Introduction
Eristalinus aeneus (Scopoli, 1763) belongs to the Syrphidae family, which is one of the largest families within the Diptera order (Thompson and Rotheray, Reference Thompson, Rotheray, Papp and Darvàs1998; Pape et al., Reference Pape, Blagoderov, Mostovski and Zhang2011). This species is present almost worldwide except for the Neotropical and Antarctica regions. It is commonly associated with eutrophicated water bodies, slow-moving streams, rivers and even coastal lagoons. In southern Europe, it has also been recorded in association with wastewater treatment plants, sewage farms and irrigation ditches, and is considered synanthropic (Pérez-Bañón et al., Reference Pérez-Bañón, Rojo, Ståhls and Marcos-García2003; Speight, Reference Speight, Speight, Castella, Sarthou and Vanappelghem2020).
The larvae of this species are aquatic and saprophagous, feeding on microorganisms and organic particles by filtering the water (Mahmoud et al., Reference Mahmoud, Bahgat, Zalat and Dewedar1999; Lobkova et al., Reference Lobkova, Barinova, Dulov and Galchenko2007). They present an elongated anal segment, the posterior respiratory process, which explains their common name of ‘rat-tailed maggots’ (Hartley, Reference Hartley1961). The larval stage presents three developmental instars (L1, L2 and L3), which are usually differentiated by the moulting events. The relevance of this immature stage resides in its role as bio-indicator of polluted aquatic environments (Sommaggio, Reference Sommaggio1999; Thompson et al., Reference Thompson, Rotheray, Zumbado, Brown, Borkent, Cumming, Wood, Woodley and Zumbado2010), as well as its efficiency as bio-decomposer of organic matter (Abou-El-Ela et al., Reference Abou-El-Ela, Taher and Nazer1978; Lardé, Reference Lardé1990).
The adults of this family are highly adapted to flower rewards such as pollen and nectar, with the subfamily Eristalinae, in particular, being considered important pollinators (Golding and Edmunds, Reference Golding and Edmunds2000; Pérez-Bañón, et al., Reference Pérez-Bañón, Rojo, Ståhls and Marcos-García2003, Reference Pérez-Bañón, Petanidou and Marcos-García2007; Ssymank et al., Reference Ssymank, Kearns, Pape and Thompson2008; Doyle et al., Reference Doyle, Hawkes, Massy, Powney, Menz and Wotton2020). Despite the scarcity of studies that focus just on the pollination efficiency of E. aeneus, the large number of references made to this species as an important member of the pollinator community in both natural and artificial environments highlights its relevance (de la Bandera and Traveset, Reference de la Bandera and Traveset2006; Saeed et al., Reference Saeed, Sajjad, Kwon and Kwon2008; Tanács et al., Reference Tanács, Benedek, Monostori and Bodnar2008; Sajjad and Saeed, Reference Sajjad and Saeed2010; Latif et al., Reference Latif, Malik, Saeed, Iqbal, Saeed, Khan, Ting and Ghramh2019).
Its importance as a pollinating agent has promoted the artificial rearing of this species. Generally, most of the larval rearing media have been based on animal manure and/or different types of decaying cereals, such as rice, wheat, barley or oats (Abou-El-Ela et al., Reference Abou-El-Ela, Taher and Nazer1978; Gladis, Reference Gladis1994; Hurtado, Reference Hurtado2013; Campoy et al., Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a). The considerable mortality at the larval stage has been stated as one of the main problems associated with the artificial rearing of E. aeneus, especially during the first instar (Campoy et al., Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a). For this reason, a better understanding of its larval feeding habits, as well as new rearing diets, are required to overcome this problem.
