LEARNING OBJECTIVES
After reading this article you will be able to:
• understand the evidence base of using psychotropic medicines in people with ASDs and how these inform current clinical guidelines
• demonstrate an increased knowledge and awareness of the individualised approach needed when prescribing psychotropic medication for people with ASDs
• understand the gaps in the literature and evolving areas of research.
Autism spectrum disorder (ASD) refers to a group of lifelong neurodevelopmental disorders characterised by significant difficulties with social interactions and social communication and stereotypical and restricted repetitive behaviours and interests (Box 1). Difficulties with sensory processing and emotion regulation and a specific thinking style are also common. The estimated prevalence of ASDs in the general population is 1–2% (Brugha Reference Brugha, Mcmanus and Bankart2011). Although outcomes vary, many individuals with ASDs experience repeated attendance at emergency departments, long-term admissions to in-patient settings and delayed discharge. In addition, comorbid mental disorders and physical health conditions are common among these individuals (Croen Reference Croen, Zerbo and Qian2015).
BOX 1 ‘Autism spectrum disorders’ in the ICD and the DSM
ICD-10 (World Health Organization 1992) continues to be the diagnostic manual used in the UK. It includes the group of ‘F84 Pervasive developmental disorders’, defined as ‘a group of disorders characterized by qualitative abnormalities in reciprocal social interactions and in patterns of communication, and by a restricted, stereotyped, repetitive repertoire of interests and activities. These qualitative abnormalities are a pervasive feature of the individual's functioning in all situations’. Within this group, several autism profiles may be diagnosed, including childhood autism, atypical autism and Asperger syndrome, as well as Rett syndrome and other childhood disintegrative or pervasive developmental disorders.
ICD-11 (World Health Organization 2018) was released in 2018 and comes into effect from 2022. This has updated the diagnostic criteria for autism to align more with DSM-5 (American Psychiatric Association 2013). The category ‘6A02 Autism spectrum disorder’ includes difficulties in interaction and social communication and restricted interest and repetitive behaviours. It does not include language problems. It also includes Asperger syndrome, childhood disintegrative disorder and other generalised developmental disorders. Unlike DSM-5, it will also include guidance on distinguishing between autism with and without an intellectual disability.
Current clinical guidance aims to reduce overprescription of antipsychotic medication in people with ASDs (Box 2). Nonetheless, there may be circumstances when prescription is warranted, such as in the context of psychiatric comorbidity and/or for challenging behaviour that cannot be managed with psychosocial interventions alone. Although ‘challenging behaviour’ is not a diagnosis in and of itself, it is described in the UK's National Institute for Health and Care Excellence (NICE) guidelines (National Institute for Health and Care Excellence Reference National Institute for Health and Care Excellence2015) as behaviours that are challenging to services, family and carers. It may manifest as aggression, self-injury, social withdrawal, repetitive and disruptive behaviours, as well as often serving a purpose as a means of communication and providing sensory stimulation.
BOX 2 STOMP
STOMP stands for ‘stopping the over-medication of people with a learning disability, autism or both’ (Health Education England 2016). The aim of this national project is to help stop the overuse of psychotropic medicines in these patient groups.
STOMP was initiated in 2016 by NHS England, the Royal College of Psychiatrists, the Royal College of Nursing, the Royal College of General Practitioners, the Royal Pharmaceutical Society and the British Psychological Society. It has since expanded to involve numerous other bodies.
In December 2018, STOMP-STAMP (Supporting Treatment and Appropriate Medication in Paediatrics) was launched to help better support children and young people with an intellectual disability (known as learning disability in UK health services), autism or both.
Such clinical situations are not uncommon in secure and custodial settings, and may require doctors to take the responsibility of accurately prescribing unlicensed (i.e. ‘off-label’) psychotropics. However, there is little in the way of formal clinical guidance on how the management of comorbid mental disorders may differ in those with ASDs compared to those without. For example, peripheral hypersensitivity may mean that many individuals with ASDs experience high levels of pain if undergoing blood tests or administered injectable medicines, as well as potentially being more sensitive to the therapeutic and adverse effects of psychotropics, necessitating different patterns of dose titration and a tighter balance of risk and benefit. Access to specialised neurodevelopmental disorder services for patients and specialist clinicians is also variable across the country (Parkin Reference Parkin, Long and Powell2020). This review will summarise the key evidence on the use of psychotropic medicines in people with ASDs, including those with psychiatric comorbidity, that forms the evidence base for current, albeit limited, clinical guidance. Together with some critical analysis, we hope that this will be of benefit to all mental health professionals who work with those with ASDs.
