Introduction
Chronotype, also known as circadian typology, refers to the individual difference in the preferred timing for sleep–wake patterns and diurnal rhythms.Reference Horne and Östberg 1 , Reference Adan, Archer and Hidalgo 2 It allows classifying people into three categories: morning types (MTs) are those subjects who prefer to awaken early in the morning and feel most active during the earlier part of the day; evening types (ETs), who prefer to awaken later and feel most active in the late part of the day; and intermediate or neither types (NTs), which are in the middle.Reference Adan and Horne 3 Chronotype reflects the physiological arrangement of the circadian systemReference Bailey and Heitkemper 4 , Reference Kantermann, Sung and Burgess 5 and has been associated with mental health outcomes. Specifically, a positive correlation between eveningness and depressive symptoms has been identified in both clinical populationsReference Au and Reece 6 and healthy subjects,Reference Kivelä, Papadopoulos and Antypa 7 , Reference Taylor and Hasler 8 independently from sleep disturbancesReference Kitamura, Hida and Watanabe 9 or with sleep disturbances considered as possible mediators.Reference Bakotic, Radosevic-Vidacek and Koscec Bjelajac 10 , Reference Cox and Olatunji 11Additionally, ETs tend to sleep worse than NTs and MTs,Reference Taylor and Hasler 8 show less robust rhythmicity in sleep–wake patterns,Reference Martinez-Nicolas, Martinez-Madrid, Almaida-Pagan, Bonmati-Carrion, Madrid and Rol 12 and are, in parallel, more likely to receive a diagnosis of depression and bipolar disorder (BD).Reference Au and Reece 6 , Reference Melo, Abreu, Linhares Neto, de Bruin and de Bruin 13 In patients diagnosed with BD, ET has been suggested as a trait characteristic of the disease due to its stability over timeReference Wood, Birmaher and Axelson 14 , Reference Ahn, Chang, Joo, Kim, Lee and Kim 15 and independence from mood status.Reference Seleem, Merranko and Goldstein 16 Moreover, ET has been associated with higher recurrence rates, rapid mood swings, more severe depressive symptoms, and earlier onset of the disease.Reference Mansour, Wood and Chowdari 17 , Reference Vidafar, Yocum, Han, McInnis and Burgess 18 It is considered a proxy marker of circadian system dysfunction and a discrete clinical sub-phenotype in BD.Reference Romo-Nava, Blom and Cuellar-Barboza 19
While the association between chronotype and mood disorders has been consistently reported, conversely, attempts to measure the association between chronotype and anxiety symptoms have generated inconsistent results. On one hand, some studies have identified an association between eveningness and social anxiety,Reference Azad-Marzabadi and Amiri 20 , Reference Markarian, Gildner, Pickett and Warnke 21 state anxiety,Reference Prat and Adan 22 , Reference Vardar, Vardar, Molla, Kaynak and Ersoz 23 and trait anxiety in both youngReference Pabst, Negriff, Dorn, Susman and Huang 24 , Reference Díaz-Morales 25 and adult populations,Reference Park, An and Kim 26 even after controlling for sleep disturbances.Reference Cox and Olatunji 11 Additionally, eveningness has been found to be highly prevalent in clinical populations with anxiety disorders.Reference Fares, Hermens, Naismith, White, Hickie and Robillard 27 , Reference Lemoine, Zawieja and Ohayon 28 On the other hand, other studies did not find an association between chronotype and anxiety symptoms neither in healthy subjectsReference Alvaro, Roberts and Harris 29 -Reference Taylor, Clay, Bramoweth, Sethi and Roane 31 nor in clinical populations, such as patients with fibromyalgiaReference Kantermann, Theadom, Roenneberg and Cropley 32 or those with specific anxiety disorders (social phobia, panic disorder with and without agoraphobia, generalized anxiety disorder).Reference Antypa, Vogelzangs, Meesters, Schoevers and Penninx 33 Although in BD, the comorbidity with anxiety symptoms is highly frequent and may negatively influence the disease prognosis,Reference Pavlova, Perlis, Alda and Uher 34 -Reference Provencher, Guimond and Hawke 36 to the best of our knowledge, no study has explored whether anxiety symptoms are associated with circadian typology in patients with BD. Since the proposed association between chronotype and anxiety symptoms is still inconsistent, further studies are required to disentangle the association between the two, both in the clinical realm as well as in healthy subjects.
The complexity of the anxiety spectrum may partially explain the inconsistent results regarding its possible association with chronotype. Anxiety is characterized by elevated sensitivity to threat and involves a broad constellation of symptoms that may overlap among the different subtypes of the disease.Reference Craske, Rauch, Ursano, Prenoveau, Pine and Zinbarg 37 Additionally, assessing anxiety symptoms through interviews, questionnaires, and psychophysiological measures may reveal discrepancies between physiological reactivity and the verbal report of symptoms, making anxiety disorders a multifaceted and challenging construct to operationalize.Reference Lang and McTeague 38 While the Diagnostic and Statistical Manual of Mental Disorders (DSM) provides strictly defined diagnostic criteria for mental disorders, complementary approaches to explore mental disorders, such as the Spectrum ProjectReference Frank, Cassano and Shear 39 and the Research Domain Criteria,Reference Cuthbert 40 , Reference Casey, Craddock, Cuthbert, Hyman, Lee and Ressler 41 have been proposed. In particular, the Spectrum Project uses a dimensional approach to clinical evaluation. This latter addresses the core symptoms of the diagnostic categories according to DSM criteria, as well as atypical symptoms, temperamental traits, and behavioral styles that can constitute prodromal phases or residual symptoms of a mental disorder. This approach also enables the identification of subclinical or subthreshold manifestations that, even if they do not meet the criteria for a diagnosis, can still contribute to significant maladjustment and constitute elements of vulnerability to the appearance and persistence of mental disorders.Reference Frank, Cassano and Shear 39 The dimensional approach of the Spectrum Project has led to the development of psychometric instruments for assessing anxiety and mood spectrum, such as the panic-agoraphobic spectrum (PAS-SR)Reference Shear, Frank and Rucci 42 -Reference Frank, Cyranowski and Rucci 44 and the mood spectrum (MOODS-SR),Reference Dell’Osso, Armani and Rucci 45 respectively. These instruments provide a comprehensive assessment of clinical and subclinical symptoms belonging to the anxiety and mood spectrum.
To better understand the link between ET and the risk of developing mental illnesses,Reference Taylor and Hasler 8 exploring the association between chronotype and trait characteristics related to lifetime mood and anxiety spectrum symptoms may be relevant. The association between circadian variables and lifetime symptoms belonging to the spectrum of mood and anxiety (as measured through the PAS-SR and MOODS-SR) has been explored in a previous study.Reference Smagula, Krafty, Thayer, Buysse and Hall 46 The authors found that delayed and irregular actigraphically determined sleep/wake cycles were associated with more severe lifetime mood spectrum symptoms in healthy subjects and patients with a history of depression.Reference Smagula, Krafty, Thayer, Buysse and Hall 46 However, so far, no study has evaluated the association between lifetime mood and anxiety-related spectrum symptoms and chronotype, as measured through the reduced version of the Morningness-Eveningness Questionnaire (rMEQ).3 The rMEQ is one of the most broadly used scales to define circadian typology. It evaluates the self-reported preference for daily rest-activity patterns, allowing to obtain a “trait-construct of chronotype” rather than a “state-construct of chronotype,” such as is obtained through the Munich ChronoType Questionnaire (MCTQ).Reference Roenneberg, Pilz, Zerbini and Winnebeck 47 , Reference Evans, Leocadio-Miguel and Taporoski 48 To the best of our knowledge, no study has evaluated how this subjective and trait-like measure of chronotype (rMEQ) relates to lifetime mood and anxiety spectrum symptoms. Likewise, no study has explored the associations between subjective (rMEQ) and objective actigraphic mid-sleep point, both with respect to each other and with respect to previously considered clinical outcomes. The first aim of this study is to explore possible associations between chronotype, as measured through the rMEQ, and lifetime mood and panic-agoraphobic spectrum symptoms, as measured through the MOODS-SR and the PAS-SR, in a sample of patients diagnosed with BD compared with a group of age-matched healthy controls (HCs). The second aim is to explore possible associations between the mid-sleep point measured through actigraphy and lifetime mood and panic-agoraphobic spectrum symptoms in both BD and HC. In addition, the third aim is to investigate the association between rMEQ-based and actigraphic-based circadian typologies, both in relation to each other and with regard to lifetime mood and panic-agoraphobic spectrum symptoms.
Based on previous literature indicating a high prevalence of ETReference Melo, Abreu, Linhares Neto, de Bruin and de Bruin 13 and anxiety-related symptoms in patients with BD,Reference Pavlova, Perlis, Alda and Uher 34 , Reference Carmassi, Bertelloni and Dell’Oste 49 , Reference Freeman, Freeman and McElroy 50 we expect to find differences in findings between BD and HC. Our first hypothesis is that patients with BD will present a higher prevalence of ET and more severe mood and anxiety-related symptoms than HC. Although the existing literature has consistently shown associations between chronotype and mood symptoms, the associations with anxiety symptoms have been inconsistent. By using instruments like MOODS-SR and PAS-SR, we expect to identify subclinical and temperamental traits that may broaden the spectrum and enhance the identification of psychopathological features potentially associated with chronotype. Therefore, our second hypothesis is that eveningness, measured through the rMEQ, could be associated with more severe mood (including depressive and rhythmicity) and panic-agoraphobic spectrum symptoms in both HC and patients with BD. In addition, considering previous reports that the mid-sleep point, as measured by actigraphy, may be linked to the self-reported chronotype (rMEQ),Reference Gershon, Kaufmann and Depp 51 our third hypothesis is that the mid-sleep point, as determined by actigraphy, may also be associated with mood and anxiety-related symptoms in both BD and HC. To account for potential confounding factors, we considered variables such as age and gender, which have previously been linked to circadian preference.Reference Adan, Archer and Hidalgo 2 , Reference Adan and Natale 52
Results derived from this exploratory study therefore may allows to better understand the relationship between self-reported and actigraphic based chronotype, with respect to lifetime panic-agoraphobic and mood spectrum symptoms in patients with BD and HCs.
