Choline is present in a wide variety of foods, with egg yolks and liver containing the largest amounts(Reference Niculescu and Zeisel1). There is a considerable controversy regarding the beneficial/detrimental role of dietary choline intake. It has also been proposed that lower choline consumption may alter the epigenetic regulation for a series of genes which may accelerate the atherogenic process(Reference Zaina, Lindholm and Lund2). There is also important crosstalk between choline metabolism and the pathways of insulin sensitivity, fat deposition and energy metabolism through epigenetic modifications(Reference Millard, Musani and Dibaba3). Furthermore, the metabolic pathway of dietary choline intake involving intestinal microbiota has been implicated in the pathogenesis of CVD(Reference Wang, Klipfell and Bennett4).
Some studies have shown that dietary choline consumption was inversely associated with C-reactive protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α)(Reference Rajaie and Esmaillzadeh5, Reference Detopoulou, Panagiotakos and Antonopoulou6). Therefore, there is a hypothesis that choline intake may protect against CVD, by improving the inflammatory status(Reference Mazidi, Rezaie and Kengne7).
Evidence linking choline and insulin resistance (IR) is limited and largely based on animal experiments. Only one study in humans reported improved IR in individuals with a higher choline intake(Reference Gao, Wang and Randell8). In another human study, serum choline levels were inversely associated with the risk for type 2 diabetes (T2D)(Reference Chen, Chen and Wang9). Furthermore, decreased serum choline levels served as possible predictors of impaired glucose tolerance (IGT) and IR in the pre-diabetic state(Reference Li, Chen and Liu10). Just a single study examined the effects of dietary choline intake on body composition; a high dietary choline consumption was significantly associated with a favourable body composition(Reference Gao, Wang and Randell8).
To the best of our knowledge, only two studies evaluated the link between CVD mortality and choline intake showing diverse results(Reference Zheng, Li and Rimm11, Reference Nagata, Wada and Tamura12), i.e. a Japanese study reported no association between intake of phosphatidylcholine and CVD mortality(Reference Nagata, Wada and Tamura12), whereas an American study found a positive relationship between choline consumption and CVD mortality(Reference Zheng, Li and Rimm11). The Japanese study also evaluated the association between stroke mortality and choline intake, with null findings(Reference Nagata, Wada and Tamura12). With regard to all-cause death, there is also just one study involving US adults, reporting a positive link with choline intake(Reference Zheng, Li and Rimm11). Furthermore, the Atherosclerosis Risk in Communities study (ARIC), involving 14 430 middle-aged men and women, found a non-significant association between choline consumption and CVD incidence(Reference Bidulescu, Chambless and Siega-Riz13). This finding was further supported by the Jackson Heart Study (n 3924), showing a positive relationship between choline intake and CHD incidence, but a reverse link between choline consumption and ischaemic stroke incidence(Reference Millard, Musani and Dibaba3). In contrast, in another study in 16 165 women (age range: 49–70 years), there was no significant link between CVD and choline intake(Reference Dalmeijer, Olthof and Verhoef14). Further studies are needed to adjust for some confounders, such as fibre intake(Reference Rajaie and Esmaillzadeh5, Reference Meyer and Shea15), in order to elucidate the association between choline intake and CVD.
Given the paucity of studies and the controversial findings, we analysed a large and nationally representative sample of US adults to better understand the links of dietary choline with mortality and cardiometabolic risk factors. Furthermore, changes in cardiometabolic risk factors were cross-sectionally evaluated across choline intake quartiles. Finally, we systematically reviewed the literature for studies of dietary choline in relation to total and cause-specific mortality (CVD and stroke), and performed a random-effects meta-analysis to generate summary relative risks (RR).
Methods
Original data
Population
In the present study, results from both prospective and cross-sectional studies including data from the US National Health and Nutrition Examination Survey (NHANES) were used. The National Center of Health Statistics (NCHS) Research Ethics Review Board approved the underlying protocol and written informed consent was obtained from all participants. The current study is based on the analysis of data from two 2-year NHANES survey cycles between 1999 and 2010, restricted to participants aged ≥20 years. Details on NHANES Laboratory/Medical Technologists Procedures and Anthropometry Procedures are described elsewhere(Reference Remer16, Reference Engberink, Bakker and Brink17).