Prescribing in ASD without any co-occurring psychiatric or neurodevelopmental disorder
There are no medications currently licensed for the difficulties associated with ASDs in the UK or across Europe. However, consensus guidelines have been published supporting the use of aripiprazole (from 6 years old) and risperidone (from 5 years old) for ASD-associated irritability (Howes Reference Howes, Rogdaki and Findon2018). There is also mixed evidence about the efficacy of selective serotonin reuptake inhibitors (SSRIs) in alleviating restricted repetitive behaviours that can otherwise interfere with learning, activities of daily living and may be associated with aggression.
Restricted and repetitive behaviours
The rationale for studies into SSRIs for restricted repetitive behaviours or stereotypies stemmed from their overlap in phenotype with other disorders with serotonergic dysfunction, such as obsessive–compulsive disorder (OCD). The main SSRIs studied include fluoxetine, citalopram and escitalopram (supplementary Table 1, available at https://dx.doi.org/10.1192/bja.2021.32). In 2013, the authors of a Cochrane review concluded with their support for the use of SSRIs for repetitive behaviours in adults with ASDs (Williams Reference Williams2013), although the evidence base for these pharmacological interventions continues to be limited. They did not support the use of serotonergic agents in children. Fluoxetine seems the best tolerated, but it should be noted that there have been no direct comparisons with other agents. There is also enormous variability in clinical responses between studies, including measures of efficacy and adverse effects. Although several studies report the efficacy of SSRIs at lower doses and a tendency for agitated or hyperactive adverse effects at doses considered therapeutic in non-ASD populations, it may be that inconsistent clinical responses within and between studies reflect inconsistent trial designs, as well as the biological and genetic heterogeneity of ASDs. In the absence of high-quality and sufficiently powered ASD-specific clinical trials, particularly in adults, more research is required before conclusions can be drawn. Treating those with ASDs for irritability and restricted repetitive behaviours may require lower starting doses, a slow titration regimen and follow-up at more frequent intervals for assessment of risk and benefit than would be used for the same medications in individuals without ASDs.
Agitation and irritability
Safety warnings were published in 2003–2004 by the UK's Committee on Safety of Medicines and the US Food and Drug Administration regarding an increased risk of suicidal behaviours associated with SSRIs. Since then, SSRI prescriptions have reduced and atypical antipsychotics have been prescribed more frequently. However, the evidence base for the efficacy of such pharmacological treatments in those with ASDs is scarce, particularly in forensic and custodial settings, and they are often prescribed off-label.
Risperidone appears to be most extensively studied and effective in reducing irritability, aggression, hyperactivity, self-injurious behaviour, stereotypies and restricted repetitive behaviours in children with ASDs, but it has increased risk of hyperprolactinaemia, sexual side-effects and gynaecomastia (McCracken Reference McCracken, McGough and Shah2002). A recent Cochrane review of aripiprazole for ASDs concluded that evidence from two randomised controlled trials (RCTs) suggested that it was effective and well tolerated as a short-term medication for some behavioural aspects of ASDs (Hirsch Reference Hirsch and Pringsheim2016). A small head-to-head comparison of risperidone and aripiprazole in children with ASDs showed comparable efficacy (Ghanizadeh Reference Ghanizadeh, Sahraeizadeh and Berk2014). Trials of other antipsychotics in ASDs are summarised in supplementary Table 1. To our knowledge, there are no studies that have specifically looked at the efficacy of antipsychotic agents in treating pseudohallucinatory or psychosis-like symptoms in people with ASDs.