Methods
Participants
A sample of HCs (n = 97, median age: 41.00 [interquartile range, IQR = 29.00, 55.00], males 43.3%) and patients diagnosed with BD (n = 76, median age 47.00 [IQR = 35.75, 56.00], males 55.3%) were enrolled in this study. Patients with a diagnosis of BD attending the outpatient unit of the Psychiatric Clinic of the University of Pisa (Italy) were consecutively enrolled in this study. Inclusion criteria consisted in a DSM-5 diagnosis of BD (I–II) assessed by the Structured Clinical Interview for DSM-5 (SCID-5),Reference First, Williams, Karg and Spitzer 53 in the euthymic phase confirmed by a total score ≤ 7 in the Hamilton Depression Rating ScaleReference Hamilton 54 and a total score ≤ 6 in the Young Mania Rating Scale,Reference Young, Biggs, Ziegler and Meyer 55 and aged 18–65. Exclusion criteria included a clinical history of neurological disease and a change in psychopharmacological therapy (dosages or molecules) 4 weeks before the recruitment. HCs were enrolled among the Department of Clinical and Experimental Medicine personnel of the University of Pisa and the Azienda Ospedaliero-Universitaria Pisana (AOUP). Inclusion criteria for HCs were a negative history of psychiatric or neurologic disorders. All eligible subjects were asked to provide written informed consent after receiving a complete description of the study and having an opportunity to ask questions before joining the study. The study was conducted in accordance with the Helsinki Declaration and received the approval of the Ethics Committee of AOUP, Area Vasta Toscana Nord Ovest (code 14785/2019).
Instruments
Sociodemographic, lifestyle, and clinical data
Sociodemographic information included age, sex, height, weight, occupation, and years of education. Health-related lifestyle variables included alcohol and tobacco consumption. Alcohol intake was quantified as alcohol units per week (an alcohol unit corresponds to a half-pint of beer, a glass of wine, or a measure of spirit). Tobacco intake was quantified as the number of cigarettes smoked in a week. Clinical data included the type of BD, age at disease onset, number of manic, depressive, and hypomanic episodes, admissions, and suicidal attempts.
The Mood Spectrum-Self Report-Lifetime version
The MOODS-SR questionnaire evaluates mood spectrum symptoms through 161 items coded as present or absent for one or more periods of at least 3–5 days throughout the subject’s lifetime. The items are organized into three manic and three depressive components exploring “mood”, “energy,” and “cognition”, as well as into a section that assesses disturbances in rhythmicity and vegetative functions, yielding a total of seven domains. The sum of the mood-, energy- and cognition-manic items endorsed by subjects (“yes”/positive answers) makes up the Manic component (62 items), while the sum of the mood-, energy-, and cognition-depressive items constitutes the Depressive component (63 items). The rhythmicity and vegetative functions domain (29 items) explores alterations in the circadian rhythms and vegetative functions, including changes in energy, physical well-being, mental and physical efficiency related to the weather and season, and changes in appetite, sleep, and sexual activities. The questionnaire showed good internal consistency, with a Kuder–Richardson’s coefficient ranging from 0.79 to 0.92 among single domains.Reference Dell’Osso, Armani and Rucci 45 In addition, we calculated the MOODS-SR suicidality score, as previously described,Reference Dell’Osso, Carmassi and Rucci 56 using six questions of the MOODS-SR inquiring whether the subject had ever experienced (independently of a depressive episode) suicidal ideation and attempts.
The Panic-Agoraphobic Spectrum–Self Report-Lifetime version
The PAS-SR is a questionnaire used to assess the panic-agoraphobic spectrum that comprises symptoms, traits, and behaviors commonly found in patients with panic disorder that can manifest even if it has not reached the threshold for the diagnostic criteria of the DSM. The tool consists of 114 questions that assess the presence of spectrum characteristics during the patient’s life, organized into eight domains (sensitivity to separation, typical and atypical symptoms of panic, stress sensitivity, sensitivity to substances, anxious expectation, typical and atypical agoraphobia, phobias related to illness and hypochondria, sensitivity to reassurance).Reference Shear, Frank and Rucci 42 , Reference Shear, Cassano, Frank, Rucci, Rotondo and Fagiolini 43 Based on ROC analysis conducted in the validation study on PAS-SR, a total spectrum score of 35 provided a threshold for identifying the presence of panic-agoraphobic spectrum.Reference Shear, Frank and Rucci 42
The reduced morningness–eveningness questionnaire
The rMEQ is the reduced version of the Horne-Ostberg Morningness-Eveningness Questionnaire.Reference Adan and Horne 3 It was validated for the Italian populationReference Natale, Esposito, Martoni and Fabbri 57 and provides the individual preference for daily rhythms and activities, as well as the timing of the individual sleep/wake patterns, allowing to identify chronotypes based on the total score as follows: MT = 19–25, NT = 11–18, ET = 4–10.
Actigraphic registration
A waterproof wrist actigraphic device Fitbit Flex2 (FF2) was used to register sleep–wake patterns for 7 days without interruption. Data were sampled in 1-minute epochs and digitally stored for subsequent analysis. Data derived from the FF2 were analyzed through an artificial neural network (ANN) based and certified algorithm (Dormi by sleepActa s.r.l.), validated in a sample including healthy subjects and patients undergoing a diagnostic exam for sleep disturbances.Reference Banfi, Valigi, Galante, Ascanio, Ciuti and Faraguna 58 , Reference Guarnieri, Maestri and Cucchiara 59 The Dormi software is a medical, risk class I device. As such, it is registered within the Italian Ministry of Health Data Bank of Medical Devices (CND: 217 Z12030682). For this study, the quantitative sleep parameter used from actigraphic monitoring was the mid-sleep point, which represents the middle of the sleep period between the sleep onset and final awakening. The mid-sleep point was classified into three categories (ET, NT, and MT) by the 25th and 75th percentile of the mid-sleep point distribution in the overall sample, as previously described.Reference Liu, Song and Zhang 60
Statistical analyses
Comparisons between groups (BD and HC) were performed using the Mann–Whitney U test to examine significant differences regarding the demographic variables, rMEQ scores, and lifetime mood and panic-agoraphobic spectrum symptoms. Comparisons between chronotype groups (ETs versus NTs versus MTs) were performed within each group (BD and HC) to explore significant differences in demographic data, spectrum symptoms, and the actigraphic mid-sleep point, using the Kruskal–Wallis test and post-hoc pairwise comparisons using Wilcoxon rank-sum test. Associations between categorical variables were assessed through the Fisher exact test. Spearman’s correlation test was performed within each group (BD and HC) to evaluate the correlations between the rMEQ total score on one side and spectrum symptoms on the other side and to evaluate the correlations between the mid-sleep point on one side and spectrum symptoms on the other side. We estimated standard linear models through ordinary least squares, using the scores in mood and panic-agoraphobic spectrum questionnaires (numerical variables) as responses. We included the following predictors: a binary indicator of male gender, age, actigraphic mid-sleep point classified into three categories (named actigraphic-based circadian typology ET, NT, and MT) based on the 25th and 75th percentile of the mid-sleep point distribution in the overall sample, and chronotype measured trough the rMEQ also classified into three categories (named rMEQ-based circadian typology ET, NT, and MT). Both actigraphic-based categories and rMEQ-based categories were entered in the regression equation by means of two binary indicators (reference = NT). The decision to use a categorical version of chronotype was taken post-hoc (after seeing between-group comparison and correlation analyses results), allowing us to explore possible nonlinear effects, e.g., a U-shaped association between chronotype and PAS-SR scores.
We estimated the discrepancy between the objective (mid-sleep point) versus the subjective (rMEQ) circadian classification to evaluate how objective and subjective measures of circadian typology are related. This discrepancy was condensed in a Circadian Classification Discrepancy Index (CCDI) defined as follows: a classification value (CV) of 1 was assigned to the MT classification, 0 to the NT, and − 1 to the ET. Each subject was assigned to each of these three classification groups (MT, NT, and ET) based both on the rMEQ score and on the mid-sleep point timing. The CCDI was computed for each subject by subtracting the actigraphy-based CV from the rMEQ-based CV (see Supplementary Table S1). As an example, a subject classified as ET(−1) based on the rMEQ but classified as MT(1) based on the actigraphic mid-sleep point would result in a CCDI score of −2 calculated as (−1)-(1). Instead, a subject classified as MT(1) based on the rMEQ while classified as ET(−1) based on the actigraphic mid-sleep point would result in a CCDI score of 2 calculated as (1)-(−1). The CCDI is proposed as a discrepancy index between the desired circadian typology (investigated by the rMEQ) and the actual one (explored here by the actigraphic mid-sleep point).
To explore the associations between subjective (rMEQ) and objective actigraphic circadian measures, with respect to clinical outcomes, CCDI values obtained for each participant were entered in standard linear models through ordinary least squares. The scores obtained in mood and panic-agoraphobic spectrum questionnaires (numerical variables) were used as outcomes, and the following variables as predictors: a binary indicator of male gender, age, and CCDI values (−2, −1, 0, 1, 2) entered in the model as a factor (reference = 0).
Regression models were adjusted for potentially confounding factors (gender and age). In detail, the tendency towards morning-type increases with age and in females;Reference Adan, Archer and Hidalgo 2 , Reference Adan and Natale 52 therefore, these variables were included in our regression models. A value of p < 0.05 was considered significant. All statistical analyses were conducted using R Studio.
