Introduction
Visceral leishmaniosis (VL) is a zoonotic infectious disease caused by protozoa belonging to the genus Leishmania, which affects humans and both domestic and wild animals [Reference Baneth1]. It is present in Asia, Africa, Europe and the Americas, in more than 88 countries in tropical and subtropical regions [2]. In Brazil, VL is transmitted by means of dipterous vectors of the subfamily Phlebotominae, which encompasses several species of the genus Lutzomyia [Reference Bates3]. Dogs play a fundamental role in the epidemiological chain of VL, since they are considered the main urban reservoir of the pathogen [Reference Baneth1].
The epidemiological scenario of VL in Brazil has been changing from a sporadic pattern that was eminently prevalent in rural areas to a condition with periurban epidemics that can affect all social strata of the population. It has thus become a serious threat to public health [4]. Worldwide, 310 000 new cases of VL occur every year, and 90% occur in Bangladesh, Brazil, Ethiopia, India, Nepal, South Sudan and Sudan [2]. In the state of São Paulo, Brazil, recent records show that VL transmission is present in 105 municipalities [Reference Rangel5].
A VL control plan has been implemented in Brazil, based on three strategies: early diagnosis and treatment of human cases; serological screening of dogs in endemic areas, followed by culling of seropositive animals; and use of insecticides in areas of notified human foci. Treatment of infected dogs is not recommended, given that there is some controversy regarding whether a parasitological cure can be achieved, even if a clinical cure is achieved, which would thus maintain these dogs as sources of infection [4].
Other strategies for controlling VL have been put forward, with the aim of controlling the disease in dog populations, such as the use of collars impregnated with insecticides and use of immunoprophylaxis. Use of insecticide-impregnated collars is a strategy that has shown efficacy for controlling VL in dogs, given that these collars promote insecticidal and insect-repellent effects [Reference Killick-Kendrick6–Reference Foglia Manzillo8].
Until 2014, two commercially available vaccines have already been used in field studies in Brazil. The vaccine Leishmune® (Zoetis Indústria de Produtos Veterinários, Brazil) is composed of the fucose and mannose-ligating glycoprotein complex antigen of Leishmania donovani and adjuvant saponins, which ensures the vaccinal protection of 92% and efficacy of 76% [Reference da Silva9]. However, in the year 2014, the license for manufacturing and commercialising Leishmune® in Brazil was suspended.
The other vaccine that has been distributed on the Brazilian market is a recombinant vaccine named Leish-tec® (Hertape Calier Saúde Animal, Brazil), which uses the antigen A2. This antigen is a specific protein of the amastigote stage of several species of the genus Leishmania and it induces a protective immune response against canine VL [Reference Coelho10, Reference Zanin11]. A comparative study among animals vaccinated with Leishmune® or Leish-tec® showed that there was no significant difference in parasitism between the animals in these two groups, 11 months after vaccination [Reference Fernandes12]. Subsequently, an efficacy study determined that the efficacy of the vaccine Leish-tec® was 71·4% when the animals were considered to be infected if they had a positive serodiagnosis that was confirmed through parasitological methods [Reference Regina-Silva13].
Despite the existence of an official control programme in Brazil, VL in dogs and humans continues to advance in this country. There is, therefore, an urgent need to develop effective control measures against VL to deter its advance. Achieving success for effective VL control strategies depends on knowledge of the infection dynamic parameters among the various participants of the epidemiological chain of the disease.
Thus, the present study had the objective of evaluating the effectiveness (EF) of two commercial vaccines for reducing Leishmania infection in dogs (Leish-tec® and Leishmune®), the EF of using collars impregnated with the insecticide pyrethroid (Scalibor®, MSD Animal Health) and the EF of associations between these measures. This was through a cohort study conducted in the extreme west of the state of São Paulo, Brazil, which is an endemic region [Reference Rangel5]. Knowledge of parameters relating to vaccine coverage, vaccine EF and the EF of using collars impregnated with repellent insecticides is likely to provide important support for modelling studies on infections, with a view to establishing control measures against this important zoonosis.
Material and methods
Study design
A prospective study was designed to evaluate the EF of the insecticide-impregnated collar, vaccine and the association of vaccine with collar for reducing Leishmania infection in dogs. Two immunogens, among those commercially available in Brazil at the beginning of the experiment, were employed in this study. The study was conducted in the municipality of Panorama, which is located in the west of the state of São Paulo (21°21′00″S and 51°51′36″W). The study began in August 2012 and ended in January 2014.