Violence and aggression
In some people with ASDs, agitation and irritability may progress to acts of violence and aggression. There were suggestions of a common neurodevelopmental basis for ASDs and childhood antisocial behaviour, with shared genetic and environmental factors linking ASDs with conduct disorder and oppositional defiant disorder (Kerekes Reference Kerekes, Lundström and Chang2014). However, although Heeramun et al (Reference Heeramun, Magnusson and Gumpert2017) showed an increased risk of violent behaviour in those with ASDs, this was negated after controlling for comorbid attention-deficit hyperactivity disorder (ADHD) and conduct disorder. There is therefore a lack of evidence that clearly distinguishes patterns of violence and criminality (if any) in those with ASDs compared with the general population.
A case series found that the atypical antipsychotic clozapine was effective at reducing impulsive behaviour, aggression and anger in individuals with dissocial personality disorder (Brown Reference Brown, Larkin and Sengupta2014). This was effective at plasma levels less than 350 ng/mL, lower than the target therapeutic range for treatment-resistant psychotic illnesses. One RCT of clozapine (up to 0.6 mg/kg) versus risperidone (0.05 mg/kg) in children and adolescents with conduct disorder found both agents to be effective (Juárez-Treviño Reference Juárez-Treviño, Esquivel and Isida2019). However, clozapine was more effective in improving traits of delinquency and improving global functioning. This is yet to be replicated in larger-scale trials or specifically in a cohort of people with ASDs. However, this highlights the efficacy of several agents in alleviating aggression and irritability with a symptom-based approach to treatment.
Broadly speaking, there is consensus between recommendations by NICE, the British Association for Psychopharmacology (BAP) and the Scottish Intercollegiate Guidelines Network (SIGN). SIGN (Scottish Intercollegiate Guidelines Network 2016) recommends that antipsychotics and SSRIs should not be used to manage core difficulties of ASDs, but that second-generation antipsychotics may be considered for irritability and hyperactivity as a short-term treatment, including a review after 3–4 weeks and cessation if no response at 6 weeks. However, antipsychotics may be considered for irritability and aggression in adults with ASDs if psychosocial interventions alone cannot address the severity of the behaviour. In addition, medications should not be used as a substitute for other treatment modalities. If medications are used, they must be used in conjunction with functional assessments, environmental and psychosocial interventions, be focused on improving specific signs and symptoms and be prescribed at the minimum effective dose and avoiding polypharmacy where possible.
Of significance is the absence of studies examining the risk and benefits of these pharmacological interventions in adults with ASDs. Current recommendations regarding pharmacological interventions appear to be extrapolated from the studies with children and adolescents detailed in supplementary Table 1. In addition, the heterogeneity of these studies makes any generalisations difficult, especially where there are inconsistencies in outcome measures, trial durations, sample sizes and dosing regimens. There is also often a lack of differentiation between compulsions and stereotypies. The short trial durations and small sample sizes also under-power the evidence base. Although the recommendations of clinical guidance are to use pharmacological agents in ASDs only for short periods, it remains unclear how feasible this is in clinical practice. The long-term medication risk profile for adults with ASDs is therefore unclear, including whether there is a heightened risk of behavioural activation with SSRIs and of metabolic diseases and tardive dyskinesia with antipsychotics.
Prescribing in ASD with comorbid psychiatric or neurodevelopmental disorders
In the sections below, the key evidence on the use of psychotropic agents in treating mental disorders in those with ASDs is discussed. Recommendations from clinical guidance by NICE, SIGN and the BAP are summarised in Table 1. Current clinical guidelines by NICE and SIGN do not discuss catered treatment of dementia, substance use disorders, depression, bipolar affective disorder or anxiety disorders, including OCD and post-traumatic stress disorder (PTSD) for people with ASDs. Guidance from the BAP includes some discussion on the treatment of depression, anxiety disorders and ADHD (Howes Reference Howes, Rogdaki and Findon2018).