Results
Comparisons between HC and BD
BD and HC groups showed a similar distribution of age and gender. Significant differences between groups were found in terms of BMI, alcohol, and tobacco intake, where patients with BD showed higher BMI and tobacco intake and lower alcohol intake compared with HC (see Table 1). Patients with BD showed significantly higher scores in all MOODS-SR components and PAS-SR domains than HC. Likewise, lower rMEQ scores were observed in BD compared with HC. A significantly major prevalence of subjects classified as ETs was found in the group of BD (38.2%) compared with HC (18.6%). Clinical data of patients with BD are described in Table 2.
Table 1. Demographic, Lifestyle, Spectrum Symptoms and Chronotype in Patients and Control Groups
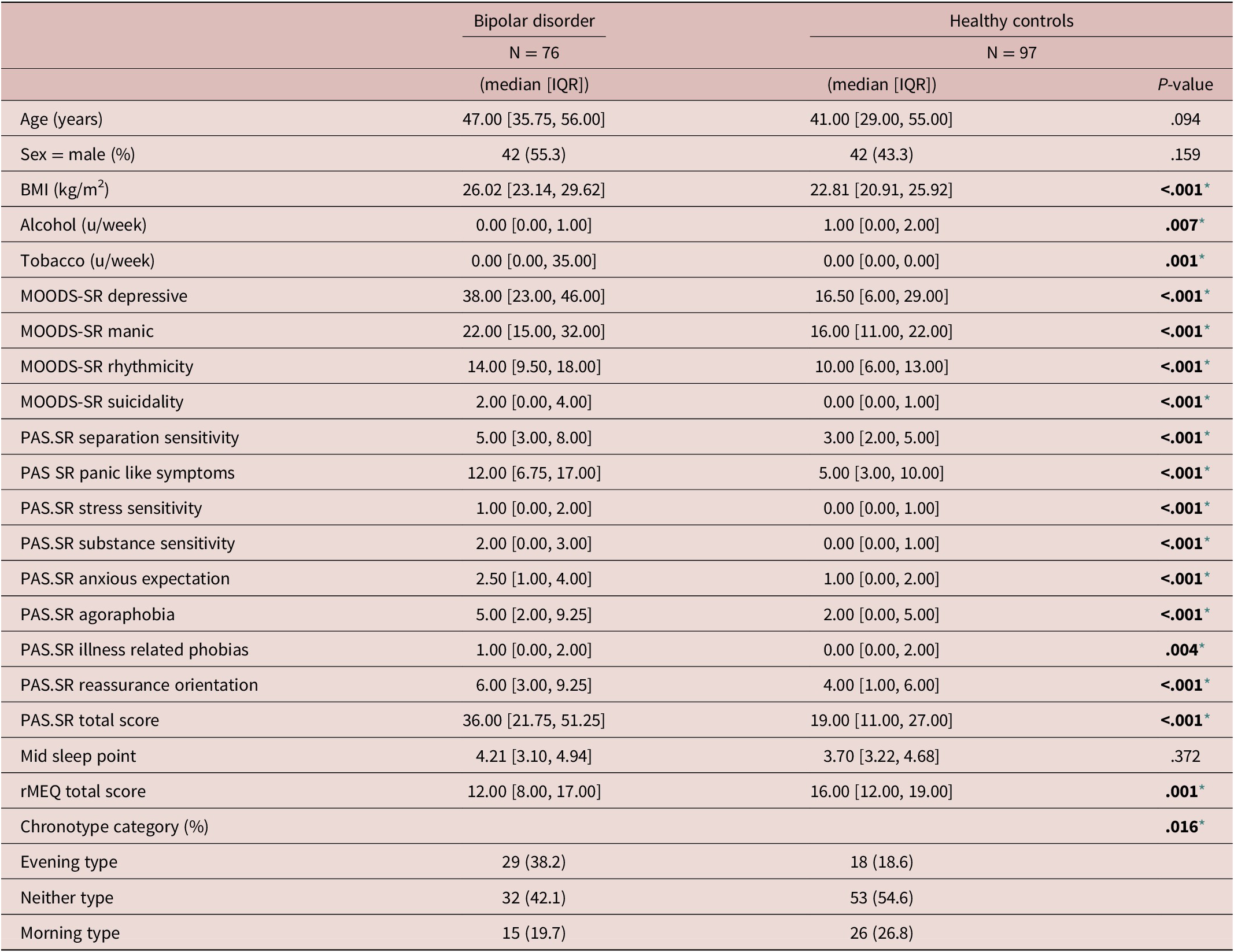
Note. Median and interquartile ranges (IQR), mean, and standard deviation (SD) are reported for the quantitative variables, frequency and percentage for the categorical ones. Numerical variables were compared using the Mann–Whitney U Test. Categorical variables were compared through the Fisher exact test.
Abbreviations: Alcohol u/w, alcohol units per week; BMI, body mass index; MOODS.SR, mood spectrum-self report-lifetime version; PAS.SR, panic-agoraphobic spectrum–lifetime version; rMEQ, morningness-eveningness questionnaire reduced version; Tobacco u/w, cigarettes units per week.
* Level of significance set at .05.
Table 2. Clinical Data
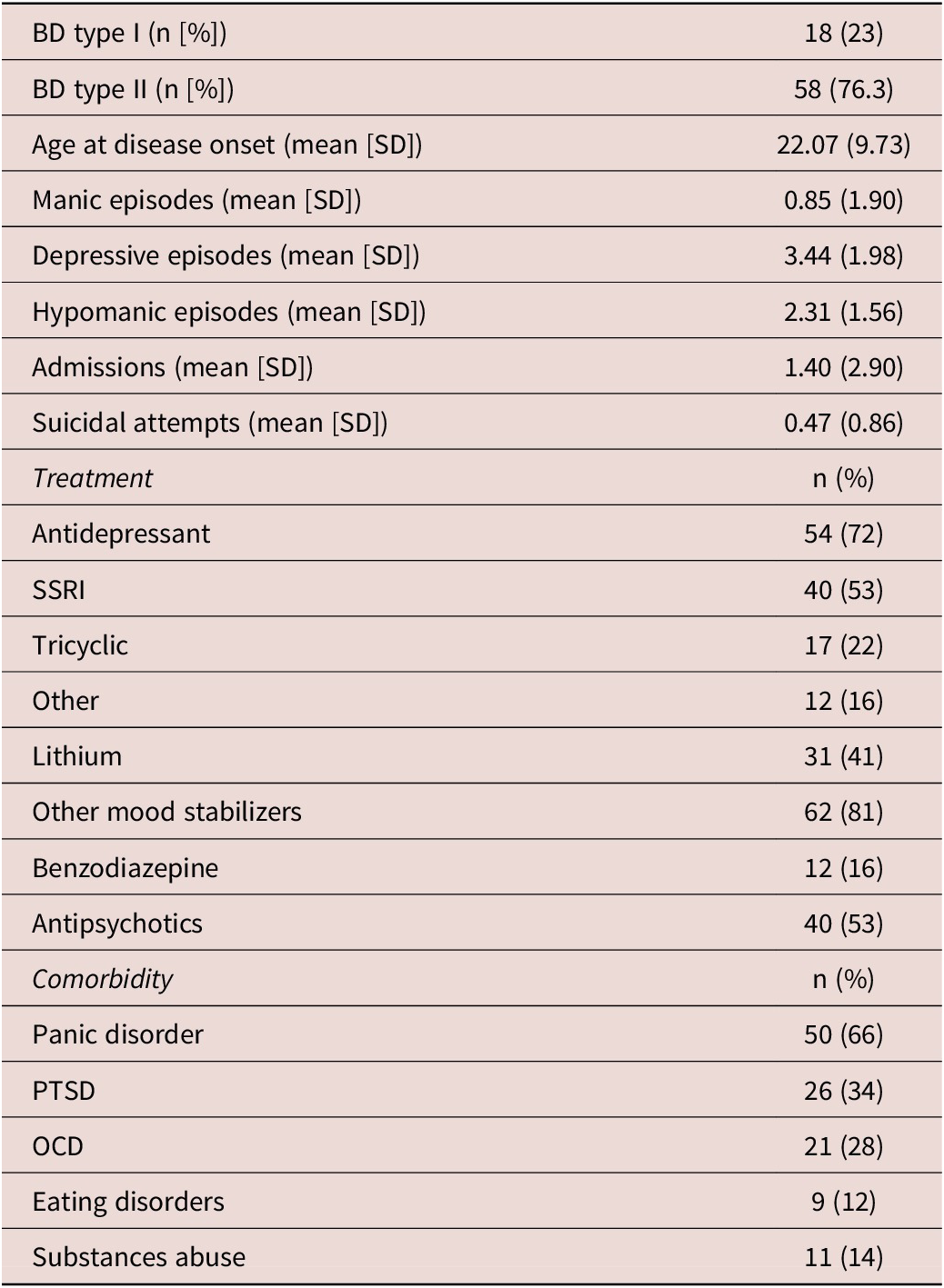
Abbreviations: BD, bipolar disorder; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder; SSRI, selective serotonin reupptake inhibitor.