Six cohorts were formed: a cohort of animals with collars impregnated with the insecticide Scalibor® (COL); a cohort of animals vaccinated with Leishmune® (V1); a cohort of animals vaccinated with Leishmune® and with Scalibor® collars (V1C); a cohort of animals vaccinated with Leish-tec® (V2); a cohort of animals vaccinated with Leish-tec® and with Scalibor® collars (V2C); and a cohort of non-collared and unvaccinated animals (CTRL).
The size of the cohorts was defined by taking the confidence interval to be 95% and the test power to be 80%. The proportion of cases in the control group was taken to be 40% [Reference Bortoletto14], and the proportion of cases in collared dogs was estimated under an efficacy of 72·3% [Reference Foglia Manzillo8]. By using these parameters, the cohort of collared dogs was estimated to be 41 individuals. For both cohorts of vaccinated animals, the efficacy was taken to be 76% [Reference da Silva9]. At the time of designing this experiment, there were no studies determining the efficacy of the vaccine Leish-tec®, and this only became known much later on [Reference Regina-Silva13]. The sample size for the vaccinated cohorts was estimated as 37 individuals. Thus, it was decided to form all the cohorts with at least 41 individuals.
Dogs were selected during a census survey conducted in 2012–2013 jointly with the technical team of the public health surveillance service of the municipality of Panorama. The technical team annually test the entire population of dogs with the Dual Path Platform rapid test (TR-DPP® Bio-manguinhos FIOCRUZ) (TR-DPP). The TR-DPP positive dogs are retested by using ELISA (ELISA for visceral canine leishmaniosis, Bio-manguinhos FIOCRUZ) and those that prove positive are culled.
To ensure that all cohorts contained animals under risk of VL infection, the following sampling design was used: the TR-DPP-positive dogs at the census survey were cartographically identified and a complete circular area of radius 100 m around the location of each seropositive dog was defined. Following this, TR-DPP-negative healthy dogs of approximately the same age and size belonging to each area were selected to participate in each cohort. The following parameters were used to define a healthy clinical state: good body score, normal mucosal coloration, the absence of skin lesions (wounds, alopecia, opaque hair and crusty lesions), the absence of onychogryphosis and normal-sized lymph nodes, as determined through palpation.
The selection of animals to form the cohorts began in August 2012 and ended in January 2013. Cohorts were then formed in both dry and rainy periods, so that climatic conditions interfered equally in all cohorts.
The TR-DPP negative dogs selected as described above were visited again 48 h after the census survey and their owners were invited to participate in the experiment. Owners who wished to participate signed a free and informed consent statement. Then, the control measures were applied and biological samples were collected from the dogs.
The following biological samples were collected: serum, whole blood in sodium citrate and popliteal lymph node aspirates. The serum samples were tested by means of TR-DPP, whilst the whole blood and lymph node aspirates were tested by means of real-time PCR (qPCR-BL and qPCR-LN, respectively) (see below). Only the animals that were negative to all the tests continued in the study. All the participating animals presented a healthy clinical state, as shown by the semiological assessment. This was the day 0 of the experiment.
The homes visited were marked with the aid of global positioning system recording equipment (GPSmap 60CS; Garmin®). Quantum GIS® software (QGIS), version 1.8.0 Lisbon, were used to plot points on a map of the municipality, thus making it possible to view all the homes from which dogs were sampled.
The animals in groups V1, V1C, V2 and V2C were revisited 21 and 42 days after application of the first dose of the vaccine, solely for the purpose of applying the second and third doses of the vaccine, in conformity with the prescriptions of the immunogen manufacturers. The animals in groups COL, V1C and V2C also received additional visits so that the collars could be changed, which was done every four months.
The incidence of Leishmania infection in dogs was estimated by means of TR-DPP, qPCR-BL and qPCR-LN 180 and 360 days after the day 0. Animals that were seronegative for the TR-DPP, qPCR-BL and qPCR-LN were considered uninfected. Those that were positive for at least one of these tests were considered infected.
All the participating animals were tagged with electronic devices for identification (Animal Tag® Mascotes, Korth RFID Ltda.) and were dewormed in accordance with the posology indicated by the manufacturer of the drug used (Vermivet® compound, Biovet SA).
This study had previously been approved by the Ethics Committee for Animal Use of the School of Veterinary Science of the University of São Paulo (FMVZ-USP), under procedural no. 2370/2011.
