Reference Suzuki, Nakamaru, Akazawa, Mizumachi, Maeda and Takagi29 In contrast, Moteki et al. examined the nasal mucosa of 10 patients with clinically and radiologically diagnosed chronic rhinosinusitis (5 patients had extra-sinonasal IgG4-related disease while the remainder were healthy controls). They found no difference in the extent of IgG4-positive plasmacyte infiltration in the nasal mucosa of those who had extra-sinonasal IgG4-related disease when compared with the control population.Reference Moteki, Yasuo, Hamano, Uehara and Usami30 To date, the exact relationship between IgG4-related disease and chronic rhinosinusitis remains uncertain.
Skull base
Immunoglobulin G4 related disease arising from the skull base is extremely uncommon. However, case reports describing IgG4-related disease in the infratemporal fossa and pterygopalatine fossa have been published. A PubMed search using the search term ‘IgG4-related disease’ yielded more than 2500 articles; only 2 papersReference Song, Choung, Park, Kim, Khwarg and Jeon31, Reference Katsura, Mori, Kunimatsu, Sasaki, Abe and Machida32 (a total of 4 patients) described individuals with IgG4-related disease involving the pterygopalatine fossa. The presence of a mass lesion in these spaces often results in compressive symptoms on the maxillary branch of the trigeminal nerve. The differential diagnoses for a mass lesion centred in the pterygopalatine fossa are extensive; carcinoma, lymphoma and tumours of neural origin have all been reported previously. Reference Aronsohn, Stringer and Brown33, Reference DelGaudio34
Treatment and prognosis of immunoglobulin G4 related disease
Recognition of IgG4-related disease in the head and neck is crucial because it usually responds favourably to corticosteroids and/or monoclonal antibody therapy. Radical excision is therefore generally not indicated. Nevertheless, results are less encouraging with steroid therapy in the later stages of the disease when extensive tissue fibrosis has developed.Reference Stone, Zen and Deshpande6, Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8 Some patients have required rituximab (a monoclonal antibody directed at B lymphocytes) and even surgical debulking to halt clinical progression. Relapse following cessation or tapering of corticosteroid doses has also been reported, leading several authors to call for long-term maintenance treatment.Reference Khosroshahi and Stone35 Maintenance of corticosteroids following remission is controversial and needs further evaluation by prospective studies. In addition, a recent report suggests that there is a risk of malignant transformation into lymphoma or carcinoma in up to 10 per cent of cases.Reference Yamamoto, Takahashi, Tabeya, Suzuki, Naishiro and Ishigami36 This highlights the importance of long-term follow up and possibly repeat biopsy.
Differential diagnosis of immunoglobulin G4 related disease
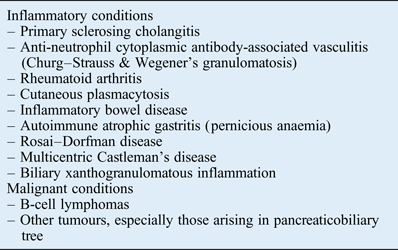
It is noteworthy that there are a number of disorders that also demonstrate elevated tissue levels of IgG4-bearing plasma cells without being regarded as part of the IgG4-related disease spectrum (Table I). Inflammatory conditions such as rheumatoid arthritis, primary sclerosing cholangitis, Rosai–Dorfman disease, sarcoidosis, Churg–Strauss syndrome, Wegener's granulomatosis and multicentric Castleman's disease not uncommonly exhibit increased tissue levels of IgG4-positive plasma cells.Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8, Reference Strehl, Hartmann and Agaimy37, Reference Umehara, Okazaki, Masaki, Kawano, Yamamoto and Saeki38 Nonetheless, these conditions typically lack the consistent and distinct histopathological features of IgG4-related disease, such as diffuse lymphoplasmacytic infiltration, storiform fibrosis and obliterative phlebitis. Therefore, over-reliance on the results of IgG4 immunohistochemical staining alone, without careful correlation with other clinical, radiological and histological findings, may result in the misdiagnosis of IgG4-related disease.Reference Stone, Zen and Deshpande6
• Immunoglobulin G4 (IgG4) related disease is a rare fibroinflammatory condition
• It can affect almost all organ systems, including the head and neck
• Biopsy is necessary for diagnosis and this condition responds favourably to systemic corticosteroids
• Tissue sampling for infratemporal or pterygopalatine fossae lesions has previously been achieved via external approaches, which are associated with morbidities
• Advances in endoscopic sinus surgery have allowed a transnasal approach
• Early recognition of IgG4-related disease is crucial and may prevent radical surgery
Table I Disorders with elevated tissue levels of immunoglobulin G4 positive plasma cells

B-cell lymphomas must also be excluded when diagnosing IgG4-related disease. An important distinguishing feature in the former is the presence of a predominantly B-cell infiltrate, whereas the inflammatory infiltrate in IgG4-related disease is chiefly composed of T cells.Reference Stone, Zen and Deshpande6, Reference Deshpande, Zen, Chan, Yi, Sato and Yoshino8 Malignant tumours, especially those arising in the pancreaticobiliary tree, may demonstrate elevated tissue levels of IgG4-bearing plasma cells, but the infiltration tends to be patchy and histological appearance is often devoid of the pathognomonic features of IgG4-related disease.Reference Yoneda, Inada, Kanayama and Shiraishi39
Conclusion
It is important to consider IgG4-related disease as a diagnostic possibility when dealing with mass lesions in the head and neck region. Neither an increase in serum IgG4 nor radiographic appearances are specific indicators for the disease; this underscores the need for histopathological diagnosis. This paper highlights the feasibility of an endoscopic, transnasal approach to the infratemporal or pterygopalatine fossae, and the role for surgery in obtaining tissue for diagnosis. It also supports the prevailing paradigm of managing this inflammatory condition with primarily systemic corticosteroids.