Comparisons between chronotypes as measured through the rMEQ - ET versus NT versus MT-
Comparisons between rMEQ-based chronotypes (ET versus NT versus MT) performed within each group (BD and HC) revealed that only in HC significant differences emerged regarding age, being ET younger than both NT and MT, and being NT younger than MT. In the group of patients with BD, no differences between chronotypes were observed regarding age distribution. No differences between chronotypes were found in terms of gender distribution and tobacco intake in HC or BD. Instead, alcohol intake was significantly different between chronotype groups in HC, reporting higher alcohol intake ETs compared with MTs. The MOODS-SR Depressive component was significantly different between chronotype groups in both BD and HC. Post-hoc analyses revealed that in the HC group, ETs showed higher scores than both NTs and MTs. Likewise, in patients with BD, subjects classified as ETs showed higher scores in the MOODS-SR Depressive component than patients with BD classified as NTs. The MOODS-SR Rhythmicity domain was significantly different between chronotype groups in HC, showing ETs higher scores than NTs and MTs. Regarding the MOODS-SR Suicidality, significant differences between chronotypes emerged only in the HC group, where ETs showed higher scores than both NTs and MTs.
In the PAS.SR total score, only in the group of patients with BD significant differences emerge between chronotypes, where NTs showed lower scores than both ETs and MTs. Instead, no significant differences between chronotype groups emerged in HC in the PAS-SR total score. Finally, the mid-sleep point was significantly different between all chronotype groups in both HC and patients with BD, showing ETs a later mid-sleep point than NTs and MTs; likewise, NTs showed a later mid-sleep point than MTs (see Table 3).
Table 3. Comparisons Between Chronotypes Within Each Group: BD and HC
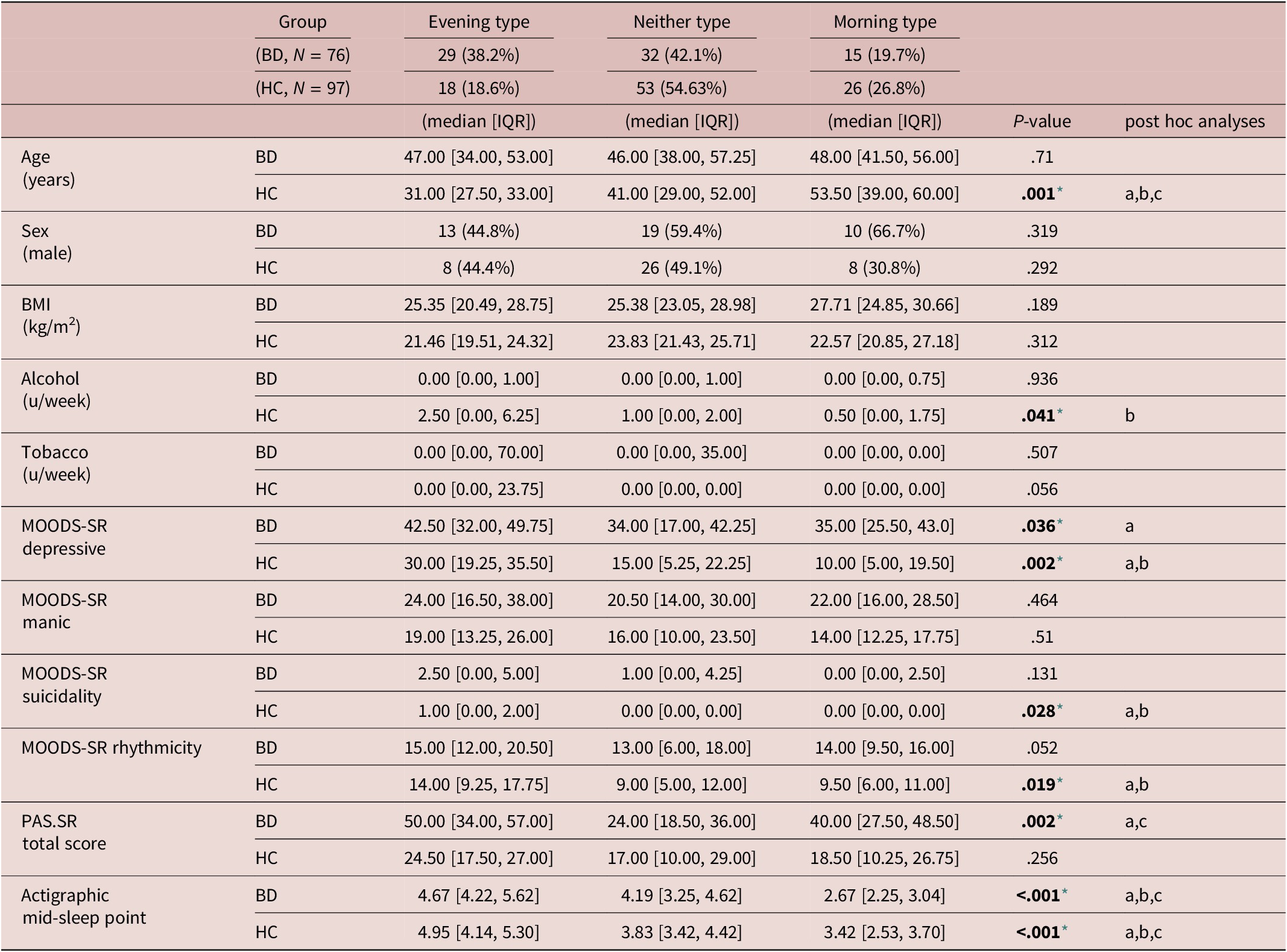
Note. Median and interquartile ranges are reported for the quantitative variables, frequency and percentage for the categorical ones. Post-hoc analyses of variables that differed significantly between chronotypes were performed through Wilcoxon rank sum test. Significant differences between groups are presented as follows:
Abbreviations: Alcohol u/w, alcohol units per week; BD, bipolar disorder; BMI, body mass index; HC, healthy controls; MOODS.SR, mood spectrum-self report-lifetime version; PAS.SR, panic-agoraphobic spectrum–lifetime version; Tobacco u/w, cigarettes units per week.
* Level of significance set at .05.
a) Significant differences between Evening type and Neither type.
b) Significant differences between Evening type and Morning type.
c) Significant differences between Neither type and Morning type.
Correlation analyses between rMEQ, mid-sleep point, and spectrum symptoms
Correlations analyses performed within each group (HC and BD) revealed that the rMEQ was negatively correlated with the MOODS-SR Depressive component in both HC (rho = −0.37, p = <0.001) and BD (rho = −0.27, p = 0.020), where higher scores in the MOODS-SR Depressive component were correlated to lower scores in the rMEQ. Only in HC the MOODS-SR Rhythmicity component was negatively correlated with the rMEQ (rho = −0.28, p = 0.010), so eveningness was correlated with higher scores in this component, suggestive of major disturbances in rhythmicity. The PAS-SR total score did not show significant correlations with the rMEQ in BD or HC (Table 4).
Table 4. Correlations Between Spectrum Symptoms and rMEQ/Mid-Sleep Point
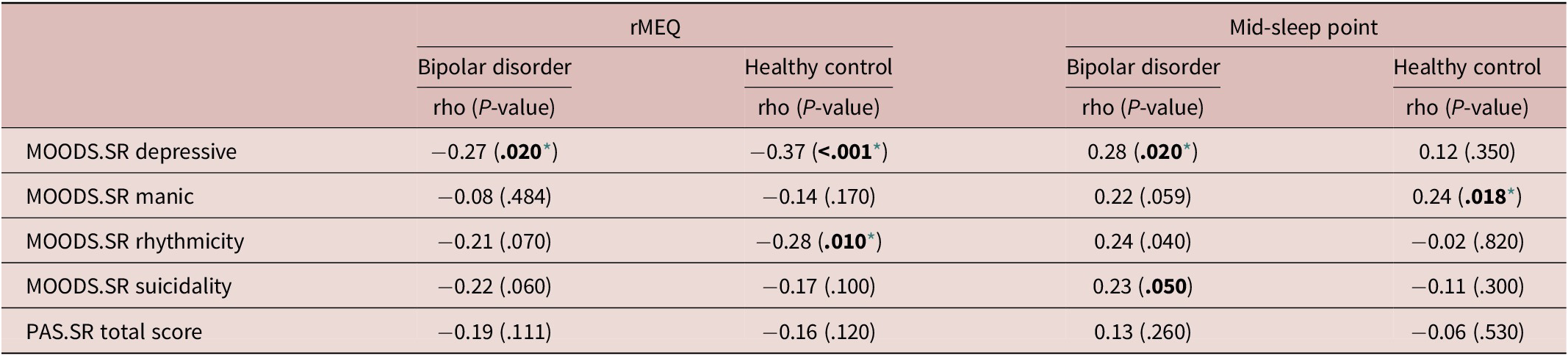
Note. Spearman’s correlation coefficients and associated P-values.
Abbreviations: MOODS.SR: mood spectrum-self report-lifetime version; PAS.SR: panic-agoraphobic spectrum–lifetime version. rMEQ: morningness-eveningness questionnaire reduced version.
* Level of significance set at .05.
Correlations analyses also showed that the actigraphic-based mid-sleep point was correlated with the MOODS-SR Depressive component (rho = 0.28, p = 0.020) and with the MOODS-SR Rhythmicity component (rho = 0.24, p = 0.040) in the sample of patients with BD. Instead, in healthy subjects, significant correlations emerged between the mid-sleep point and the MOODS-SR Manic component (rho = 0.24, p = 0.018). These results suggest that later mid-sleep point was correlated with more severe mood symptoms (Table 4).
Regression models to evaluate the associations between circadian typology and spectrum symptoms
Results obtained through comparisons and correlation analyses regarding the relationship between chronotype and the PAS-SR total score were somewhat inconsistent. Comparison analyses revealed significant differences in the PAS-SR total score between chronotype groups in patients with BD, while correlation analyses between rMEQ and PAS-SR total scores were not significant. Therefore, we estimated regression models using the rMEQ-based chronotype as a categorical variable, allowing us to explore possible nonlinear effects, e.g., a U-shaped association between chronotype and PAS-SR scores.
