Human enteroviruses (HEVs) belong to the Enterovirus genus which comprises 10 species [1]. Polioviruses (PVs) are important serotypes of HEV-C, one of the 10 enterovirus species. HEVs are transmitted by the faecal–oral route and excreted in stools for several weeks after the acute phase of the infection. PV-infected individuals, with or without symptoms, may shed the virus in the faeces for several weeks and circulation of PV can be detected in sewage, sometimes even before an outbreak is clinically apparent [Reference Hovi2]. Surveillance for PVs in sewage has therefore been included by the World Health Organization (WHO) in the Global Polio Eradication Initiative (GPEI), supplementing surveillance of acute flaccid paralysis (AFP) of cases aged <15 years, which remains the gold standard for PV surveillance [Reference Hovi2, 3]. For mass vaccination campaigns, the GPEI relies on oral poliovirus vaccine (OPV) which consists of three live attenuated PVs (Sabin strains 1–3). OPV was selected because of its low cost and easy means of delivery. A disadvantage of OPV use is, however, that virulent vaccine-derived poliovirus strains (VDPV) may emerge which can circulate for lengthy periods in the environment and eventually may cause poliomyelitis in unvaccinated individuals [Reference Zurbriggen4]. VDPVs are defined as PVs with a 1–15% nucleotide sequence difference in VP1 (viral protein 1) compared to the parental Sabin strain. Sabin-like (SL) isolates are those that display less than 1% nucleotide sequence difference from the parental strain [Reference Zurbriggen4]. Both the VDPVs and SL viruses can cause poliomyelitis but at different rates. The emergence of VDPVs are a growing concern, particularly in settings of low vaccination coverage [Reference Minor5]. Many countries have therefore changed from OPV to inactivated polio vaccines (IPV) for routine vaccination in order to maintain vaccination levels [6]. In the Slovak Republic (SR), PV surveillance includes sampling water from the sewage treatment plants in addition to screening AFP cases for PV. The aim of this study is to describe and compare the circulation pattern of PVs and NPEVs in the SR, as a component of the PV surveillance programme. The study extends from the period before the vaccination strategy was changed from oral (OPV) to inactivated vaccine (IPV) to 6 years after implementation of the change.
Sewage sample collection: every 2 months, a sample was drawn from each of 48 fixed localities in three regions covering the whole SR: the Western region (24 localities) with an average population size of 2 475 000, the Central region (10 localities) average population size 1 353 000 and the Eastern region (14 localities) average population size 1 570 000. From January 2004 to April 2005, sampling in the Western region was performed on a weekly basis because VDPVs were isolated [Reference Cernakova7]. One-litre samples were concentrated by centrifugation using the two-phase method of separation with 29% polyethylene-glycol 6000 (Serva, Germany) and 22% dextran (Serva) both prepared in 5 m NaCl as recommended and further modified by the WHO [3, Reference Cernakova7, 8].
Besides PV surveillance in sewage, AFP cases aged <15 years were reported and screened for HEVs. Paired stool samples were collected from AFP cases according to the WHO's definition. Virus isolations were performed according to standard WHO procedures for PV surveillance by using two cell lines, RD (human rhabdomyosarcoma cells) and L20B (mouse L cells expressing the human poliovirus receptor CD155) [Reference Cernakova7, 8]. Isolates were identified by virus neutralization whenever a cytopathic effect was observed.
Typing of all isolates was performed by microneutralization test using type-specific antisera [Lim Benyesh-Melnick pools (A–H), National Institute for Public Health and Environment, Bilthoven (RIVM), The Netherlands] and also by an indirect fluorescence test as described in detail in the WHO manual [Reference Cernakova7, 8]. Typing was performed at the National Reference Centre (NRC) for Poliomyelitis in Bratislava. Poliovirus isolates and unidentified isolates were submitted to the WHO Regional Reference Laboratory, Helsinki, Finland.
During 2001–2011, 538 HEVs (113 PVs, 425 NPEVs) were isolated and identified in sewage water plants. In 2001, PV1, PV2 and PV3 (SL strains) were isolated from the Eastern and Western regions. PV2 and PV3 (SL strains) were again isolated in the same localities of the Eastern and Western regions in 2002. In 2003, a PV2-VDPV strain and also PV2 and PV3 (SL) strains were isolated in the Western region. During this time in Eastern Slovakia, PV2 and PV3 (SL) strains were isolated. In 2004, PV1, PV2, PV3 (SL strains) and a PV2-VDPV strain were isolated in Western Slovakia whereas in the Central region PV1 and PV2 (SL) were isolated. Since 2005 in Western Slovakia a reduction in PV isolations was observed although PV2-VDPV and SL strains of PV3 were still isolated. The last poliovirus, a PV1 (SL) strain, was isolated in 2009 in Western Slovakia.