Anthropometric data
A digital scale was used to measure weight to the nearest 100 g and a fixed stadiometer to measure height to the nearest mm. BMI was calculated as weight in kg divided by the square of height in m. Waist circumference (WC) was measured at the iliac crest to the nearest mm, using a steel tape(18).
Biochemical parameters
A blood specimen was drawn from an antecubital vein. Glycated Hb (HbA1c) was measured using a Tosoh A1C 2.2 Plus Glycohemoglobin Analyzer (Tosoh Bioscience). Fasting blood glucose was measured by a hexokinase method using a Roche/Hitachi 911 Analyzer and Roche Modular P Chemistry Analyzer. Insulin was measured using an ELISA immunoassay (Mercodia)(Reference Mazidi, Michos and Banach19). Other laboratory-test details are available in the NHANES Laboratory/Medical Technologists Procedures Manual(20). Details on CRP measurement are available elsewhere(18). The anthropometrically predicted visceral adipose tissue (apVAT) was calculated with sex-specific validated equations that included age, BMI, WC and thigh circumference(Reference Samouda, Dutour and Chaumoitre21). The equation for men was 6 × WC – 4·41 × proximal thigh circumference +1·19 × age – 213·65; and the equation for women was 2·15 × WC – 3·63 × proximal thigh + 1·46 × age + 6·22 × BMI −92·713(Reference Samouda, Dutour and Chaumoitre21).
Dietary assessments
We have previously described the dietary assessments of NHANES(Reference Mazidi, Kengne and Mikhailidis22). Dietary intake was assessed via 24-h recall obtained by a trained interviewer, with the use of a computer-assisted dietary interview system with standardised probes, i.e. the United States Department of Agriculture (USDA) automated multiple-pass method (AMPM)(Reference Ahluwalia, Andreeva and Kesse-Guyot23, Reference Ahluwalia, Dwyer and Terry24). Briefly, the type and quantity of all foods and beverages consumed in a single 24-h period before the dietary interview (from midnight to midnight) were collected with the use of the AMPM. The AMPM is designed to enhance complete and accurate data collection while reducing respondent burden(Reference Ahluwalia, Dwyer and Terry24, Reference Moshfegh, Rhodes and Baer25). The USDA estimated the choline contents of NHANES foods in recipes by linking the ingredients in survey food recipes to food composition data provided by the USDA National Nutrient Database for Standard Reference. Total choline is the sum of free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine and sphingomyelin.
Mortality
The anonymised data of NHANES 1999–2010 participants were linked to longitudinal Medicare and mortality data using the NHANES assigned sequence number. Mortality follow-up data are available from the date of the survey participation until 31 December 2011. We examined all-cause mortality, as well as mortality due to CVD (I00–I09, I11, I13, I20–I51, I60–I69) and cerebrovascular disease (I60–I69). Cause of death was determined using the 10th revision of the International Classification of Diseases (ICD-10).
Statistical analysis
The guidelines set by the Centers for Disease Control and Prevention(26) were applied for analysis using a SPSS® complex sample module version 22.0 (IBM Corp). Continuous and categorical demographic variables were compared across the choline consumption quartiles using ANOVA and χ2 tests, respectively. Adjusted (for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre and meat) means of cardiometabolic factors across choline consumption quartiles were calculated using ANCOVA. Adjusted (for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre and meat) logistic regression was also performed to determine the risk of hypertension and T2D across choline intake quartiles; the results are expressed as OR and 95 % CI. The first quartile (Q1) was always used as reference for all models.
Multivariable Cox proportional hazards were applied to determine the hazard ratios (HR) and 95 % CI of mortality (total, CVD and stroke) for choline consumption; the first quartile (Q1) was always used as reference for all models. To derive HR and 95 % CI, two different models were used i.e. Model 1: Adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre, polyunsaturated to saturated fat, saturated fat and folate; Model 2: Adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre, PUFA, SFA, folate, BMI, hypertension and T2D. A two-sided P < 0·05 was considered significant.
Systematic review and meta-analysis
Literature search and study selection
The present meta-analysis was designed, conducted and reported according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines(Reference Stroup, Berlin and Morton27). The primary exposure of interest was choline consumption, while the primary outcome of interest was changes in total and cause-specific mortality subsequent to choline intake. Prospective cohort studies published up to January 2018 (without language restriction) were searched using PubMed, Embase, and Scopus databases; the query syntax of searching is shown in Table S1 in the online Supplementary material. This was complemented by hand searches of the reference list of eligible articles and email correspondence with authors for additional data, where relevant.