Regression analysis using the rMEQ-based chronotype as a predictor and the MOODS-SR Depressive component as outcome adjusted for potentially confounding factors (age and gender) showed that the MOODS-SR Depressive component was associated with chronotype in both BD (ET versus NT, β = 8.49; p = 0.030) and HC (ET versus NT, β = 9.44; p = 0.006). These results indicate that being classified as ET compared with being classified as NT was associated with higher depressive symptoms (see Table 5, Figures 1 and 2).
Table 5. Regression Models to Estimate the Effect of Chronotype Measured Through the rMEQ (as predictor) on MOODS.SR and PAS-SR Scores (As Outcomes), Adjusting for Potentially Confounding Factors
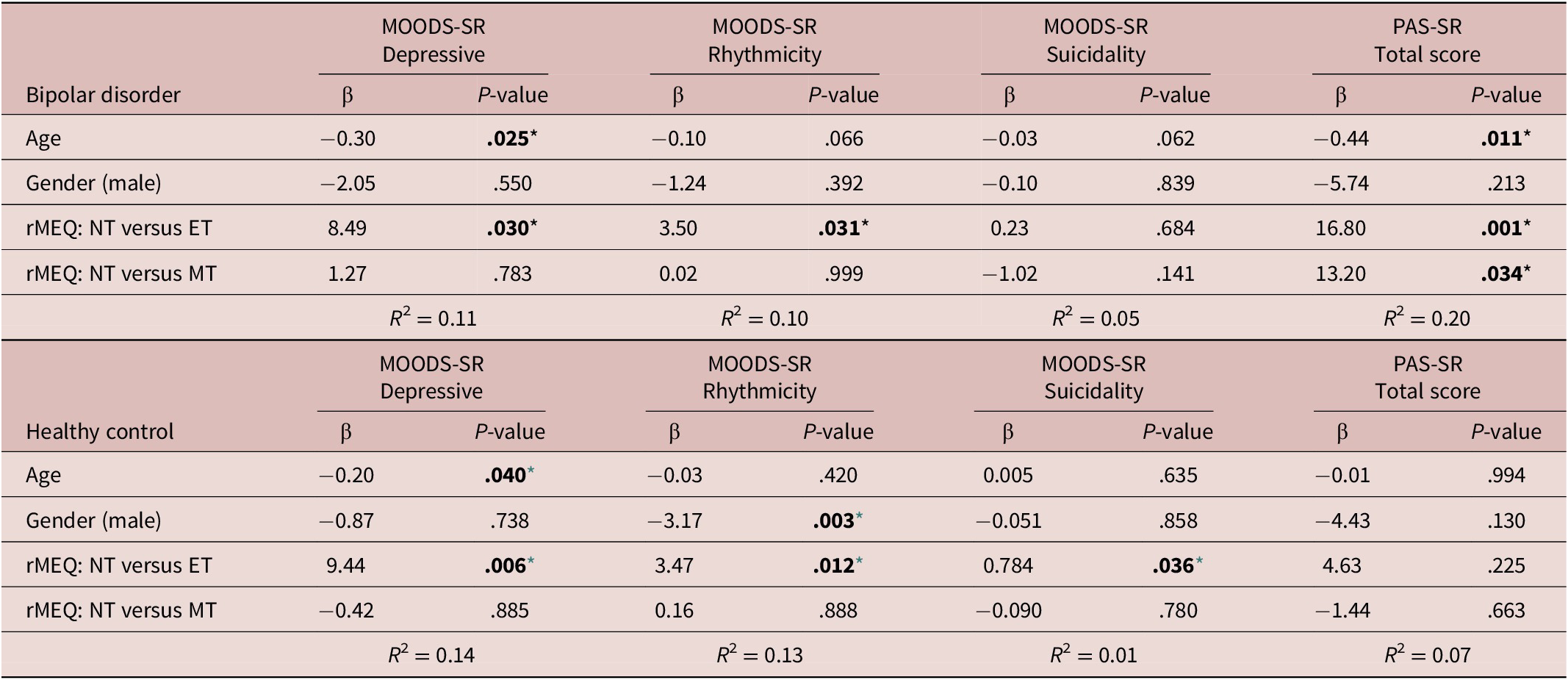
Note. Each model was performed individually and was adjusted for age and gender.
Abbreviations: ET, evening type; MEQ, morningness–eveningness questionnaire; MOODS.SR, mood spectrum-self report-lifetime version; MT, morning type; NT, neither (intermediate) type; PAS.SR, panic-agoraphobic spectrum–lifetime version.
* Level of significance set at .05.
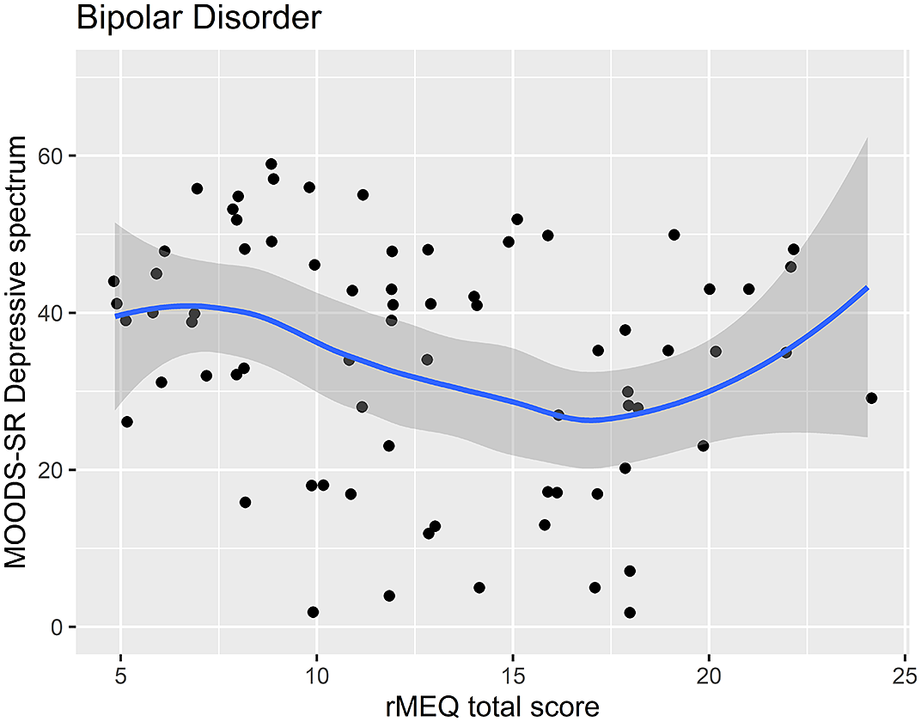
Figure 1. Scatter plot of the rMEQ and MOODS-SR Depressive spectrum symptoms in bipolar disorder.
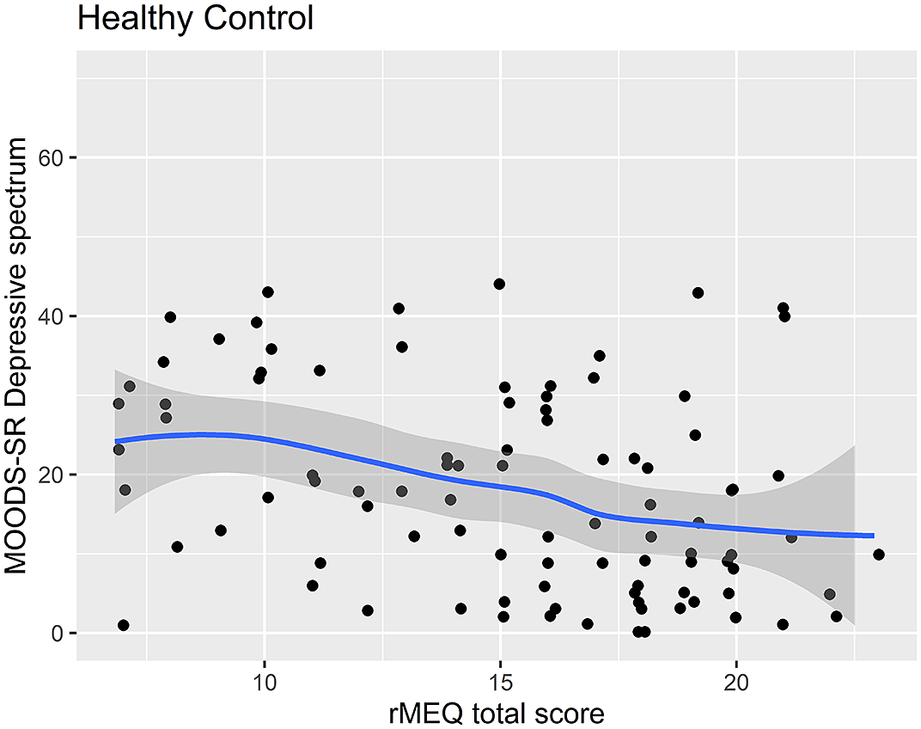
Figure 2. Scatter plot of the rMEQ and MOODS-SR Depressive spectrum symptoms in healthy controls.
Regression analysis using the MOODS-SR Rhythmicity as outcome and the rMEQ-based chronotype as predictor, showed that the MOODS-SR Rhythmicity was associated with chronotype in both BD (ET versus NT, β = 3.50; P = .031) and HC (β = 3.47; P = .012). These results show that being classified as ET compared with NT was associated with higher scores in this domain, indicative of higher disturbances in rhythmicity (see Table 5). Regression analysis using the MOODS-SR Manic as outcome and the rMEQ-based chronotype as predictor showed no significant effect of chronotype in BD and HC (data not showed).