Collection of biological samples
Blood samples were collected aseptically in two aliquots: one with and the other without the addition of anticoagulant (sodium citrate). Serum was then obtained from the blood aliquot without anticoagulant, transferred to Eppendorf tubes of volume 1·5 ml and was kept at −20 °C until the time of the analyses, as also were the aliquots of whole blood. The popliteal lymph node aspirates were collected from the animals aseptically, after the skin in the area had been shaved and disinfected using 70% alcohol with 2·5% iodation. The aspirates were performed with the aid of a syringe and needle, and the biopsy product was immediately resuspended in 500 µl of 0·9% physiological saline, in Eppendorf tubes of volume 1·5 ml. These microtubes were then stored at −20 °C until the time of the analyses.
Diagnostic tests: TR-DPP, qPCR-BL and qPCR-LN
As stated earlier, the TR-DPP test was used for the serodiagnosis. This test is based on the reaction of IgG from the animal tested, to the antigen K28, which is specific for Leishmania (Leishmania) infantum chagasi immobilised in the solid phase.
For the qPCR-BL and qPCR-LN, DNA was extracted from whole blood and from the suspension of popliteal lymph node aspirate by means of the DNeasy Blood & Tissue Kit (Qiagen®, Hilden, Germany). The sample size used was 200 ml of whole blood or popliteal lymph node aspirate suspension, in accordance with the protocol recommended by the manufacturer for DNA extraction from the blood. Ten microlitres of DNA extracted from each sample were amplified with the aid of the LightCycler® 480 Probes Master kit (Roche Diagnostics Ltd, Switzerland) in microplates for real-time PCR, using the LightCycler 480II equipment (Roche Diagnostics Ltd, Switzerland). The hybridisation primers and probes described by Francino et al. [Reference Francino15] were used: primers LEISH-1 (5′– AAC TTT TCT GGT CCT CCG GGT AG – 3′), LEISH-2 (5′ – ACC CCC AGT TTC CCG CC – 3′) and hydrolysis probe FAM – 5′– AAA AAT GGG TGC AGA AAT – 3′– BHQ1 non-fluorescent quencher. The samples were tested in duplicate. The amplification curves were analysed using the LightCycler 480 SW 1.5.1 software, using the results from calculating the maximum second derivative as the criterion, in accordance with the software manual.
Statistical analysis
Relative risk (RR) and 95% confidence interval of RR (95% CI–RR) were calculated by using Cox proportional-hazards regression model [Reference Morgenstern, Kleinbaum and Kupper16]. This was through a cohort study considering the incidence and time of total exposure over a period of 1 year (method to estimate the incidence of infection was based on days of observed exposure). The incidence of Leishmania infection in dogs was estimated by means of serological and molecular diagnostic tests (as described above), 180 and 360 days after the application of the control measures. The EF of the control measures was EF = 1 − RR. Data were analysed by using the software R [17] survival package [Reference Therneau18].
Results
Three hundred and eighty-one TR-DPP-negative dogs were selected to integrate the six cohorts. However, 81 of them were excluded because they were found to be positive for at least one of the three tests used (TR-DPP, qPCR-LN and qPCR-BL) at the day 0 (Table 1). Among these 81 samples, 73 were negative for TR-DPP and positive for qPCR-LN or qPCR-BL, five were positive for TR-DPP and negative for qPCR-BL and qPCR-LN, and three were positive for all the tests. Thus, the total number of participant animals was reduced to 300.
Table 1. Frequencies of Leishmania-infected dogs in each cohort over the course of the experiment and the effectiveness (EF) of the control measures
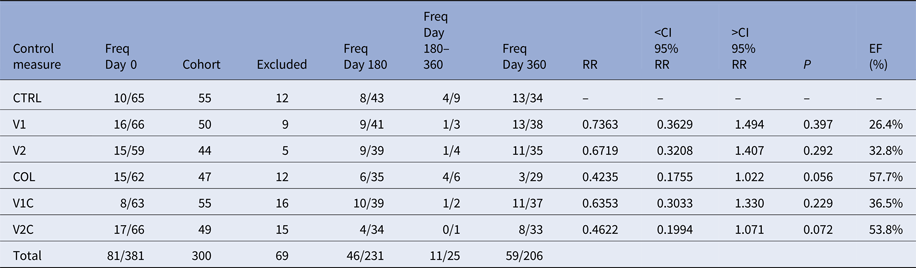
Freq Day 0, frequency of Leishmania-infected dogs at day 0; Cohort, number of dogs forming each cohort after exclusion Leishmania-infected dogs at day 0; Excluded, number of dogs excluded from the experiment between days 0 and 180; Freq Day 180, frequency of Leishmania-infected dogs among the survivors at day 180; Freq Day 180–360, frequency of Leishmania-infected dogs among the dogs that left the experiment between days 180 and 360 of the experiment; Freq Day 360, frequency of Leishmania-infected dogs among the survivors at day 360; RR, relative risk; <CI 95% RR, inferior confidence interval 95% of relative risk; >CI 95% RR, superior confidence interval 95% of relative risk; P, P-value; EF (%), effectiveness of the control measure.