After excluding duplicates and based on titles and abstracts, we excluded studies on animals, or populations with baseline age ≥18 years, prior CHD, T2D or other chronic diseases. Eligible studies were selected by using predefined inclusion criteria of prospective cohort studies, healthy populations and original articles on the association of choline consumption and all-cause and cause-specific mortality (CHD and stroke). In addition, supplementary hand searching of reference lists of previous reviews or meta-analyses was conducted. Of eleven eligible full articles, two articles met the inclusion criteria (Fig. 1).
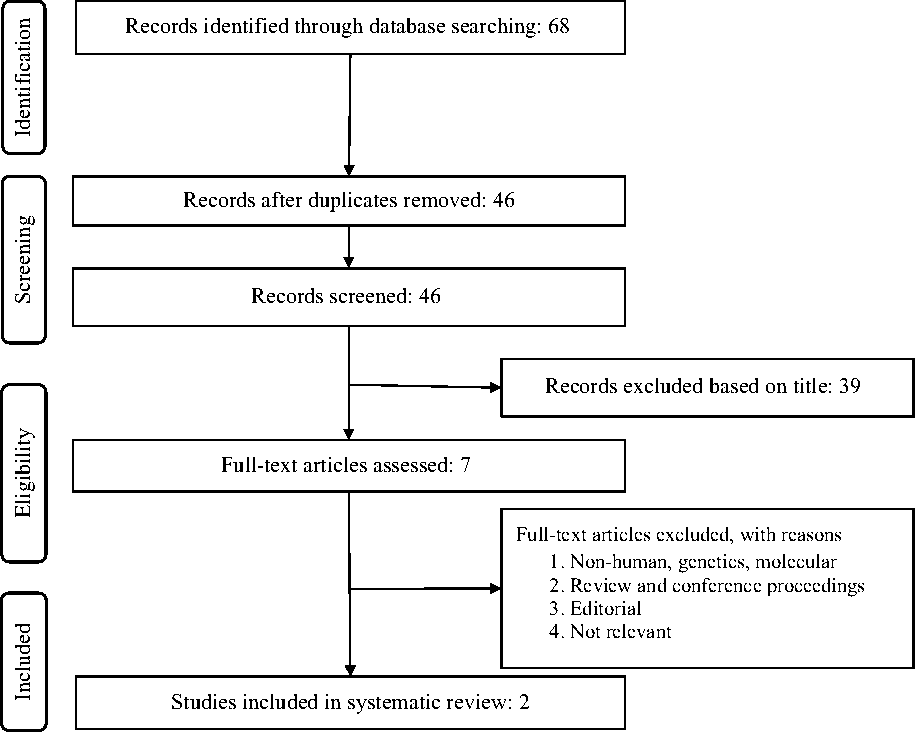
Fig. 1. Flow chart of the literature search for the meta-analysis on the relationship of choline consumption with overall and cause-specific mortality.
Study selection
Study selection started with the removal of duplicates, followed by screening of titles and abstracts by two reviewers (M. M. and M. B.). To avoid bias, they were blinded to the names, qualifications or institutional affiliations of the authors. The agreement between the reviewers was excellent (κ index: 0·91; P < 0·001). Disagreements were resolved at a meeting between reviewers prior to selected articles being retrieved (a flow chart is available in Fig. 1). We included studies if they met all the following criteria: (1) the point of interest was choline intake; (2) the studies were population-based cohort studies and reported cancer mortality data; (3) RR, HR or OR estimates with 95 % CI adjusted for multivariable factors were available or could be calculated. Studies were excluded according to the following criteria: (1) reviews, letters, unpublished results or comments; (2) those published in languages other than English; (3) not population-based cohort studies; (4) RR, HR or OR estimates with 95 % CI were not available or could not be calculated. Narrative reviews, comments, opinion papers, editorials, letters or any other publications lacking primary data and/or explicit method descriptions, were also excluded.