Regression analysis using the PAS-SR total score as outcome and the rMEQ-based chronotype as predictor, adjusting for potentially confounding factors (age and gender), showed that only in patients with BD, the PAS.SR total score was associated with chronotype in both contrasts: ET versus NT (β = 16.80; P = .001), and MT versus NT (β = 13.20; P = .034). These results suggest that being an extreme chronotype was associated with more severe panic-agoraphobic symptoms, as compared with NTs (see Table 5 and Figures 3 and 4).
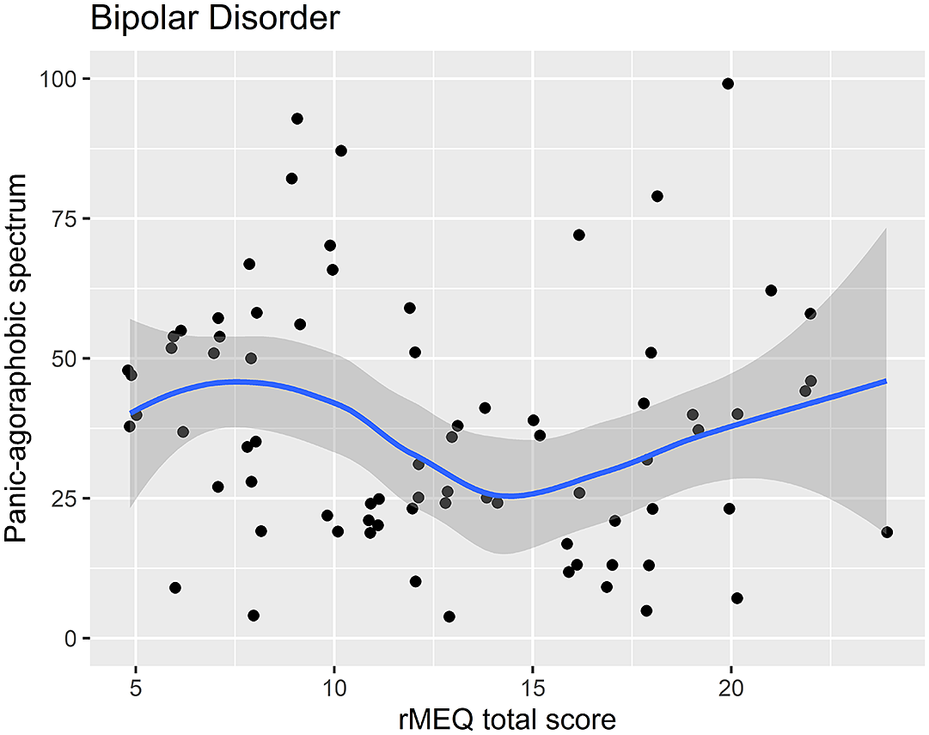
Figure 3. Scatter plot of the rMEQ and panic-agoraphobic spectrum symptoms in bipolar disorder.
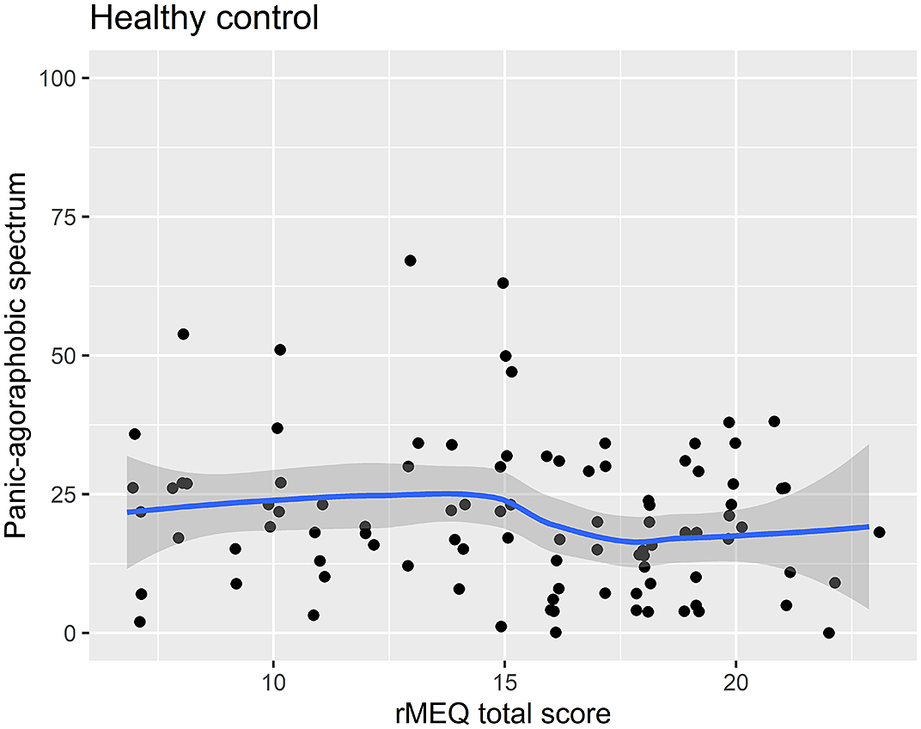
Figure 4. Scatter plot of the rMEQ and panic-agoraphobic spectrum symptoms in healthy controls.
Regression analyses to estimate the associations between the actigraphic-based circadian typology and mood and anxiety-related outcomes entered the actigraphic-based circadian typology as a categorical variable. To this aim, the three categories were defined by the 25th and 75th percentile of the mid-sleep point distribution in the overall sample as follows: actigraphic-based MT: mid-sleep point earlier than 3.18; actigraphic-based NT: mid-sleep point between 3.18 and 4.73 (intermediate); and actigraphic-based ET: mid-sleep point later than 4.73. These regression analyses adjusting for potentially confounding factors showed that only in HC patients the actigraphic-based circadian typology was significantly associated with the MOODS-SR depressive domain (β = 10.01; P = .001), suggesting that compared with the actigraphic-based NT, the actigraphic-based MT group showed higher scores in the MOODS-SR Depressive. Regression analysis using the MOODS-SR rhythmicity and vegetative functions domain, the MOODS-SR suicidality score, and the PAS-SR total score as outcomes and the actigraphic-based circadian typology as predictor adjusting for potentially confounding factors (age and gender) showed no significant associations in either BD or HC (Table 6).
Table 6. Regression Models to Estimate the Effect of the Actigraphic-Based Circadian Typology (As Predictor) on MOODS.SR and PAS-SR scores (As Outcomes), Adjusting for Potentially Confounding Factors
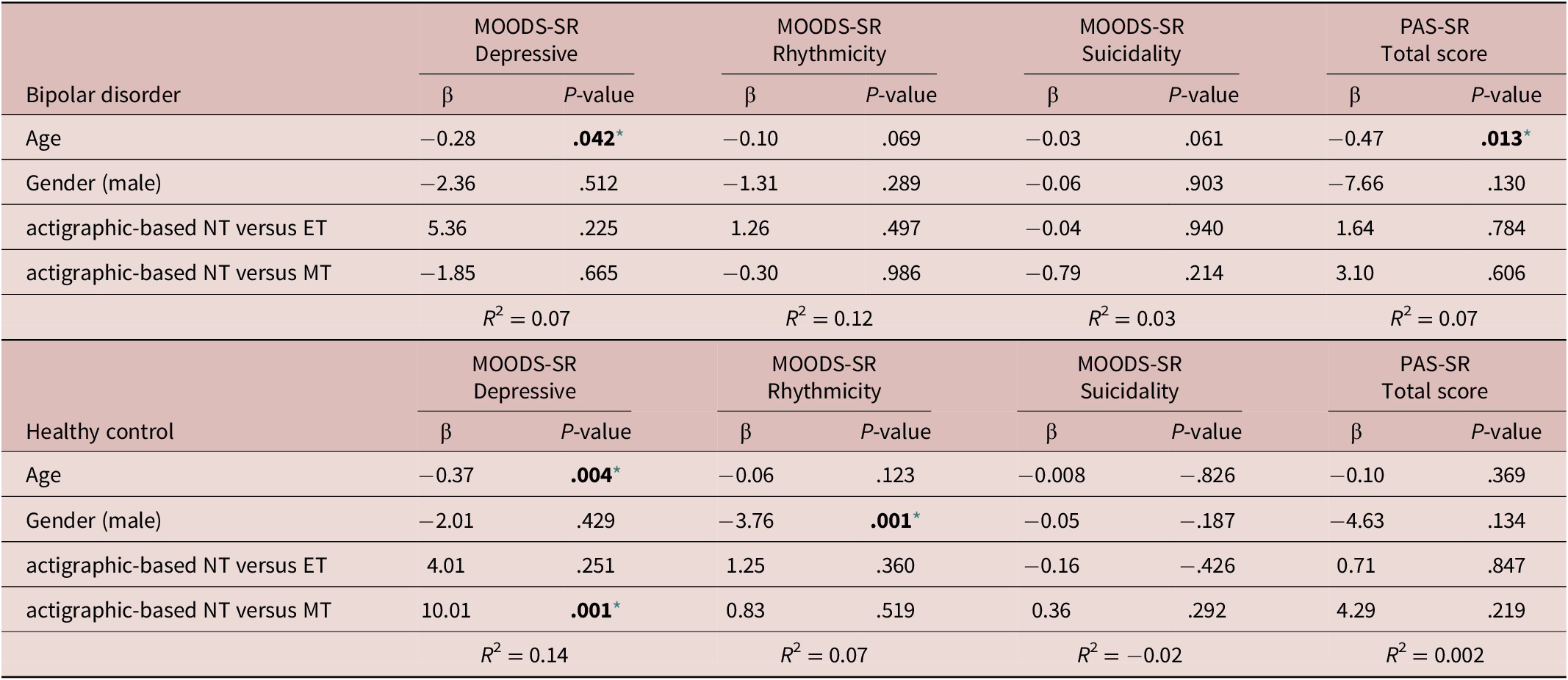
Note. The actigraphic-based circadian typology was determined using the 25th and 75th percentile of the mid-sleep point (defined as the midpoint between bedtime and wake-up time) in the overall sample. The three categories corresponds to: Morning type (MT): mid-sleep point earlier than 3.18; Neither type (NT): mid-sleep point between 3.18 and 4.73 (intermediate); and Evening type (ET): mid-sleep point later than 4.73. Each model was performed individually and was adjusted for age and gender.