TABLE 1 Summary of clinical guidelines on the pharmacological management of comorbid mental disorders in individuals with autism spectrum disorders (ASDs)
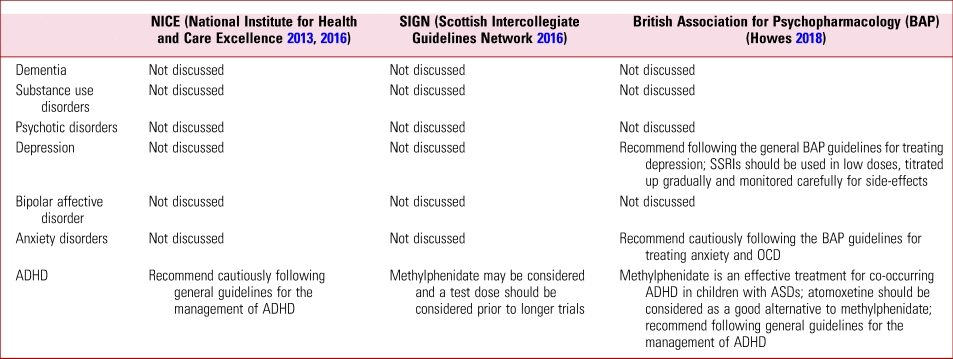
SSRI, selective serotonin reuptake inhibitor; OCD, obsessive–compulsive disorder; ADHD, attention-deficit hyperactivity disorder.
Each section will primarily focus on the treatment of psychiatric comorbidity in the context of an ASD. However, we acknowledge that some treatments used for psychiatric comorbidity may also lead to improvements in difficulties associated with core or associated features of ASDs. Given this and the difficulties in accurate diagnosis of comorbidity in this group, we will also include discussion on more symptomatic approaches to management where such overlaps in symptom aetiology may arise.
Dementia
Deficits in cholinergic neurotransmission have been described in some individuals with ASDs (Perry Reference Perry, Lee and Martin-Ruiz2001). This has led to studies investigating the role of acetylcholinesterase inhibitors such as donepezil, rivastigmine and galantamine in ameliorating distressing symptoms associated with ASDs. We would direct readers to a systematic review by Rossignol & Frye (Rossignol Reference Rossignol and Frye2014) for a more detailed discussion of the evidence base of individual studies. No acetylcholinesterase inhibitor or glutamate receptor antagonist is recommended in clinical guidelines for those with ASDs. The evidence of anti-dementia drugs being efficacious in those with ASDs is often conflicting and inconsistent, but it is reassuring that they appear well tolerated.
Although anti-dementia drugs have been trialled to target symptoms associated with ASDs, to our knowledge there are no similar trials on the use of such drugs to treat established dementia in those with ASDs and whether and how such treatment may differ compared with the general population. Those with dementia and an ASD with an intellectual disability are more likely to be treated with off-label agents such as antipsychotics and benzodiazepines than acetylcholinesterase inhibitors (Axmon Reference Axmon, Kristensson and Ahlström2017); such prescribing is perhaps perpetuated by behavioural changes often being an initial manifestation of a neurodegenerative illness in this group and a symptom-based approach to management. This highlights a discrepancy in clinical practice, with this group therefore less likely to receive more catered treatment. It is not clear whether these data can be extrapolated to those without an intellectual disability. This reinforces a need for clear clinical guidelines on the catered management of dementia in those with ASDs across the intellectual spectrum. Until more specific data in this group become available, treatment should proceed using existing clinical guidelines for the management of dementia.
Substance use disorders
The prevalence of substance use disorders in those with ASDs may be higher than once thought, particularly with co-occurring ADHD (Butwicka Reference Butwicka, Långström and Larsson2017). In a review of available literature we failed to find any studies that have investigated the use of replacement therapies such as methadone, buprenorphine or nicotine replacement therapies in those with comorbid ASDs and substance use disorders, nor studies on aversion pharmacotherapies such as disulfiram, anti-craving agents such as naltrexone, nalmefene or acamprosate or the opioid receptor antagonist naloxone. There also appear to be no studies examining the treatment of acute alcohol or opioid withdrawal in those with ASDs. It is therefore not known whether broader guidelines on the treatment of these conditions can be extrapolated to people with ASDs and whether this group may be more sensitive to the sedating effects of pharmacotherapies such as opioid replacement therapy or benzodiazepines. There are no clinical guidelines from NICE, SIGN or the BAP regarding the pharmacological management of substance use disorders in individuals with ASDs.