Data extraction and management
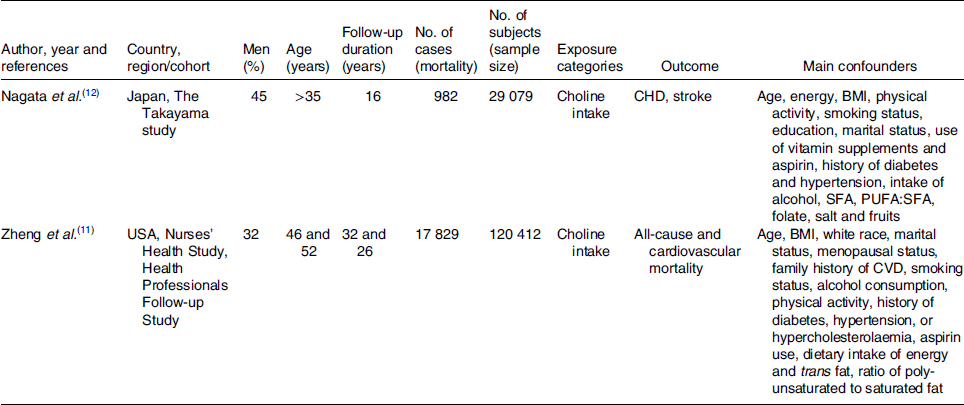
The full text of studies meeting inclusion criteria was retrieved and screened to determine eligibility by two reviewers (M. M. and N. K.). The study quality assessment was performed according to the Newcastle–Ottawa Scale (Table S2 in Supplementary material)(Reference Stang28). By evaluation of selection, comparability and outcome, the rating system scores studies from 0 (highest degree of bias) to 9 (lowest degree of bias). Additionally, we investigated the funding sources of all of the eligible studies. Following assessment of methodological quality, the two reviewers extracted data using a purpose-designed data extraction form and independently summarised what they considered to be the most important results from each study. These summaries were compared and any difference of opinion was resolved by discussion and consultation with a third reviewer (M. B.). Any further calculations on study data considered necessary were conducted by the first reviewer and checked by the second reviewer. Information extracted from each eligible study included the following items: author, year and references, country, study name, men (%), age, follow-up time (years), no. of cases, no. of subjects, exposure categories, outcome and main confounders (Table 1).
Table 1. Characteristics of the prospective cohort studies included in the meta-analysis
Data synthesis and statistical analyses
For studies that reported results from different multivariable-adjusted models, the model with the majority of confounding factors was extracted from the meta-analysis. The random-effects model was applied to calculate pooled RR, 95 % CI and P value for heterogeneity. RR comparing the highest with the lowest category were combined across studies to generate summary associations. The extent of heterogeneity across studies was examined using the I 2 test(Reference Mazidi, Rezaie and Gao29, Reference Mazidi, Kengne and Banach30); I 2 >50 % together with two-sided P < 0·05 indicated significant heterogeneity(Reference Mazidi, Rezaie and Gao29, Reference Mazidi, Kengne and Banach30).
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation and Egger’s weighted regression tests. The Duval and Tweedie trim method was used to adjust the analysis for the effects of publication bias(Reference Duval and Tweedie31). The present meta-analysis was conducted using the Comprehensive Meta-Analysis (CMA) V3 software (Biostat)(Reference Borenstein, Hedges and Higgins32).
Results
Original data
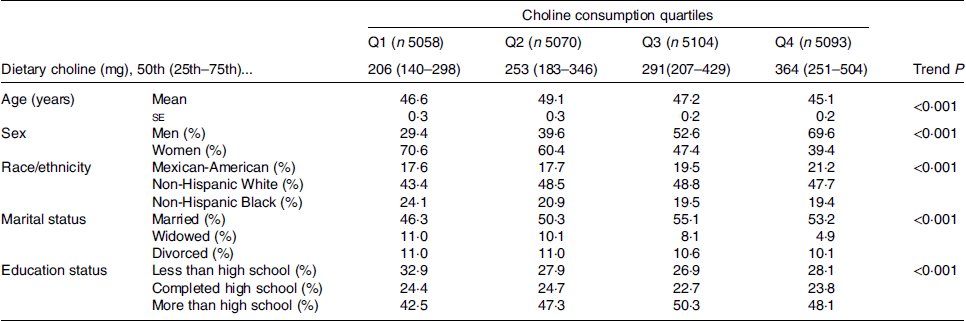
Overall, 20 325 individuals were included, with a mean age of 47·4 years and comprising 48·7 % men and 51·3 % women. Demographic characteristics of the participants are shown in Table 2. There was a significant age difference between quartiles of choline dietary intake; individuals in the highest quartile (Q4) were significantly younger than in the other quartiles (P < 0·001, Table 2). Of note, from the lowest quartile (Q1) of choline dietary intake to the highest quartile (Q4), the percentage of men significantly increased (Q1: 29·4 v. Q4: 69·6 %), while the proportion of women decreased (Q1: 70·6 v. Q4: 39·4 %) (Table 2).