A promising alternative rearing medium consists of by-products from other industries, for instance, brewing, which would reduce the cost associated with insect production while contributing to circular economy practices (Fillaudeau et al., Reference Fillaudeau, Blanpain-Avet and Daufin2006; Thomas and Rahman, Reference Thomas and Rahman2006; Kerby and Vriesekoop, Reference Kerby and Vriesekoop2017). Some by-products from this industry are brewery spent grain (BSG) or yeast, which have already been used in the animal feed sector, mainly for ruminants, but also for poultry, fish and pigs (Szponar et al., Reference Szponar, Pawlik, Gamian and Dey2003; Mussatto et al., Reference Mussatto, Dragone and Roberto2006; Aliyu and Bala, Reference Aliyu and Bala2011). BSG is an abundant low-cost by-product, with about 3.4 million tonnes being produced in Europe every year (Stojceska et al., Reference Stojceska, Ainsworth, Plunkett and Ibanoglu2008), and its management is a matter of concern at local, national and international levels (LIFE-Brewery, 2016). It is rich in proteins (around 20%), carbohydrates, fibre, minerals and vitamins, among other beneficial nutrients, as well as being full of microorganisms (Kanauchi et al., Reference Kanauchi, Mitsuyama and Araki2001; Mussatto, Reference Mussatto, Singh-Nee and Pandey2009; Aliyu and Bala, Reference Aliyu and Bala2011). The main problems associated with the use of BSG as animal feed are the high moisture content (80–85%) and its susceptibility to spoilage by microbial growth (Stojceska et al., Reference Stojceska, Ainsworth, Plunkett and Ibanoglu2008; Aliyu and Bala, Reference Aliyu and Bala2011). However, both issues can be used for the rearing of eristaline syrphids since the larval stage is aquatic and microorganisms are their main source of food (Hartley, Reference Hartley1961; Rotheray, Reference Rotheray1993).
In order to test and compare the efficiency of different rearing media, it is essential to conduct accurate monitoring and analysis of population performance. For this purpose, life table methods have proven to be appropriate (Carey, Reference Carey1993; Eliopoulos, Reference Eliopoulos2006). These methods provide a wide range of data related to the life cycle, survival rates, fecundity and population dynamics. Age-stage, two-sex life table theory, which was developed by Chi and Liu (Reference Chi and Liu1985) and Chi (Reference Chi1988), has been shown to be a suitable tool for conducting these studies (Chi et al., Reference Chi, You, Atlıhan, Smith, Kavousi, Özgökçe, Güncan, Tuan, Fu, Xu, Zheng, Ye, Chu, Yu, Gharekhani, Saska, Gotoh, Schneider, Bussaman, Gökçe and Liu2020), as it addresses some of the common problems associated with traditional life table analysis (Lewis, Reference Lewis1942; Leslie, Reference Leslie1945; Birch, Reference Birch1948).
The objectives of this study were: (1) to study the biology and population dynamics of E. aeneus under artificial conditions; (2) to compare the biological parameters of E. aeneus when reared with two different larval media, aiming to discover which medium is more suitable for artificial rearing.
Materials and methods
Rearing method for preimaginal stages (egg, larva and pupa)
This research was conducted at the University of Alicante, where an artificial rearing protocol for E. aeneus is being developed. For the experiments, six random egg batches from the second laboratory generation were collected. Three egg batches were placed in a plastic container (15 × 23 × 10.5 cm3) with 200 g of BSG and the other three batches in a different container with 200 g of soaked oat grains (SOG). The methodology followed with the two populations was identical, replicating the experiment conducted by Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a). The whole experiment was carried out in a rearing chamber at 25 ± 1°C, with 50% RH and a 12:12 (L:D) hours photoperiod.
After the first instar larvae hatch, 200 individuals from each container were collected with a soft paint brush and separated into groups of ten larvae. Each group was kept in a plastic container (9.5 × 15 × 6 cm3) with 60 g of their corresponding rearing medium and water, and in total 20 containers were used for each rearing medium. These containers were checked every day, which involved recording the mortality and monitoring the remaining larvae. When the larvae reached the third instar, the containers were placed inside plastic cages (15 × 23 × 10.5 cm3) containing a layer of sawdust, in which the larvae pupated once their development was finished. These pupae were collected and separated individually in Petri dishes every day until the adults emerged.
Rearing method for the adult stage
The adults were marked with a coloured dot on their thorax to identify the date of emergence, thus facilitating their monitoring. These dots were applied with a small paint brush using harmless waterproof paint of different colours. The marked adults were released in a bug-dorm (40 × 40 × 40 cm3) until the first egg batch was laid. At that moment it was assumed that most of the females had mated. During this period, the adults were provided with 5 g of fresh pollen, 10 g of sugar, 30 ml of honey, water and 15 g of SOG as oviposition medium. Food and water were replaced every 2 days, while the oviposition medium was checked daily.
At this point, the males and females were separated into plastic cages (14.5 × 22.5 × 13.5 cm3). These adults were provided with 2 g of fresh pollen, 4 g of sugar, 5 ml of honey, water and 5 g of oviposition medium (SOG). Both fecundity and mortality were measured daily. Fecundity was recorded as the number of oviposition and the number of eggs per oviposition.