Psychotic disorders
Schizophreniform disorders appear to be more common in people with ASDs compared with the general population (Zheng Reference Zheng, Zheng and Zou2018). ASDs and schizophreniform pathology share similar symptomatology, notably the negative symptoms, including deficits in social communication and social-emotional reciprocity. Those with ASDs can also experience transient positive psychotic symptoms induced by severe psychological stress, that may be responsive to antipsychotics (Box 3). There has also been the suggestion that individuals with ASDs may be at increased risk of acting on hallucinations and delusional beliefs (Wachtel Reference Wachtel and Shorter2013). There is little evidence about how to treat psychosis that coexists with an ASD and whether and how this may differ from treating psychosis without an ASD. There have been concerns that those with an ASD and a comorbid psychotic disorder have a higher burden of negative and cognitive symptoms (Kästner Reference Kästner, Begemann and Michel2015) and a twofold increase in treatment failure (Downs Reference Downs, Lechler and Dean2017).
BOX 3 Case vignette 1: pseudohallucinatory experiences
A 19-year-old man with an autism spectrum disorder (ASD) has become socially withdrawn, appears dishevelled and has been seen responding to auditory hallucinations.
This scenario aims to first highlight that not all voice-hearing is psychotic in nature. Pseudohallucinatory experiences are common in people with ASDs, particularly when emotionally overwhelmed. In addition, those who are non-verbal may not be able to communicate other first-rank symptoms, such as thought alienation and passivity phenomena. The treatment of schizophrenia comorbid with ASDs should be the same as for the general population. However, clinicians must be mindful that those with ASDs may be more susceptible to the sedating effects of antipsychotics; they should also consider agents with depot formulations if there are concerns over non-adherence to medication.
Clinicians should have a low index of suspicion of comorbid psychosis in people with ASDs who show changes in speech and behaviour (Box 4). Without more catered clinical guidelines, comorbid psychotic disorders in those with ASDs should be treated as per existing NICE guidelines. In addition, given the fluid boundaries and overlap between ASDs and chronic psychotic disorders, a multimodal symptom-based approach to treatment might be appropriate, as might close monitoring, in light of some evidence suggesting increased rates of treatment failure and adverse effects. It is unclear whether clinical responses occur at similar doses, and in the case of clozapine at similar plasma concentrations, to those in people without comorbid ASDs. This cautious approach would help to ensure that the minimum therapeutic dose is used that prevents oversedation and the metabolic side-effects that may contribute to both polypharmacy and an increased risk of mortality and morbidity. High rates of non-adherence to medication may also be a problem.
BOX 4 Case vignette 2: sudden change in behaviour
A 35-year-old woman with an autism spectrum disorder who is non-verbal presents with a sudden increase in agitation and irritability. She is repeatedly throwing items, gesturing in threatening ways towards others and has stopped eating and drinking at times of distress.
This is a common scenario in which a wide differential diagnosis is needed. From a biological perspective, one needs to be mindful of pain or physical illness, which in someone who is non-verbal may be communicated through sudden changes in behaviour or the precipitation of delirium. Such behaviours are commonly triggered by changes in environment and disruption to routine. Excluding organic causes and managing behaviours using psychosocial approaches (such as the Antecedent–Behaviour–Consequences Checklist) are the first-line interventions. Psychotropic medication such as aripiprazole and risperidone should be reserved for where psychosocial interventions have been inadequate or there is an imminent risk to self or others. If used, they should be reviewed at regular intervals and only continued if there is clear evidence of clinical benefit.
Depression
Depression is common across the age range in individuals with ASDs, affecting everyday functioning and increasing the burden on carers. Much like the other comorbidities discussed, mood disorders can present differently in ASDs, notably through changes in sleep and behaviour – especially if individuals are non-verbal. SSRIs are the established first-line pharmacotherapy for depression. As previously highlighted, SSRIs are widely used in those with ASDs, despite conflicting evidence and variable levels of tolerability. There are also no RCTs examining the use of SSRIs in targeting depression in any age group of individuals with ASDs. Clinical guidelines, including those recommended by the BAP and NICE, are therefore extrapolated from trials in children and adults with depression without ASDs. Given the variable SSRI tolerability found in children with ASDs treated for restricted repetitive behaviours, the use of low doses with careful monitoring for efficacy and adverse effects is warranted.