Table 2. Characteristics of the study participants by choline consumption*
(Mean values with their standard errors; percentages)
* Groups across the quartiles compared by either χ2 or ANOVA.
We also calculated adjusted (for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre and meat) mean levels of cardiometabolic risk factors across increasing choline intake quartiles (Table 3). It was found that as individuals consumed more choline, they had a worse lipid profile (i.e. TAG and HDL-cholesterol) and glucose homeostasis (fasting blood glucose, HbA1c) but lower levels of inflammatory markers (CRP), while there was no difference in anthropometric parameters (BMI, WC and apVAT) and blood pressure (both systolic and diastolic, Table 3). For example, from the first (Q1) to the top quartile (Q4) of choline intake, CRP varied from 4·5 to 3·5 mg/l, respectively (P = 0·026, Table 3), whereas fasting blood glucose and HbA1c increased from 98·6 mg/dl (5·5 mmol/l) and 5·48 % to 100·6 mg/dl (5·6 mmol/l) and 5·72 %, respectively (P < 0·028 for both comparisons, Table 3).
Table 3. Characteristics of the study participants by choline consumption adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre and meat*
(Mean values with their standard errors)
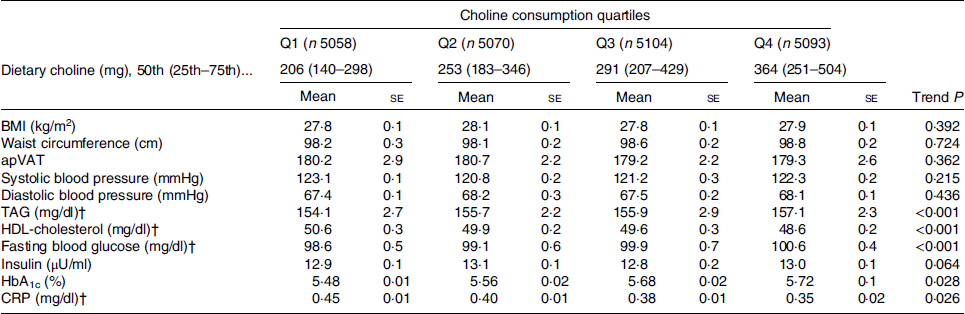
apVAT, anthropometrically predicted visceral adipose tissue; HbA1c, glycated Hb; CRP, C-reactive protein.
* Adjusted means were compared across choline consumption by using ANCOVA.
† To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert HDL in mg/dl to mmol/l, multiply by 0·0259. To convert glucose in mg/dl to mmol/l, multiply by 0·0555. To convert CRP in mg/dl to mg/l, multiply by 10.
By applying multiple logistic regressions adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, BMI, CRP, alcohol consumption, intake of fibre and meat, we found that individuals with the highest choline intake (Q4) had a greater likelihood of T2D (OR 1·34, 95 % CI 1·10, 1·99, P-trend < 0·001) compared with the first quartile (Q1), while no link was observed between the risk of hypertension and choline consumption (P = 0·421).
During the follow-up period of 76·4 months, 3431 total deaths were recorded, including 967 CVD and 233 stroke deaths. The distribution of total and cause-specific mortality across choline intake quartiles is shown in Table 2.
Results from multivariable Cox regression models for risk of death (total, CVD and stroke) across choline intake quartiles are shown in Table 4. With regard to total mortality, in model 1, individuals in the highest (Q4) choline intake quartile had a 48 % greater risk of total mortality (RR 1·48, 95 % CI 1·06, 2·05, P < 0·001) compared with those in the first quartile (Q1); this association persisted even after further adjustment in model 2 (RR 1·23, 95 % CI 1·09, 1·38, P < 0·001, Table 4). We found a positive and significant association between choline intake and CVD mortality, as individuals in the highest choline intake quartile had a 78 % higher risk of CVD mortality compared with those in the first quartile; the same trend was found in model 2 (RR 1·33, 95 % CI 1·19, 1·48, P < 0·001, Table 4). Dietary choline consumption was also positively and significantly associated with stroke mortality. For example, in model 2, individuals in the highest choline intake quartile had a 30 % greater risk of stroke mortality compared with those in the first quartile (RR 1·30, 95 % CI 1·02, 1·66, Table 4).