Life table analysis
The age-stage, two-sex life table theory (Chi and Liu, Reference Chi and Liu1985) and the method described by Chi (Chi, Reference Chi1988; Chi et al., Reference Chi, You, Atlıhan, Smith, Kavousi, Özgökçe, Güncan, Tuan, Fu, Xu, Zheng, Ye, Chu, Yu, Gharekhani, Saska, Gotoh, Schneider, Bussaman, Gökçe and Liu2020) were used to analyze the raw data from both experiments. The analysis was carried out by means of user-friendly software, namely TWOSEX-MSChart (Chi, Reference Chi2020), which was designed in Visual BASIC (Version 2020.06.16) for the Windows operating system, available at http://140.120.197.173/Ecology/prod02.htm (Chung Hsing University, Taichung, Taiwan). Different parameters were estimated for both populations of E. aeneus: the age-stage specific survival rate (sxj; where x is age and j is the stage), the mortality distribution (pxj), the age-specific survival rate (lx), the age-stage specific fecundity (fx4), the age-specific fecundity (mx), the age-specific maternity (lxmx), the total preoviposition period (TPOP), the adult preoviposition period (APOP), the age-stage life expectancy (exj), and the reproductive value (vxj). Additionally, four population parameters were obtained: the intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (R0) and the mean generation time (T).
The main formulas used by the software to calculate these parameters are shown in table 1. The mortality distribution (pxj), age-specific survival rate (lx) and age-specific fecundity (mx) were obtained following the equations presented by Chi and Liu (Reference Chi and Liu1985) and Chi (Reference Chi1988). The iterative bisection method based on the Euler-Lotka equation was used to calculate the intrinsic rate of increase (r), with age indexed from 0 (Goodman, Reference Goodman1982; Burden and Faires, Reference Burden and Faires2005). The age-stage life expectancy (exj) was estimated according to the formula of Chi and Su (Reference Chi and Su2006). The reproductive value (vxj) (Fisher, Reference Fisher1930) was obtained following the method of Huang and Chi (Reference Huang and Chi2011) and Tuan et al. (Reference Tuan, Lee and Chi2014a, Reference Tuan, Lee and Chib).
Table 1. Main parameters and formulas used to calculate them

Statistical analysis
The TWOSEX-MSChart software was also used to calculate means and standard errors by using the bootstrap technique. A total number of 100,000 bootstrap resamplings were conducted to ensure precise results (Akköprü et al., Reference Akköprü, Atlihan, Okut and Chi2015). The paired bootstrap test based on confidence interval (Efron and Tibshirani, Reference Efron and Tibshirani1993; Smucker et al., Reference Smucker, Allan and Carterette2007; Reddy and Chi, Reference Reddy and Chi2015; Sedighi et al., Reference Sedighi, Aghdam, Imani and Shojai2017) was used to compare the larval survival, pupal survival, preadult survival, developmental time, mean life history duration, adult longevity, fecundity, number of oviposition, APOP, TPOP and population parameters between the two tested rearing media. Differences between male and female longevity for each rearing medium were also compared.
Results
Life cycle
The time of development for each stage, the adult longevity of both sexes and the mean life history duration were calculated for each population of E. aeneus using two different rearing media (table 2). Significant differences were only found between the larval stage and the total preadult stage (egg, larva and pupa) (P < 0.05), with these being shorter in general when SOG was used as the rearing medium.
Table 2. Developmental time and adult longevity of E. aeneus reared with brewery spent grains (BSG) and soaked oat grains (SOG)
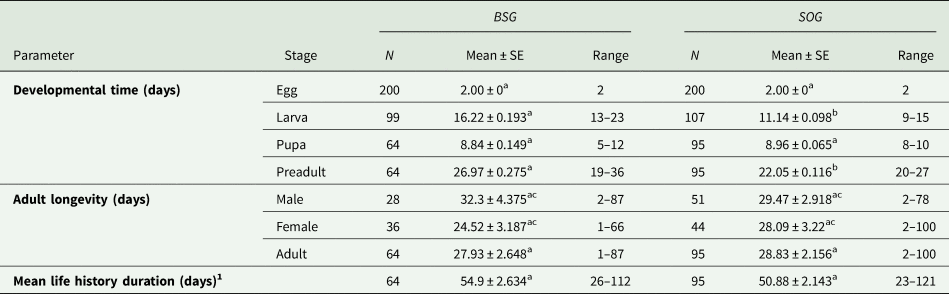
Means within rows followed by different letters (a or b) are significantly different (P < 0.05), as well as the longevity of male and female adults when reared using the same medium (c or d). Differences between parameters were evaluated by using paired bootstrap test.