Figure 1 shows comparison of PVs, coxsackie B viruses (CBVs) and echoviruses. After 2006 a relative increase was observed in NPEVs: mainly CBVs (15/36 isolates, 42%) and echoviruses (20/36 isolates, 56%). Until 2010, the percentage of CBVs remained high (32/40 isolates, 80%) but in 2011, the incidence of echoviruses rose to 59% (26/44 isolates).

Fig. 1. Comparison of polioviruses (PV), coxsackie B viruses (CBV) and echoviruses (Echo) in sewage water during 2001–2011. * The year 2004 has been put on a different scale as the frequency of sewage water sampling was increased to weekly sampling instead of every 2 months. Other non-polio viruses have not been included in this comparison as they constituted only 2% of the total isolates.
During the whole surveillance period, the most dominant HEVs were CBVs (213/538 isolates, 40%) and echoviruses (200/538 isolates, 37%). PV isolates made up 21% (113/538). The most frequently identified CBVs were CBV5 (15%) and CBV2 (11%), and to a lesser degree CBV3 (8%) and CBV4 (6%). The most frequently identified echoviruses were echovirus 3 (13%), 11 (7%) and 7 (5%). The other echoviruses namely 3, 4, 6, 9, 20, 21, 25, 29, 30 constituted the remaining 12%. Coxsackie A viruses (CAVs) 7, 10, 16, 21 and 24 made up the remaining 2% of HEV isolates during the 11 years. The PV/NPEV ratio showed a marked change after 2006. Only one enterovirus 90 isolate was found comprising 0·2% of the total isolates. Furthermore, the highest percentage of HEV isolates was found in the Western region and the lowest in the Central region (Fig. 2).

Fig. 2. Geographical spread of human enteroviruses isolated from sewage water during 2001–2011. Population size in thousands and number of polio viruses and non-polio enteroviruses isolated for the self-governing regions in the Western, Central and Eastern regions. The Central region border is indicated by red contour lines. PV, Polioviruses; NPEV, non-polio enteroviruses. BB, Banska Bystrica; PO, Presov; ZA, Zilina; NR, Nitra; KE, Kosice; TN, Trencin; TT, Trnava; BA, Bratislava.
For AFP surveillance in children aged <15 years, stool samples were tested which yielded 20 HEV isolates: 12 PVs and 8 NPEVs, all PVs being isolated before the change in the vaccination strategy. HEV isolates from AFP cases corresponded to the viruses isolated from sewage water although VDPVs were not isolated from AFP cases. The incidence of AFP cases in the population aged <15 years varied between 1·3 and 0·1/100 000 per year (Table 1). AFP can occur also from non-poliomyelitis causes such as Guillain–Barré syndrome which has an estimated incidence of 1/100 000 per year. The low numbers in Table 1 suggest suboptimal surveillance in those particular years, indicating the necessity to improve AFP surveillance in the SR.
Table 1. Human enterovirus isolations from acute flaccid paralysis cases in the Slovak Republic during 2001–2011
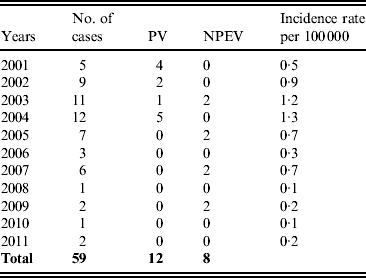
PV, Polioviruses; NPEV, non-polio enteroviruses.
The WHO European Region was declared and certified as polio free in 2002 and has maintained this status despite a large outbreak which occurred in Tajikistan and three other Member States in 2011 [9]. The AFP surveillance in Member States contributed critically to the timely detection and action to contain this outbreak [9]. Environmental surveillance of PV has recently been added as a supplementary tool to AFP surveillance. Combined, these two surveillance activities provide useful data also on the circulating non-polio HEVs [9].