Table 4. Sex-stratified multivariable-adjusted hazard ratios for mortality across choline consumption
(Hazard ratios (HR) and 95 % confidence intervals)
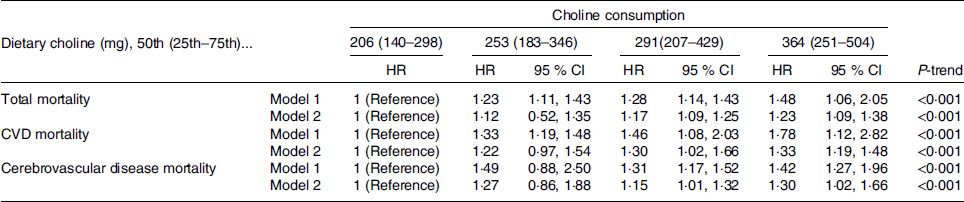
* Model 1: adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre, polyunsaturated to saturated fat and saturated fat.
† Model 2: adjusted for age, sex, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, intake of fibre, polyunsaturated to saturated fat, saturated fat, BMI, hypertension and diabetes.
Systematic review and meta-analysis
Overviews of the characteristics of the two prospective cohort studies are shown in Table 1. A total of 149 491 participants with 18 811 mortality cases were included in the analysis. The duration of follow-up was 16(Reference Nagata, Wada and Tamura12) and 32(Reference Zheng, Li and Rimm11) years, respectively, with a mean follow-up of 24·4 years. Results of quality assessment are shown in Table S2 in Supplementary material, with both studies scoring 9.
Results of pooling data from prospective studies that evaluated the link between choline consumption, overall and cause-specific mortality are shown in Figs. 2–4. A positive and significant association was observed between choline intake and total mortality (RR 1·12, 95 % CI 1·08, 1·17, P < 0·001, n 3 studies, Fig. 2), with no heterogeneity (I 2 = 2·9, P = 0·882). Furthermore, choline intake predicted CVD mortality (RR 1·28, 95 % CI 1·17, 1·39, P < 0·001, n 5 studies, Fig. 3), i.e. individuals consuming more choline had a 28 % greater risk of dying from CVD; no heterogeneity was observed (I 2 =9·6, P = 0·864). However, the positive association between stroke mortality and choline consumption became non-significant (RR 1·18, 95 % CI 0·97, 1·43, P = 0·092, n 3 studies, Fig. 4, I 2 = 1·1, P = 0·934).
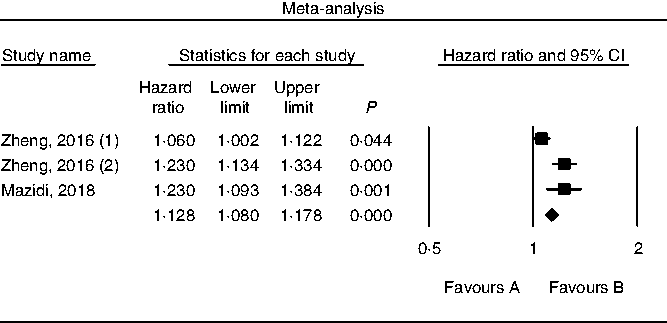
Fig. 2. Forest plot of choline consumption and risk of total mortality.
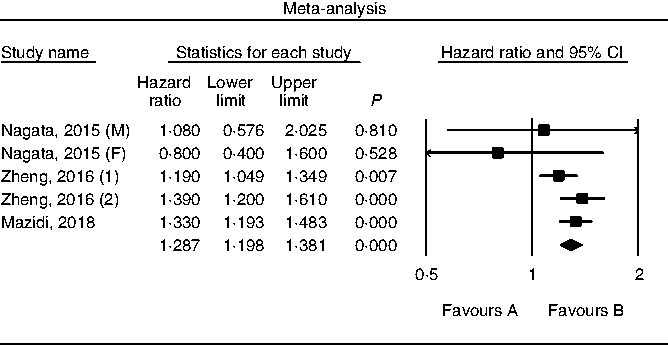
Fig. 3. Forest plot of choline consumption and risk of CVD mortality. M, male; F, female.