Abbreviations: MOODS.SR, mood spectrum-self report-lifetime version; PAS.SR, panic-agoraphobic spectrum–lifetime version.
* Level of significance set at .05.
Comparison between actigraphic-based and rMEQ-based circadian typology
The rMEQ and the mid-sleep point were negatively correlated (rho = −0,58, p < 0.001), see Supplementary Figure S1. A negative correlation is expected. Still, the strength of the association is not particularly high. The discrepancy between actigraphic-based circadian typology and rMEQ-based circadian typology was estimated using the CCDI (see “Methods” section). We found that 97 subjects (56.06%) were correctly classified (CCDI = 0), 38 subjects (21.96%) were self-reported evening, classified as intermediate by actigraphy (CCDI = −1), three subjects (1.7%) were self-reported evening, classified as MT by actigraphy (CCDI = −2), 33 subjects (19,07%) were self-reported morning and classified as intermediate by actigraphy (CCDI = 1), while two subjects (1.15%) were self-reported morning, classified as ET by actigraphy (CCDI = 2).
Regression models to estimate the effect of the CCDI (as predictor) on MOODS.SR Depressive component (as outcome), adjusting for potentially confounding factors, showed that, only in HC, the CCDI was significantly associated with the MOODS-SR Depressive component. The results presented in Table 7 suggest that, compared with subjects with congruent circadian typology (CCDI = 0), being classified as MT by actigraphy but having a self-reported ET by rMEQ (CCDI = −2) was associated with significantly higher scores in the MOODS-SR Depressive component (β = 23.00; P = .005). In contrast, being classified as NT by actigraphy but having a self-reported MT (CCDI = 1) was associated with significantly lower scores in the MOODS-SR Depressive component (β = −7.21; P = .010). Models performed to estimate the effect of CCDI on other mood and anxiety-related parameters did not show significant associations.
Table 7. Regression Models to Estimate the Effect of the CCDI as Predictor on MOODS.SR Depressive Score (As Outcome), Adjusting for Potentially Confounding Factors
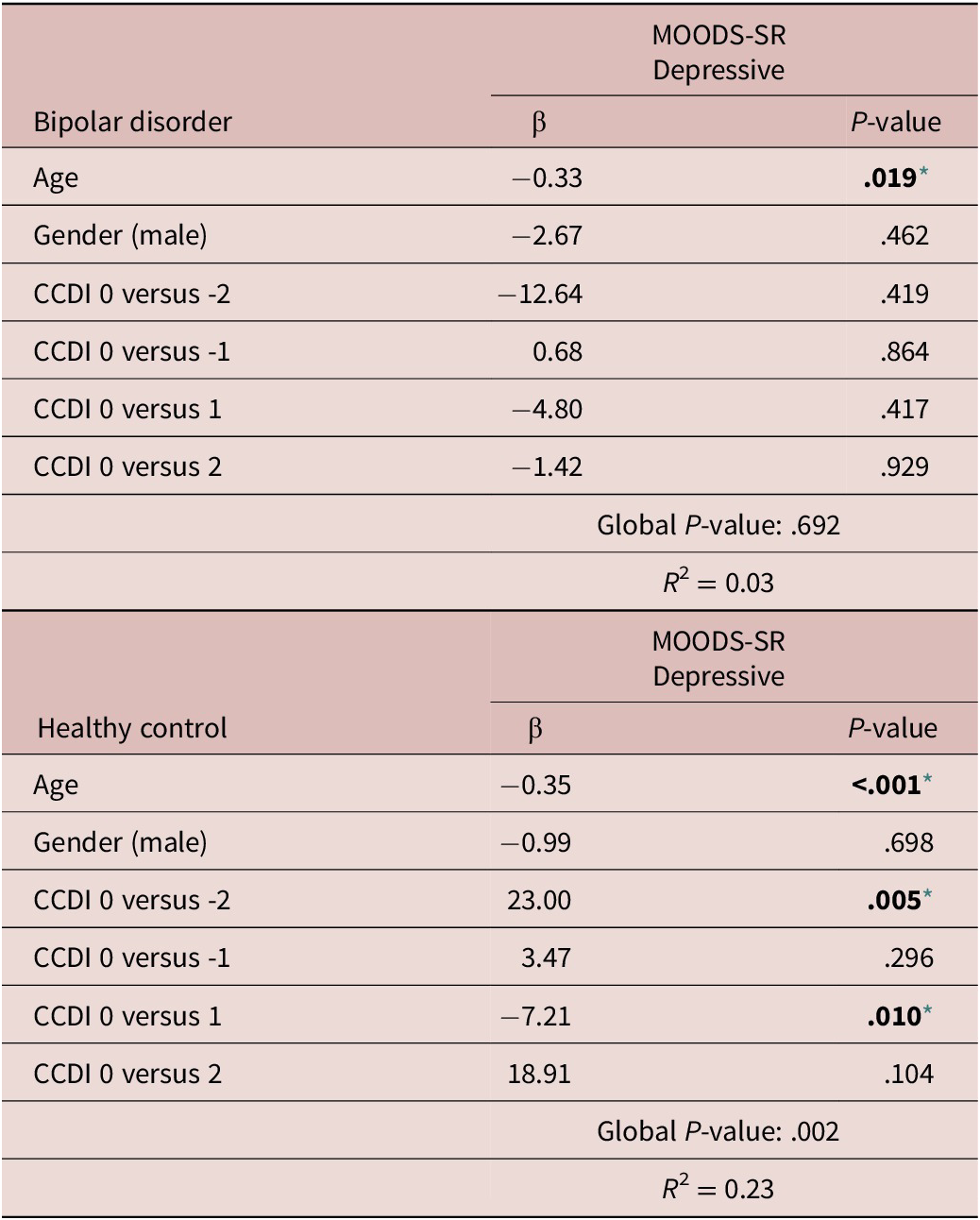
Note. Model was performed individually and was adjusted for age and gender.
Abbreviation: CCDI: Circadian Classification Discrepancy Index.
* Level of significance set at .05.
Discussion
In this study, we explored for the first time the associations between lifetime panic-agoraphobic and mood spectrum symptoms with respect to chronotype, measured through the rMEQ, and the actigraphic mid-sleep point in patients with BD and HCs. We found that more severe lifetime depressive symptoms and disturbances in rhythmicity were consistently associated with eveningness in both HC and patients with BD. Instead, in patients with BD only extreme chronotypes––ETs and MTs––were significantly associated with more severe panic-agoraphobic symptoms when compared with NTs. Regarding the objective measurements, we observed that only in HCs, the actigraphic mid-sleep point was significantly associated with the MOODS-SR depressive component. Specifically, compared with NTs, those with earlier mid-sleep point (MTs) reported higher depressive symptoms. In addition, the discrepancy between actigraphic-based and rMEQ-based circadian typology was associated with the MOODS-SR Depressive component only in the HC group. This result suggests that the misalignment between the preferred circadian typology (rMEQ) and the actual one (explored here by the actigraphic mid-sleep point) could negatively affect mental health, particularly mood-related outcomes, in HCs.
The literature suggests a possible role of ET as a risk factor for developing a wide range of psychiatric disorders.Reference Kivelä, Papadopoulos and Antypa 7 In particular, individuals with mood disorders have a marked trend towards eveningness.Reference Au and Reece 6 , Reference Wood, Birmaher and Axelson 14 , Reference Ahn, Chang, Joo, Kim, Lee and Kim 15 Accordingly, we found a higher prevalence of ET in BD (38.2%) than in HC (18.6%), confirming our first hypothesis regarding the increased tendency to evening preference in patients with BD. Likewise, in both HC and patients with BD, higher disturbances in the MOODS-SR Depressive component and more disruptive rhythmicity and vegetative functions were associated with greater eveningness. Moreover, we also found that subjects classified as ETs tended to show higher scores in the MOODS-SR suicidality index, reaching statistical significance in the group of HC. These results partially confirm our second hypothesis stating that eveningness, as measured through the rMEQ, could be associated with more severe mood spectrum symptoms in both HC and patients with BD. While the exact mechanisms linking mood disturbances and circadian disruptions remain to be elucidated, common neuroanatomical and neurochemical pathways that regulate circadian processes and mood may explain the intrinsic association between mood symptomatology and disturbances in circadian rhythmicity.Reference von Schantz, Leocadio-Miguel, McCarthy, Papiol and Landgraf 61 -Reference Benca, Duncan, Frank, McClung, Nelson and Vicentic 64
The relevance of the associations between eveningness and depressive symptoms is further highlighted by our results showing significantly increased suicidality symptoms, as measured through the MOODS-SR, in HC classified as ET compared with both NTs and MTs. A relationship between ET, decreased rhythmicity, and suicidal thoughts has been identified in several studies.Reference Rumble, Dickson and McCall 65 Moreover, reduced rhythmicity and greater vegetative symptoms have been significantly associated with an increased risk of lifetime suicide attempts in patients with BD and borderline personality disorder.Reference Balestrieri, Rucci and Sbrana 66 At the same time, subthreshold manic/hypomanic features may also be associated with higher suicidality.Reference Dell’Osso, Carmassi and Rucci 56 Reduced sleep is one of the core symptoms of manic episodes, and in parallel, a decrease of even just 1 hour of sleep has been associated with a significantly greater likelihood of suicide-related outcomes in teenagers.Reference Winsler, Deutsch, Vorona, Payne and Szklo-Coxe 67 These previous studies may suggest that both regularity and duration of sleep could contribute to suicidal-related symptoms. Indeed, a recent meta-analysis found that sleep disturbances, including insomnia, prospectively predicted suicidal thoughts and behaviors.Reference Liu, Steele and Hamilton 68 Considering the evidence in the literature and our results, further studies could explore whether, in ETs, objective measurements of sleep duration, sleep regularity, and sleep-phase may mediate the appearance of mood disturbances, including symptoms related to suicidal ideation in both clinical and nonclinical populations.