1 To calculate the mean life history duration, only those individuals that reached the adult stage were considered.
No statistical differences were found in adult longevity, even when comparing males and females separately (P > 0.05). The total mean life history duration was also similar regardless of the rearing media (P > 0.05) (table 2).
Survival rate, distribution of mortality and life expectancy
Both the age-stage survival rate (sxj) (fig. 1) and the distribution of mortality (pxj) (fig. 2) revealed that the highest mortality in the two populations is found in the larval stage (50.5% in BSG and 46.5% in SOG), although the mortality distributions are different. When SOG was used as the main larval diet, the greatest mortality by far was on the 3rd day (2nd day in larval stage, L1), when 27.5% of the whole population died. In the case of BSG, the mortality was more scattered, with 12.5% dying on the 3rd day (2nd day in larval stage, L1), followed by a continuous increment of mortality at the end of this stage, reaching its maximum on the 19th day, when 6% of the population died. The mortality distribution was more noteworthy in the pupal stage when 17.5% of the BSG population died in contrast with the figure of 6% found in SOG. The overlapping observed in figs 1 and 2 corresponds to the variable development among individuals, which results in them reaching the next developmental stage at different ages. Despite the variability in the mortality distribution, no significant differences were found in the larval survival rate using the two-rearing media (P > 0.05). However, differences between the populations were observed when comparing the survival rate of the pupal stage and the total preadult stage (P < 0.05).
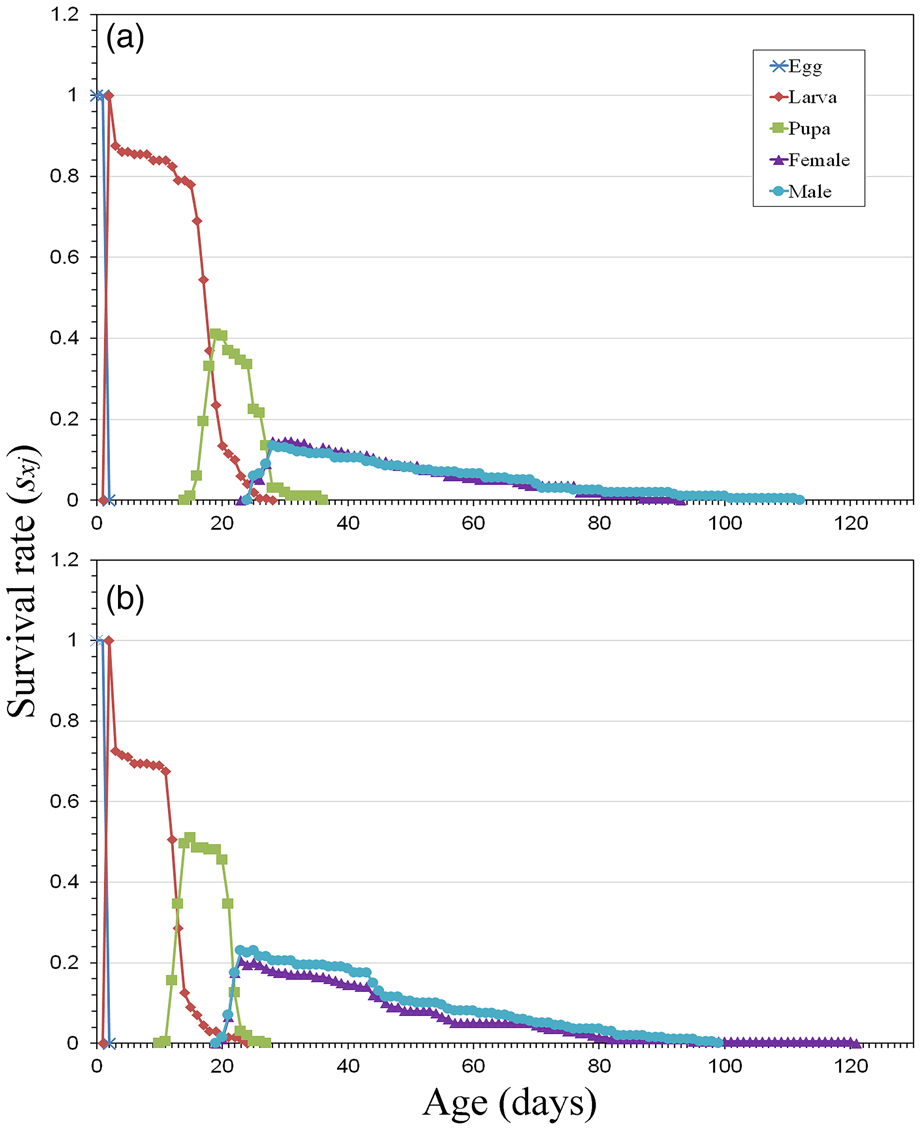
Figure 1. Age-stage survival rate (sxj) of E. aeneus reared with brewery spent grain, BSG (a) and soaked oat grains, SOG (b).