Over the course of the experiment, 69 animals left the study for a variety of reasons, such as loss of the animal (death for any reason, ran away or disappeared), owner moving home and non-adherence of the owner to the experiment. The number of animals per cohort excluded for these reasons ranged from five to 16, depending on the cohort that the animal belonged to (Table 1). In 27 of these cases, the use of collars was discontinued and in other two cases the owners decided to fit collars in their dogs. In these two cases, the dogs belonged to cohorts in which collars were not supposed to be used.
Two hundred and thirty-one dogs survived until day 180 of the study, 25 dogs were lost between days 180 and 360 and 206 dogs survived until day 360. The numbers of VL-infected dogs in each group are shown in Table 1.
In all cohorts, the estimates of the relative risks were not significant. Nevertheless, the data showed a tendency that the use of collar is the most effective measure to Leishmania-infection control in dogs. From the results, EF the Leish-tec® vaccine was between 0% and 67·9% with an expected value of 32·8%. Leishmune® vaccine had EF between 0% and 63·7% with an expected value of 26·4%, and the collar had an expected EF of 57·7% with a confidence interval between 0% and 82·5% (Table 1).
Discussion
This study reports the results from a prospective survey aiming at the determination of the EF of control measures against infection by a causative agent of VL in dogs, using cohorts that were defined based on previous knowledge on the efficacy and protection values of these measures. In this study, animals were not clinically evaluated, thus we did not infer how effective the control measures were in terms of protection against VL. We measured the capability of the vaccines and collars to protect against infection, but not a disease.
All the VL control measures showed EF values with poor performance, even though the amplitude of the 95% CI–RR was large in all cases. To make the 95% CI–RR narrower, the cohort size would have to be increased. The cohorts of this experiment were formed with numbers of participants that were typically larger than the number that had been defined through the sample calculation. However, the number of animals that were eliminated during the experiment was surprisingly high and the reduction in cohort's sizes was greater than expected. On the other hand, none of the owners dropped out of the study. Except for the 27 cases of misuse of the collar, the owners were collaborative. This shows that dog owners are willing to cooperate through participating in surveys of this nature.
Even in the absence of significant statistical difference in RR, it was notable, from the 95% CI–RR, that the performance of the collars impregnated with insecticide had a tendency to be better than that of measures based solely on immunoprophylaxis. In a survey conducted in a region with a canine prevalence of 3·1%, the use of insecticide-impregnated collars has been shown to be effective in reducing the incidence of infection both in dogs and in humans [Reference Camargo-Neves, Rodas and Pauliquévis Junior19]. In canine leishmaniasis-endemic regions in Italy, delthametrin-impregnated collar reduced the incidence of the infection in 72 and 84% within 1 year of observation [Reference Foglia Manzillo8, Reference Ferroglio, Poggi and Trisciuoglio20]. However, in these two field studies the detection of the infection was performed by using only serological methods. Brianti et al. in Italy [Reference Brianti21] also reported a reduction of incidence of infection after treatment of dogs with delthametrin-impregnated collar for 1 year. In this case, the EF of the collar was 62% and the dogs were tested by means of PCR, bone marrow smears and serology. In our study, we also used PCR and serology to detect the infection and the EF of the collars was even lower than that reported by Brianti et al. [Reference Brianti21]. This indicates that using molecular methods in addition to serology might give a more realistic estimate of the EF of the control measure in preventing infection and might explain, in addition to other epidemiological reasons, the difference in the results between this study and others.
Use of collars has been shown to have a greater impact in canine populations in which the infection transmission rate is lower and it may be an efficient control measure in areas of high endemicity if owners collaborate through correct use of this measure, with the efficient replacement of the device in the event of loss [Reference Reithinger22]. In the present experiment, the animals that were fitted with impregnated collars were followed up every four months and the results were only taken into account if the device had been correctly used.
The efficacy of the vaccine Leish-tec® was determined in a field study with the aid of a combination of the ELISA and the Imunnoflorescent Antibody Test, which used to be the official diagnostic methods for VL in Brazil. The diagnosis of seropositive animals was confirmed by means of parasitological methods. Using this criterion, Regina-Silva et al. [Reference Regina-Silva13] found that the efficacy of the immunogen was 71·4%. After including the xenodiagnosis, the positivity for VL increased, thus leading to a decrease in the efficacy of the vaccine Leish-tec® to 58·1%. In this case, by increasing the sensitivity of the criterion for determining the positivity of the animals, the efficacy of the measure was drastically reduced, just as in the present study, in which a criterion of high diagnostic sensitivity was used to classify infected animals. Vaccines against VL in Brazil have been shown to have better performance than what was shown in the present study. However, it needs to be taken into consideration that the differences between the diagnostic tools used for VL diagnosis might be a determining factor with regard to variations in the results.