We had also hypothesized that ET, as measured through the rMEQ, could be associated with more severe panic-agoraphobic spectrum symptoms in both HC and patients with BD. However, we only found significant associations in the group of BD, and interestingly, we found that both ETs and MTs were associated with higher panic-agoraphobic symptoms when compared with NTs. This nonlinear (e.g., U-shaped) association between chronotype and PAS-SR scores can explain that simple correlation coefficients did not show any significant association while using chronotype as a categorical predictor in a regression model generated significant results. Although in previous literature reports MTs have been associated with mental health outcomes less frequently than ETs,Reference Kivelä, Papadopoulos and Antypa 7 , Reference Taylor and Hasler 8 a recent study investigating the relationship between personality dimensions and chronotype found that Neuroticism-Anxiety traits were more common in men classified as MTs and in women classified as NTs. At the same time, the ET group did not show a significant association with this dimension.Reference Muro, Gomà-I-Freixanet and Adan 69 This result is noteworthy because the Neuroticism-Anxiety traits correspond to symptoms also evaluated through the PAS-SR, including anticipatory anxiety, insecurity, interpersonal sensitivity, and separation anxiety. We also found associations between these anxiety-related symptoms and MTs in the group of BD. Likewise, a recent study found that patients with BD during euthymic phase who had attempted suicide (SA) showed an earlier “M10 onset” (a circadian phase marker actigraphically determined) than patients without SA and even earlier than HC, suggesting that patients with BD and SA tend to be active earlier than the groups of reference.Reference Benard, Etain and Vaiva 70 This association between an advanced circadian phase and SA could suggest that an extreme morning tendency could also be related to worse clinical outcomes in BD. Accordingly, our results suggest that patients with BD with an extreme MT could experience more severe lifetime anxiety spectrum symptoms than NTs.
Regarding objective measurements of circadian typology, we found that the mid-sleep point was significantly correlated with MOOD-SR depressive and rhythmicity components in patients with BD and with the MOODS-SR manic component in HC. This result partially confirmed our third hypothesis, which suggested that later mid-sleep point may be related to more severe mood and anxiety symptoms in both HC and patients with BD. However, contrary to our expectations, correlations with anxiety symptoms did not emerge. Moreover, the aforementioned associations did not remain significant in regression analyses adjusting for potentially confounding factors. The fact that the mid-sleep point showed fewer associations with lifetime spectrum symptoms than the rMEQ may be explained by the state versus trait nature of the measurements. Lifetime mood and panic-agoraphobic symptoms, as measured through the MOODS-SR and PAS-SR scales, are focused on temperamental traits that could be more consistently associated with a trait measurement of chronotype (MEQ) than with a state measurement of the circadian typology (actigraphic mid-sleep point). Therefore, further studies should replicate our analyses using both state and trait measurements of mood and anxiety-related outcomes.
In our study, the mid-sleep point, measured by actigraphy monitoring, was correlated with the self-reported chronotype (rMEQ), confirming previous reports in the literature.Reference Gershon, Kaufmann and Depp 51 Still, the strength of the association was not particularly high. Indeed, we identified a discrepancy between actigraphic-based and rMEQ-based circadian typologies in 43.88% of cases. This discrepancy was estimated using the CCDI, an index that we proposed to measure the potential misalignment between the desired circadian typology and the actual one. Interestingly, we found that the discrepancy, as measured through the CCDI, was associated with higher depressive spectrum symptoms in HCs. This result could suggest that the misalignment between the subjective and objective circadian typology may negatively affect mental health, particularly when actigraphy classifies subjects with a self-reported evening preference as MTs. It could be in accordance with previous reports in the literature suggesting that circadian misalignment experienced by ETs may contribute to their susceptibility to mental health disturbances.Reference Wittmann, Dinich, Merrow and Roenneberg 71
The main limitation of our study is the sample size; however, to optimize the scope of our estimates, the multivariate analyses were adjusted for potentially confounding factors. Our study is observational and presents the typical limitations of observational studies, such as lack of generalizability and inability to generate a direct causal interpretation. Unobserved background variables may affect both chronotype and the appearance of psychopathological symptoms. For instance, exposure to traumatic events may be associated with severe mood disturbances,Reference Stratta, Capanna, Riccardi, Carmassi, Piccinni, Dell’Osso and Rossi 72 and simultaneously, the reaction to traumatic events may be worse in subjects with mood disorders,Reference Dell’Osso, Stratta, Conversano, Massimetti, Akiskal, Akiskal, Rossi and Carmassi 73 particularly those with evening preference.Reference Carmassi, Cruz-Sanabria, Gravina, Violi, Bonelli, Dell’Oste, Pedrinelli, Frumento, Faraguna and Dell’Osso 74 Therefore, trauma exposure could be evaluated in further studies with the aim of exploring associations between chronotype mood and anxiety. Moreover, as chronotype has been associated with resilience levelsReference Bazzani, Bruno, Frumento, Cruz-Sanabria, Turchetti and Faraguna 75 and resilience operates as a protective factor from stress symptom development,Reference Stratta, Capanna, Dell’Osso, Carmassi, Patriarca, Di Emidio, Riccardi, Collazzoni and Rossi 76 further studies in the field could also evaluate individual resilience levels. Another potential limitation of the present study is the cross-sectional design that does not evaluate the progression of mood and panic-agoraphobic spectrum symptoms. Despite these limitations, results derived from our study provide evidence of the strong associations between mood disturbances and eveningness in BD and HC and suggest potential associations between extreme chronotypes and anxiety-related symptoms in BD. In addition, we found that the discrepancy between subjective and objective circadian typology could negatively affect mood.
Despite the belief that circadian preference is invariant, previous reports in the literature have shown successful interventions in modifying circadian preference in healthy subjects by manipulating light exposureReference Harvey, Hein and Dolsen 77 or using a Transdiagnostic Sleep and Circadian Intervention.Reference Wright, McHill, Birks, Griffin, Rusterholz and Chinoy 78 Additionally, in patients with mood disorders, bright light therapy, melatoninergic agonists, and behavioral interventions have been effective.Reference Gottlieb, Benedetti and Geoffroy 79 -Reference Chan, Chan and Li 81 Further studies could evaluate the discrepancy between objective and subjective measurements of circadian typology to identify subjects with a potential misalignment between desired and actual sleep patterns that could benefit from chronotherapy. Moreover, further studies with a longitudinal design could identify risk factors such as trauma exposure and substance use, among others, evaluating the role of these factors on the association between circadian typology and the risk of suffering mood and anxiety-related disturbances.
Conclusions
This exploratory study investigated the relationship between chronotype, mid-sleep point, and lifetime panic-agoraphobic and mood spectrum symptoms in patients with BD and HCs. Our findings confirm that in patients with BD there is a higher prevalence of ET compared with HCs. Additionally, we found that eveningness was associated with depressive mood and altered rhythmicity in both HCs and patients with BD. Conversely, only in patients with BD were both extreme chronotypes (morning and ETs) associated with anxiety symptoms.
Our results highlight the role of chronotype as a relevant phenotype associated with mood symptoms in HCs and patients with BD and with a higher comorbidity burden in BD. Furthermore, the objective mid-sleep point was associated with depressive symptoms in the group of HCs, and the discrepancy between actigraphic-based and rMEQ-based circadian typology was associated with depressive symptoms only in HC.
Based on our findings, further studies are required to confirm whether extreme MT is linked with worse clinical outcomes in BD, particularly related to anxiety symptoms. Furthermore, additional research could explore whether objective measurements of sleep duration, sleep regularity, and mid-sleep point may mediate the appearance of mood disturbances, including symptoms related to suicide in subjects classified as ET, including both clinical and nonclinical populations. Studies comparing both trait and state measurements of mood and anxiety symptoms, with respect to circadian typology are also required. Finally, the applicability of the CCDI should be examined in other settings, including clinical and nonclinical populations. Our findings pave the way to interventional studies targeting circadian typology in an attempt to prevent or treat mental health disorders.
Acknowledgments
The authors thank the participants and their relatives for their generous contribution in this research.
Financial support
This work has been partially supported by Italian Ministry of Health under grant-RC 1.21 “Monitoraggio e tele-monitoraggio del sonno in età evolutiva e in pazienti adulti” and the 5 × 1000 voluntary contributions. Fondazione Arpa Onlus, a nonprofit organization (https://fondazionearpa.it).
Author contributions
Conceptualization: F.C-S., C.C., Data curation: F.C-S., M.V., L.M., C.A.B., V.D.; Formal analysis: F.C-S., P.F.; Funding acquisition: U.F.; Investigation: F.C-S., M.V., S.B., L.M.; Methodology: F.C-S., A.B., Project administration: L.D., U.F., C.C., Resources: L.D., U.F., C.C.; Software: S.B., P.F., U.F.; Supervision: U.F., L.D., C.C.; Validation: L.D.; Writing—review & editing: F.C-S., A.B., S.B., C.A.B., V.D., P.F., U.F., C.C.; Writing—original draft: F.C-S.
Disclosures
U.F. is co-founder and president of SleepActa S.r.l, a spin-off company of the University of Pisa operating in the field of sleep medicine. All other authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852923001207.