Figure 2. Mortality distribution (pxj) of E. aeneus reared with brewery spent grain, BSG (a), and soaked oat grains, SOG (b).
The age-stage life expectancy (exj) of a newborn was equal in the two experiments (28.65 d) (fig. 3). When BSG was used, the highest life expectancy was found to occur 2 days after the emergence of the male adults (27th day), 32.93 d, followed by the day after the first mortality event in the larval stage (4th day), 29.31 d (fig. 3a). When E. aeneus was reared with SOG, the highest life expectancy in the adult stage was found to occur on the day of adult emergence (20th day) in both sexes, 29.64 d and 31.37 d for females and males, respectively. However, the highest peak during the whole life cycle was found in the larval stage, 35.37 d (fig. 3b), one day after the critical mortality event mentioned above (4th day).

Figure 3. Age-stage life expectancy (exj) of E. aeneus reared with brewery spent grain, BSG (a), and soaked oat grains, SOG (b).
Reproductive parameters
Different reproductive parameters were calculated for both populations: the mean age-stage specific fecundity (fx4), the maximum daily fecundity, the maximum total fecundity and the mean number of oviposition (table 3). Mean fecundity and the number of oviposition were higher in the population reared with BSG, in fact, the total egg production of this population was 19,242 eggs, meanwhile the population reared with SOG only laid 15,116 eggs, yet no significant differences were found between the two populations (P > 0.05). The number of females that reached fertile age was similar: 21 out of 36 in the case of the population reared with BSG, and 25 out of 44 when reared using SOG.
Table 3. Reproductive parameters of E. aeneus reared with brewery spent grains (BSG) and soaked oat grains (SOG)
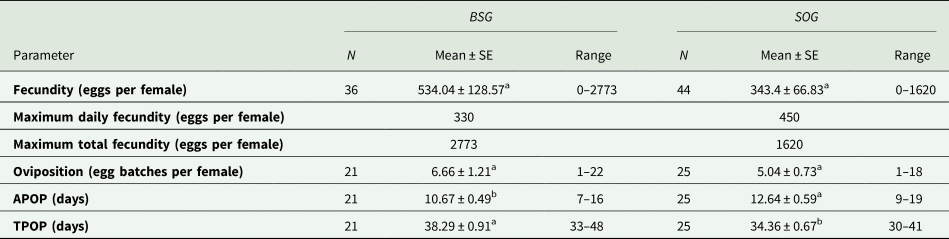
Means within rows followed by different letters (a or b) are significantly different (P < 0.05). Differences between parameters were evaluated by using paired bootstrap test.
The APOP and TPOP were significantly different (P < 0.05) between the populations (table 3). Despite the APOP being shorter when using BSG, pointing at a faster reproductive adult maturation, the TPOP was longer, considering the extended preadult developmental time found when this rearing medium was used.
The age-specific survival rate (lx), the age-stage specific fecundity (fx4), the age-specific fecundity (mx) and the age-specific maternity (lxmx) of E. aeneus reared with SOG and BSG are shown in fig. 4. The population reared with BSG displayed a fertile age-range between the 33rd and 81st day, with a large egg contribution between the 37th and 60th day, followed by two important fecundity events, with the highest fecundity being 79.14 eggs per female. This period ran from the 30th to the 97th day when SOG was used. In this case, most of the egg production occurred between the 30th and 47th day, and the highest fecundity was 38.38 eggs per female.