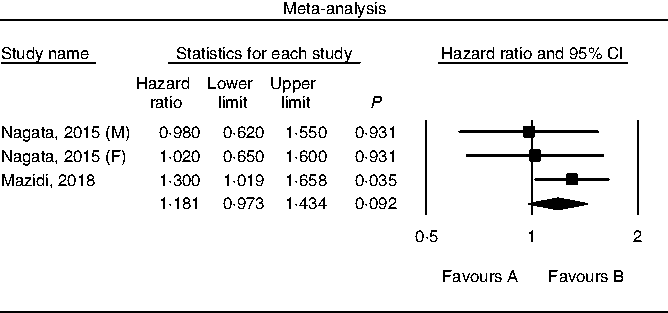
Fig. 4. Forest plot of choline consumption and risk of stroke mortality. M, male; F, female.
Sensitivity analysis
In leave-one-out sensitivity analyses, the pooled effect estimates remained similar for the associations of choline consumption with total (RR 1·12, 95 % CI 1·08, 1·17, P < 0·001), CVD (1·28, 95 % CI 1·17, 1·39, P < 0·001) and stroke mortality (RR 1·18, 95 % CI 0·97, 1·43, P = 0·092). This confirms that the significant differences between the studied groups were the overall effect of all included studies.
Publication bias
Visual inspection of the funnel plot symmetry suggested no potential publication bias for the comparison of choline consumption and total mortality (Fig. S1 in Supplementary material). Furthermore, both Egger’s linear regression (intercept –1·10, 95 % CI –4·02, 1·81, two-tailed P = 0·314) and Begg’s rank correlation test (Kendall’s tau with continuity correction = –1·01, z = 0·244, two-tailed P = 0·806) indicated no publication bias. After adjustment of the effect size for potential publication bias using the ‘trim and fill’ correction, no potentially missing studies were imputed in the funnel plot (Fig. S2 in Supplementary material). Furthermore, the ‘fail-safe N’ test showed that thirty-two studies would be needed to bring the weighted mean difference down to a non-significant (P > 0·05) value.
Discussion
The present study evaluated the associations of dietary choline intake with overall and cause-specific mortality in a large, randomly selected, nationally representative US population. A positive link between total, CVD and stroke mortality with choline intake was observed, a finding that was further confirmed by conducting a meta-analysis. Furthermore, higher choline intake was associated with worse cardiometabolic risk factors, as well as a greater risk of T2D.
To the best of our knowledge, this is the first time that a positive link between choline intake and stroke mortality in US adults is reported. In contrast, a study performed among Japanese adults (13 355 males and 15 724 females, aged >35 years, 16 years follow-up) found no significant association between choline consumption and stroke mortality(Reference Nagata, Wada and Tamura12). Furthermore, in the present study, dietary choline intake was positively associated with total and CVD mortality. Until now, only a single study evaluated the link between all-cause mortality and choline intake; the authors examined the association between habitual dietary intake of phosphatidylcholine (which is the major dietary source of choline) with all-cause mortality among 80 978 women and 39 434 men followed for up to 32 years. They concluded that a higher intake of phosphatidylcholine was associated with an increased all-cause mortality (RR 1·11, 95 % CI 1·06, 1·17)(Reference Zheng, Li and Rimm11). Another two studies evaluated the link between dietary choline intake and CVD mortality, one of them in a Japanese population with null findings (RR 0·80, 95 % CI 0·40, 1·60) and another one in US adults with positive and significant results (RR 1·26, 95 % CI 1·15, 1·39)(Reference Zheng, Li and Rimm11, Reference Nagata, Wada and Tamura12).