Bipolar affective disorder
Bipolar disorder appears more common in adults with ASDs than in the general population (Croen Reference Croen, Zerbo and Qian2015). Perinatal exposure to the mood stabiliser sodium valproate may also be a risk factor for the future development of ASDs. Sodium valproate has been shown to be efficacious and well tolerated in alleviating restricted repetitive behaviours and irritability in children with ASDs (Hollander Reference Hollander, Soorya and Wasserman2006). There are no RCTs on the use of valproate in treating bipolar disorder in people with ASDs. Although there are no RCTs on the efficacy and/or safety of lithium in those with ASDs, Mintz & Hollenberg (Mintz Reference Mintz and Hollenberg2019) undertook a retrospective chart review and found that adjunctive lithium therapy was efficacious in mood stabilisation and in attenuating maladaptive behaviours and restricted repetitive behaviours in adolescents and adults with ASDs. It was also well tolerated. However, this study was not randomised and had a small sample. The relationship between plasma lithium levels, clinical response and adverse effects also remains unclear.
Anxiety disorders
The prevalence of comorbid anxiety disorders has been estimated to be approximately 40% in children (van Steensel Reference van Steensel, Bögels and Perrin2011) and 34–50% in adults with ASDs (Hofvander Reference Hofvander, Delorme and Chaste2009). More specifically, there are high prevalence rates of comorbid generalised anxiety disorder, social phobia, OCD and separation anxiety disorder. SSRIs have been shown to be efficacious in adults with generalised anxiety disorder, social phobia, OCD, panic disorder and PTSD in the general population. To our knowledge, there have been no trials of pharmacological agents specifically targeting anxiety disorders or OCD in people with ASDs, with most trials focusing on possible shared features of both ASDs, anxiety and OCD. Much like their off-label use in treating restricted repetitive behaviours, the evidence for SSRIs in the treatment of anxiety disorders and OCD is limited and inconsistent. BAP guidelines report that there is little to no evidence to support the use of SSRIs, risperidone or clomipramine in the treatment of anxiety or OCD symptoms in children or adults with ASDs (Howes Reference Howes, Rogdaki and Findon2018). They therefore recommend cautiously following existing BAP guidelines for the treatment of anxiety disorders and OCD in those without ASDs.
Attention-deficit hyperactivity disorder
ADHD is a common comorbid neurodevelopmental disorder in those with ASDs. A meta-analysis of four studies concluded that methylphenidate is an effective treatment in children with ASDs and comorbid ADHD (Reichow Reference Reichow, Volkmar and Bloch2013). However, it appeared to be less efficacious than in those with ADHD alone and was associated with more adverse effects and subsequent discontinuation. There are no studies on amphetamines (including dexamfetamine and lisdexamfetamine). Atomoxetine had effect sizes comparable with those of methylphenidate in children with ASDs and comorbid ADHD (Research Units on Pediatric Psychopharmacology Autism Network 2005). Guanfacine has been shown to have similar efficacy to methylphenidate, albeit with more frequent treatment-limiting adverse effects in those with ASDs than has been seen in non-ASD populations (Biederman Reference Biederman, Melmed and Patel2008). We would direct readers to a systematic review by Joshi et al (Joshi Reference Joshi, Wilens and Firmin2021) for a more detailed discussion about the individual studies on the treatment of ADHD in children with ASDs. There are also no RCTs on the use of ADHD medications in adults with ASDs.
The comparable efficacy between stimulant and non-stimulant medication means that a lower threshold for the use of the latter may be warranted in those with adverse effects to stimulants. However, when interpreting the data one must be mindful that they come from a small number of studies, with small numbers of participants and heterogeneous outcome measures. In addition, the largest of the studies included a substantial number of non-verbal participants or those with intellectual disability (Research Units on Pediatric Psychopharmacology Autism Network 2005). Psychiatric comorbidities such as affective disorders were not an exclusion criterion in all studies and the persistence of these may have masked improvements in underlying features of ADHD. It is therefore unclear whether the lower efficacy and tolerability may represent greater baseline comorbidity, in which case extrapolation to those with higher-functioning ASDs may not be indicated.