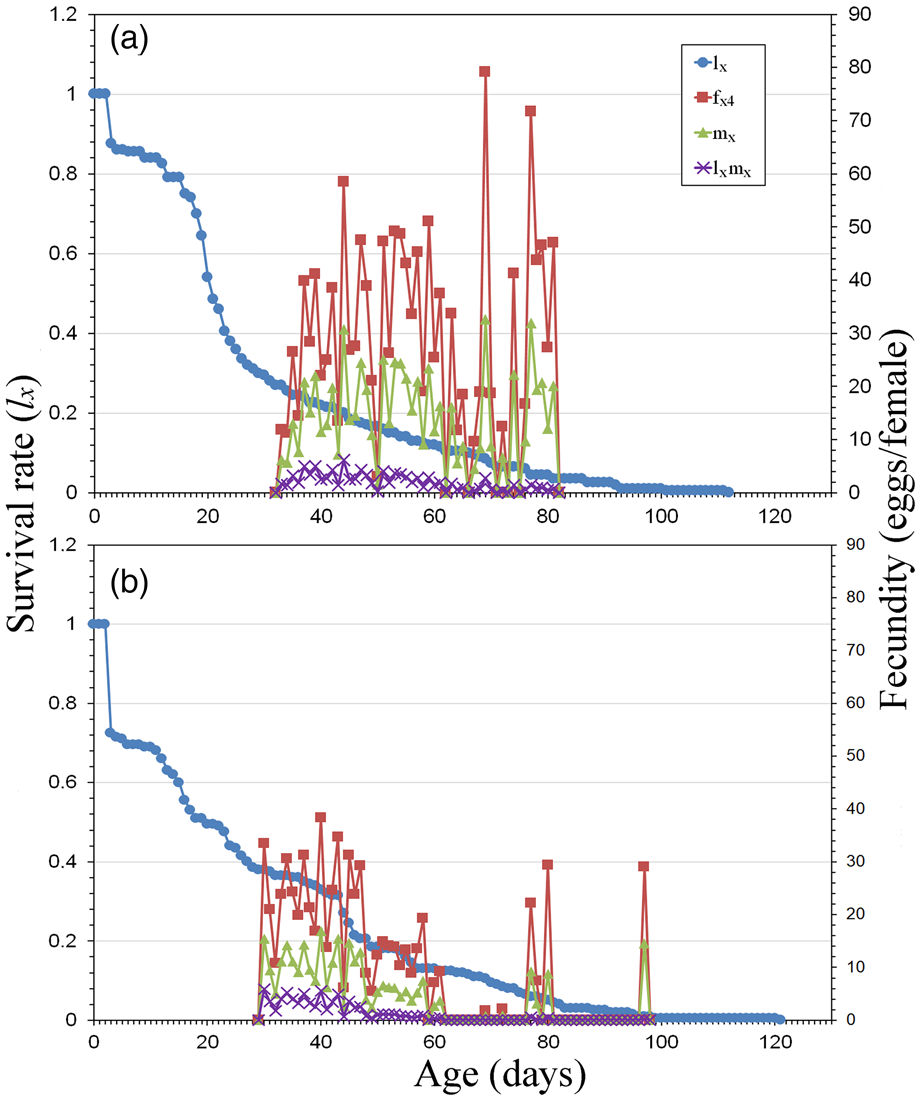
Figure 4. Age-specific survival rate (l x), age-stage fecundity (f x4), age-specific fecundity (m x) and age-specific maternity (l xm x) of E. aeneus reared with brewery spent grain, BSG (a), and soaked oat grains, SOG (b).
Although the reproductive value (vxj) considers the whole population (except for the male adults), higher values are usually found to be associated with females (fig. 5). Their contribution to population growth was located between the 24th and 81st day when BSG was used, with the highest peaks being reached on the 37th (265.29 d−1) and 44th (265.83 d−1) days. Additionally, a high reproductive value was found in the pupal stage, reaching its maximum on the 35th day (222.19 d−1) (fig. 5a). This peak corresponds to late-emerging females which contributed to the next generation. The population reared with SOG displayed a population growth between the 20th and 97th day, reaching the highest peak on the 30th day (183.35 d−1) (fig. 5b).