Monitoring of sewage water showed that different HEV serotypes were endemic in the SR during a period of 11 years (2001–2011). Relatively high percentages of PV strains (SL and VDPV) were observed in sewage water during 2001–2006. Thereafter, PV isolates were gradually replaced by NPEVs, a transition that was due to the change in the childhood vaccination programme in which OPV was substituted by IPV. This change occurred because of a relatively high incidence of VDPV strains including PV2-VDPV during 2003–2004 [Reference Cernakova7, 10]. In particular, finding PV2-VDPV in a world devoid of wild-type PV2 was of concern as it is a serious risk for re-introduction of outbreaks with a revertant PV2. This concern strengthened the decision to switch from OPV to IPV [Reference Cernakova7, 10].
After the first VDPVs were isolated in the Western region, the sampling frequency of sewage water was temporary increased to weekly sampling from January 2004 to April 2005 [Reference Cernakova7], causing an increase in number of isolates and a relative increase of isolates in this region. In addition, the high population density of the capital Bratislava and the greater number of sewage plants (n = 24) in the Western region may explain the high percentage of HEV isolates in that region throughout the entire study period. In the first two years (2001–2002), the number of isolates was relatively low compared to later years. We have no explanation for this observation, since techniques remained unchanged during the entire observation period.
Of the NPEVs, CBV isolates predominated of which CBV5 and CBV2 were most commonly identified. Echovirus serotypes 3, 11 and 7 were most frequent. CAVs accounted for less than 2% of the total number of isolates, the prevailing serotypes being CAV7, 10, 16, 21 and 24. Slovakia is a small country sharing borders with five other countries: Ukraine, Poland, Czech Republic, Austria and Hungary. The only comparable data were from Hungary. The circulating HEV serotypes in Hungary from 2000 to 2008 differed from those in the SR. Furthermore, in Hungary, echovirus serotypes 2, 4, 6, 7, 9, 11, 13, 25 were isolated, denoting more variation in the circulating echoviruses than in the SR. Only three CAV and CBV serotypes and a single enterovirus 71 were observed in Hungary, which differed from the Coxsackievirus serotypes isolated in the SR. CBV5 was observed in both countries [Reference Kapusinszky11].
This is the first long-term HEV surveillance reported for the SR which includes the period before and after the change in vaccination strategy. Environmental surveillance of sewage water for PVs can also serve as a tool for studying the NPEVs in circulation. In Slovakia, present detection of HEVs is entirely dependent on the AFP and sewage surveillance of the WHO and this is the only means for detecting NPEVs. The study has some limitations for NPEV surveillance as the focus of the WHO is to detect PVs. The two cell lines recommended for PV isolation (RD and L20B) are less suitable for isolation of NPEVs. Our data may therefore under-represent the actual number of NPEVs. On the other hand, the methodology has remained constant throughout the study. Patterns observed for PV and NPEV remain valuable and are comparable with those from PV surveillance in other countries. The change of vaccine type from OPV to IPV took place at different times in different countries. Of the bordering countries (Austria, Czech Republic, Hungary, Poland, Ukraine) only Poland and Ukraine still continue giving OPV booster doses [Reference Bonnet and Dutta12]. We failed to obtain data showing a comparison of the circulating HEVs before and after to the change of vaccination strategy. CBV5, CBV2, echovirus 3 and SL PV2 were the most frequently isolated serotypes during the study period. As expected, the changes in the vaccination programme from OPV to IPV tipped the balance of circulating serotypes from SL PVs and VDPVs towards NPEVs.
New imports of PVs in polio-free countries pose a continued threat to the momentum of the GPEI. Large outbreaks occurred in the European region and in the Republic of Congo in 2010 [Reference Pfeiffer13]. HEVs are capable of undergoing mutations and recombination, a change from OPV to IPV will lower specific and non-specific local mucosal gut immunity to PVs [14]. These factors and others such as increases in international travel and globalization can be expected to serve as an ideal foundation for drifting of NPEVs and even newly emerging HEVs. HEV surveillance should therefore be continued, even after successful eradication of PV is achieved.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
Supported by Ministry of Health of the Slovak Republic, project MZSR 2007/03-RUVZBB-01 received by C.K., and project MZSR 2005/23-SZU-01 received by S.B. Data has been regularly submitted to the Regional laboratory of WHO. We are grateful for the advice, sera and confirmations to Associate Professor Merja Roivainen, and Professor Tapani Hovi, Regional Reference Laboratory, WHO in Helsinki, Finland and Dr Harrie van der Avoort, National Institute of Public Health and the Environment, Bilthoven, The Netherlands.