Sleep disorders
Sleep disorders are common in those with ASDs, including both dyssomnias and parasomnias (Devnani Reference Devnani and Hegde2015). The aetiology is unclear but is most likely to be multifactorial and may have a detrimental impact on daytime functioning and behaviour. The use of melatonin for sleep disorder is supported in children with ASDs. It has been shown to increase the duration of sleep and decrease the latency to sleep onset compared with placebo but with no changes in night-time waking (Rossignol Reference Rossignol and Frye2011). This aligns with recommendations made by the BAP (Wilson Reference Wilson, Anderson and Baldwin2019) and NICE (National Institute for Health and Care Excellence 2020). A more recent multicentre open-label uncontrolled trial of 26 weeks of melatonin supplementation for children with neurodevelopmental disorders and sleep disorder found that, in addition to a reduction in sleep onset latency, there was also an improvement in RRBs, irritability, hyperactivity and inappropriate speech (Yuge Reference Yuge, Nagamitsu and Ishikawa2020). There are no clinical trials on the use of melatonin in adults with ASDs. Otherwise, clinical recommendations for the use of melatonin in adults with ASDs is extrapolated from studies in children or adults without ASDs.
Uncertainties and gaps in the literature
Most RCTs for the pharmacological treatment of ASDs and psychiatric comorbidities have focused on children and adolescents. In addition to being very heterogeneous in design and outcome measures, these studies have also focused on symptom-based approaches to management rather than clearly distinguishing between ASD symptoms and comorbid disorder. The application of the results from these studies to adults therefore requires caution. There is a need for more pharmacological research that includes adults with ASDs.
The high prevalence of comorbidities in people with ASDs may mean that they are more frequently excluded from clinical trials in the name of internal validity and, in turn, this may limit the generalisability of the subsequent findings. Concerns over capacity to consent, particularly in people with comorbid intellectual disability, may also lead to exclusion from recruitment. Those with ASDs who are not verbally fluent may require ‘ASD-friendly’ versions of written information or data collection tools, or the use of technology to consent and participate. Such tools may not be readily available. A recent meta-analysis (Russell Reference Russell, Mandy and Elliott2019) found that not only were people with comorbid ASDs and intellectual disability more likely to be excluded from studies, but that numerous studies failed to report information about participants’ intellectual ability.
Recent developments in the understanding of the biological basis of ASDs have led to clinical trials of novel and repurposed agents, several of which are currently underway and summarised in Table 2. The emerging treatments being developed include targeting neuropeptides such as oxytocin, cannabinoid receptors, neurosteroids and allopregnanolone (a potent positive allosteric modulator of the GABAA receptor), and modulators of neuroinflammation and the brain–gut axis.
TABLE 2 Current UK clinical trials on pharmacotherapies for autism spectrum disorders (ASDs)
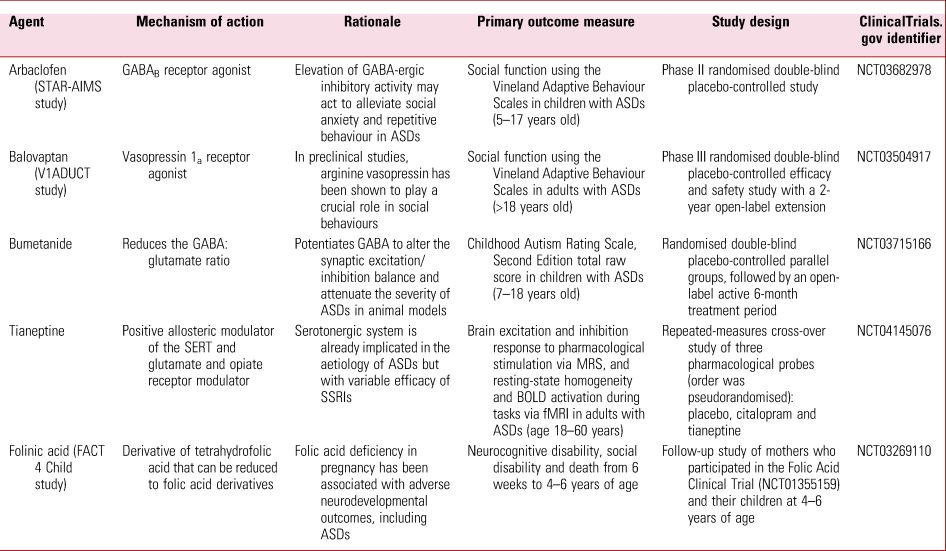
GABA, gamma-aminobutyric acid; SERT, serotonin reuptake transporter; SSRI, selective serotonin reuptake inhibitor; MRS, magnetic resonance spectroscopy; BOLD, blood oxygen level dependent; fMRI, functional magnetic resonance imaging.