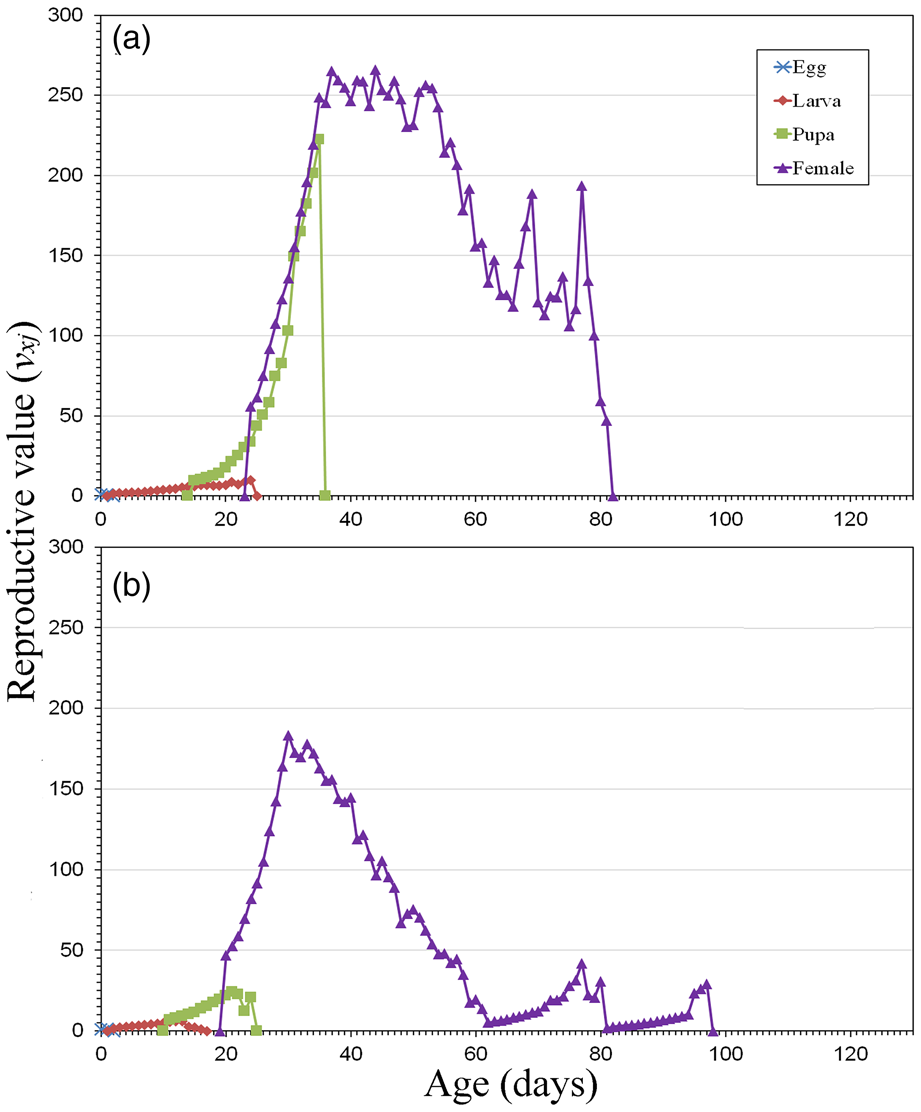
Figure 5. Age-stage reproductive value (vxj) of E. aeneus reared with brewery spent grain, BSG (a), and soaked oat grains, SOG (b).
Population parameters
The following population parameters were calculated for both populations: the intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (R0) and the mean generation time (T) (table 4). Both the intrinsic and the finite rates of increase indicated the positive growth of the two studied populations. The net reproductive rate was slightly higher in the population reared with BSG due to its higher fecundity, although no significant differences were found when comparing the intrinsic rate of increase, the finite rate of increase or the net reproductive rate (P > 0.05). However, differences were observed when comparing the mean generation time (P < 0.05), this being shorter when E. aeneus was reared using SOG.
Table 4. Population parameters of E. aeneus reared with brewery spent grains (BSG) and soaked oat grains (SOG)

Means within rows followed by different letters (a or b) are significantly different at P < 0.05. Differences between parameters were evaluated by using paired bootstrap test.
Discussion
Life cycle
The chosen rearing media had a direct influence on the larval developmental time, and thus on the total preadult duration. In general, the population reared with SOG displayed a faster larval development, allowing E. aeneus to reach the adult stage 4.92 d earlier (table 2). This difference could be explained as a variable nutritional content between both media, especially the microorganism populations that are present, as well as a totally different media consistency, although these factors were not assessed in this experiment. The population of E. aeneus reared by Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a), using SOG under the same conditions (25°C, 50% RH and 12:12 (L:D) h) and methodology, presented a larval and preadult duration (14.79 d and 25.24 d, respectively) similar to the results obtained using BSG and slightly longer than those obtained with SOG in this study. This comparison confirms that fermented cereals, though effective, do not provide consistent results and the variability among populations cannot be underestimated. These differences between the two populations reared with SOG (in this study and Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a)) could correspond to the variable fermentation process, different populations of microorganisms or the genetic variability among individuals. However, since these factors have not been evaluated, no conclusions can be drawn in this regard. Future studies analysing the medium composition could shed light on this question.