Although qPCR has been shown to be an appropriate method for detecting asymptomatic infected animals [Reference Carson23], the role played by qPCR-positive asymptomatic dogs in VL transmission needs to be better assessed [Reference da Costa-Val24–Reference Laurenti26]. Infectiousness of dogs to the sandfly vector is associated with high parasite numbers [Reference Ribeiro27, Reference Courtenay28] and qPCR is capable of detecting very small quantities of the parasite in infected dogs [Reference Francino15, Reference Quaresma29]. Here, parasite burdens were not measured, and thus infectiousness of the qPCR-positive dogs was not assessed. Therefore, it is worthwhile stressing that cohorts were not compared in terms of infectiousness of their dogs.
With regard to the differences between studies on the EF and efficacy of vaccine performance, it is important to differentiate that in the first case, the control measures are evaluated without taking into consideration the animals’ ‘window of susceptibility’, i.e. the period prescribed by the manufacturer of the product during which the protection has not yet reached its maximum value. It is noteworthy mentioning that vaccines should prevent against disease after the third dose, i.e. 42 days after application of the first dose, while collars reach full EF 4 weeks after application [Reference David30]. The present study evaluated EF such that the vaccinated animals and those fitted with collars were exposed to risk immediately after the measures had been applied. This difference in approach may explain the difference in vaccine performance in relation to what has been described in other studies [Reference da Silva9, Reference Regina-Silva13]. Considering the control measures on a population-based scale, EF values should be taken to be those that realistically determine the success of the measures applied.
According to the prescriptions of manufactures of immunogens distributed in Brazil, animals that are serologically negative for VL are eligible for immunisation. However, use of serodiagnosis methods alone for distinguishing between infected and uninfected animals does not seem to be the most appropriate method, because many false-negative animals may be subjected to immunisation. Therefore, only non-reactive animals for the three tests (TR-DPP, qPCR-BL and qPCR-LN) participated in the experiment. In a recent paper, we have evaluated the diagnostic tests employed here: 975 dogs from this population were surveyed by using TR-DPP, qPCR-BL and qPCR-LN for the diagnosis of VL. When TR-DPP-negative dogs were tested by qPCR applied in blood and lymph node aspirates, 174/887 (19·6%) were positive in at least one sample [Reference Lopes31].
As part of the study caveats, we should remark on the estimate of prepatency of the infection in dogs. Although only dogs negative on qPCR-BL, qPCR-LN and TR-DPP were selected to form the cohorts, the number of animals that were actually infected in spite of being tested negative is unknown. In leishmaniasis endemic region in Brazil, the expected time from exposure to serological and parasitological detection was estimated at about 200 days [Reference Quinnell32], but the estimated time from exposure to serological and molecular detection is unknown.
Here, a significant proportion of the individuals had to be withdrawn from the study after diagnoses were made on the samples collected at day 0. According to the Brazilian official classification criterion for infected animals, dogs that are serologically negative through the TR-DPP are considered free from infection. These that are positive for this test are retested using ELISA and only with a positive result from this second test is the animal considered to be infected [4].
Caution is needed in the interpretation of results presented here, because of the consequences imposed by the small sample size in each cohort. Even so, under the conditions used for this study, none of the immunogens for VL control was shown to be sufficiently effective with regard to protection against infection. Despite the drawbacks presented in this investigation, none of the superior limits of 95% CI–RR was close to what would be desirable for satisfactory EF. The absence of significance of RR might be associated with the lack of power of the test aggravated by the losses of individuals in the cohorts. However, it is possible to infer that the EF of the Leish-tec® vaccine is significantly different from 71·4% [Reference Regina-Silva13]. This suggests that the performance of the vaccines distributed in Brazil is inferior to the values typically reported from other studies.
In conclusion, under the conditions of this study, none of the immunogens for VL control was sufficiently effective to protect dogs against infection. On the other hand, use of collars impregnated with insecticide seems to constitute a method with better prognosis, corroborating other studies in this field.
Acknowledgements
This study was sponsored by FAPESP, grant number 2011/21796-2. F. F., C. M. N. and E. A. B. G. are the recipient of productivity fellowship from CNPq. E. G. L. received the scholarships from FAPESP (2011/14892-5).
Declaration of Interest
None.