Additional questions include whether potential pharmacokinetic differences in those with ASDs contribute to variable tolerability and efficacy of psychotropics, particularly the commonly used SSRIs. An association has been found between the total plasma risperidone and 9-hydroxyrisperidone levels and attenuation of irritability in children with ASDs (Kloosterboer Reference Kloosterboer, De Winter and Reichart2021). However, whether the therapeutic window is the same as in those without ASDs is unclear, as is the role of therapeutic drug monitoring. The pharmacokinetic parameters of the participants were similar to those of children and adults without ASDs. There was no significant effect of CYP3A4 or CYP3A5 gene polymorphisms on risperidone pharmacokinetics. It has not been established whether there is a higher prevalence of cytochrome enzyme polymorphisms in those with ASDs to account for variable psychotropic efficacy and tolerability. Variable tolerability, and therefore a higher burden of adverse effects, can lead to poor adherence to psychotropic medication. To our knowledge, there are no studies that have investigated the use of long-acting intramuscular formulations of antipsychotic agents such as risperidone, paliperidone and aripiprazole to improve adherence. Additional considerations here would include the long duration of action if intolerable adverse effects were to emerge, and whether adjunctive measures such as topical local anaesthetic agents would be advisable to mitigate the tactile hypersensitivity that is characteristic of those with ASDs.
Conclusions
This review highlights the paucity of studies and guidance on prescribing psychotropic medicines in people with ASDs, whether this be to alleviate associated difficulties or to manage comorbidities. This lack of guidance is particularly apparent for adults. We argue that this may lead prescribers to make clinical practice decisions based on inferences and extrapolation from low-quality evidence in non-representative populations.
Within the context of the limited body of available evidence suggesting that several psychotropic classes have variable efficacy and tolerability in those with ASDs, we would advocate a ‘start low and go slow’ approach to prescribing. This, along with more frequent monitoring of benefit and risk than would usually be warranted in those without ASDs, may help clinicians find each person's minimum therapeutic dose and a therapeutic window for treatment that may be different from that in people without ASDs.
It is vital that future studies address the selection bias against those with ASDs across the spectrum of intellectual ability and include those with psychiatric comorbidity. This will help improve the external validity of larger-scale trials to ensure generalisability beyond the non-ASD population. Their inclusion in such research will aid a better understanding of the epidemiology of psychiatric comorbidity, prescribing practices, potential pharmacokinetic differences and trials using a wider range of psychotropic medicines. We hope this will lead to updated clinical guidance based on primary research in more representative populations.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bja.2021.32.
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
Acknowledgements
We thank Lauren Boniface, undergraduate psychology student from the University of Cardiff on placement at Broadmoor Hospital, who helped with the literature search.
Author contributions
All authors reviewed and contributed to the construction of the manuscript.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
MCQs
Select the single best option for each question stem
1 Core symptoms of ASDs include:
a restricted and repetitive behaviours
b pseudohallucinations
c impaired short-term memory
d vocal tics
e flight of ideas.
2 The estimated prevalence of ASDs in the general population is:
a <1%
b 1–2%
c 2–5%
d 5–10%
e >10%.
3 Which antipsychtic medications are effective in the treatment of agitation and irritability in ASDs?
a olanzapine and risperidone
b clozapine and haloperidol
c quetiapine and aripiprazole
d olanzapine and aripiprazole
e risperidone and aripiprazole.
4 Independent of psychiatric diagnosis, anti-aggression effects have been shown with:
a olanzapine
b zuclopenthixol
c haloperidol
d clozapine
e risperidone.
5 As regards ASDs:
a people with ASDs are less likely to develop substance use disorder than the general population
b stimulant medications should not be used in people with ASDs and comorbid ADHD
c comorbid ADHD increases the risk of substance use disorder in people with ASDs
d the use of psychotropic agents increases the risk of substance use disorder in people with ASDs
e stimulant medications are licensed for use in treating ADHD in children under 5 years old.
MCQ answers
1 a 2 b 3 e 4 d 5 c






eLetters
No eLetters have been published for this article.