A few studies (n 4) evaluated the relationship between CVD events and choline consumption. For example, Dalmeijer et al. (Netherlands, n 130 667)(Reference Dalmeijer, Olthof and Verhoef14) and Millard et al. (USA, n 35 316)(Reference Millard, Musani and Dibaba3) reported no significant associations between choline intake and CVD incidence (RR 1·04, 95 % CI 0·75, 1·45 and RR 0·97, 95 % CI 0·86, 1·09, respectively)(Reference Millard, Musani and Dibaba3, Reference Dalmeijer, Olthof and Verhoef14). The same studies(Reference Dalmeijer, Olthof and Verhoef14) found no link between choline intake and stroke incidence (RR 0·86, 95 % CI 0·51, 1·44 and RR 0·94, 95 % CI 0·80, 1·11, respectively)(Reference Millard, Musani and Dibaba3, Reference Dalmeijer, Olthof and Verhoef14). Overall, several studies reported inconsistent associations between dietary choline intake and risk of CHD(Reference Detopoulou, Panagiotakos and Antonopoulou6, Reference Bidulescu, Chambless and Siega-Riz13, Reference Dalmeijer, Olthof and Verhoef14).
The pathophysiological links between choline intake and mortality are not clear. Phospholipid phosphatidylcholine is a major dietary source of choline and trimethylamine, which are metabolised into circulating trimethylamine-N-oxide (TMAO) via intestinal microbiota-dependent mechanisms(Reference Wang, Klipfell and Bennett4). Higher circulating TMAO concentrations have been related to an increased risk of CVD and mortality(Reference Wang, Klipfell and Bennett4). Wang et al. found that plasma concentrations of metabolites (including TMAO) from dietary phosphatidylcholine via a gut microbiota-related nutrient metabolism pathway were highly predictive of CVD risk(Reference Wang, Klipfell and Bennett4). Our study extends these findings by suggesting that the dietary sources of such gut microbiota-related metabolites may also be related to mortality. We found that the highest quartile (Q4) of choline consumption had a higher HR for overall, CVD and stroke mortality. Taken together our study (showing a significant and positive relationship between choline intake and CVD mortality) and other studies that evaluated the link between choline consumption and CVD incidence (reporting attenuated or non-significant associations)(Reference Detopoulou, Panagiotakos and Antonopoulou6, Reference Bidulescu, Chambless and Siega-Riz13, Reference Dalmeijer, Olthof and Verhoef14), we could suggest that the effects of dietary choline intake may be greater on CVD prognosis than on CVD development.
It seems that high choline intake would increase expression and activity of betaine-homocysteine methyltransferase enzyme (BHMT) in the liver and kidney; this enzyme, by transferring a methyl group, would convert homocysteine to methionine and would lead to reduction in circulating homocysteine levels(Reference Hajer, van der Graaf and Olijhoek33). Choline is also a betaine precursor which may explain why high choline intake is associated with reduced homocysteine concentrations(Reference Schwab, Torronen and Toppinen34). The TAG-raising effect of phosphatidylcholine may contribute to the positive association with the risk of CVD(Reference Olthof, van Vliet and Verhoef35). Furthermore, intestinal microbial metabolism of dietary phosphatidylcholine has been implicated in the pathogenesis of atherosclerosis(Reference Wang, Klipfell and Bennett4).
The present study has several limitations. Although our analysis was adjusted for multiple factors, including socio-demographic characteristics, lifestyle habits, physical activities and energy intake, genetic and dietary nutrient factors absorbed when consuming choline-rich foods and unknown or poorly measured factors could not be completely ruled out. Furthermore, we need to mention that due to our observational design, even we adjusted for known confounders, there is still a chance of residual bias. This limitation might be addressed in the future by randomised control trials. It would also be interesting to have studies based on the source of choline intake. However, our findings may have public health implications, addressing the extent to which associations between TMAO, cardiometabolic risk factors and mortality may influence dietary advice related to the consumption of the TMAO precursor, i.e. choline.
In conclusion, we found that individuals consuming more choline had worse lipid profile and glucose homeostasis, but lower CRP levels and no significant differences in anthropometric parameters and blood pressure, compared with those consuming less choline. Furthermore, individuals in the highest quartile (Q4) of choline intake had a greater overall, CVD and stroke mortality than those in the lowest quartile. Our findings revealed that a higher choline intake could have detrimental effects on health but further research is needed in this field.
Acknowledgements
There are no acknowledgements to be made.
The material presented in this manuscript is original and has not been submitted for publication elsewhere.
N. K. has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, NovoNordisk, Sanofi and WinMedica. D. P. M. has given talks and attended conferences sponsored by MSD, AstraZeneca and Libytec. The other authors have no conflict of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001065