Regarding adult longevity, no significant differences were found between the two populations, assuming that none of the chosen media negatively affects this parameter (table 2). In general, no significant differences were found between males and females, although it was found to be significant in the study of Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a).
Survival rate, mortality distribution and life expectancy
The mortality rate displayed by the larvae of both populations was similar, although its distribution varied (figs 1 and 2). This mortality was critical just after hatching, at the first larval instar, especially when SOG was used as the rearing medium (figs 1b and 2b). This phenomenon was also observed by Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a). In the case of BSG, the mortality of the first instar was not as pronounced, but a steady increase in mortality was observed, reaching a second peak during the third larval instar (figs 1a and 2a). This is probably due to an adverse fermentation of the medium, which in this case had a harmful effect on the larvae in the long term. This fact was corroborated by the peak in mortality found at the beginning of the pupal stage, coinciding with the pupariation process, when hardening and tanning of the puparium takes place before reaching the actual pupal stage (Fraenkel and Bhaskaran, Reference Fraenkel and Bhaskaran1973; Campoy et al., Reference Campoy, Aracil, Pérez-Bañón and Rojo2020b). In this research, the prepupal phase (between pupariation and larval-pupal apolysis) was included as part of the pupal stage.
Although the highest life expectancy is commonly associated with the first day of the population, in these two media the highest peaks are found after noteworthy mortality events (Tuan et al., Reference Tuan, Chang, Saska, Atlihan and Chi2017), for instance on the 4th day (figs 2 and 3), one day after the critical mortality detected in the first larval instar in both populations, especially when SOG was used. The population reared with BSG stands out for being the one in which the male adults present the highest life expectancy since this was the long-lived stage. There are also two big troughs in the pupal stage. The first coincides with the mortality found in the pupariation process mentioned above, and the second corresponds to a high adult emergence event (fig. 3a). The population reared with SOG presents three unusual peaks in the female adult stage on the 57th, 82nd and 95th days. They correspond to three periods of stability (without mortality), with the 95th day being the most noteworthy when a single female survived for 25 days (fig. 3b).
Reproductive and population parameters
The main significant differences between the two populations were found in the APOP and TPOP (table 3). The TPOP indicates that the population reared with SOG reached the fertile age significantly earlier. This difference can be mainly attributed to the shorter larval stage duration displayed by this population since female adults from the BSG medium showed a shorter APOP.
Even though no significant differences were found in the rest of the parameters analysed, the total production of eggs was 21.44% larger in the population reared with BSG (table 3). This difference suggests that adult fecundity could somehow be related to the larval diet; the fact that nutrient consumption and the conditions in which the larvae grow have an effect on adult performance and development has already been demonstrated (Aguila et al., Reference Aguila, Hoshizaki and Gibbs2013; Gobbi et al., Reference Gobbi, Martínez-Sánchez and Rojo2013; Weldon et al., Reference Weldon, Mnguni, Démares, du Rand, Malod, Manrakhan and Nicolson2019). When the populations reared with SOG in this experiment and in Campoy et al. (Reference Campoy, Sáez, Pérez-Bañón and Rojo2020a) are compared, we observe that the fecundity parameters in the latter study are much lower. In this case, where the larval medium and methodology are very similar, the main differences could be associated with other factors such as diet (the inclusion of honey in this study) or genetics (laboratory generation or genetic loss). Furthermore, the temperature also has an undeniable effect on fecundity, and especially on the maturation period. For example, Abou-El-Ela et al. (Reference Abou-El-Ela, Taher and Nazer1978) found an APOP of 4 days at 32°C, whereas Hurtado (Reference Hurtado2013) reported 17.23 d at 20°C.
The figures for fecundity and reproductive value (figs 4 and 5) provide visual information about the abundance and distribution of egg production by females. The highest reproductive values are usually found between female adult emergence and the TPOP, highlighting the period when the greatest contribution to the next generation is expected. The population reared with SOG reached the highest reproductive value 7 days earlier than the population reared with BSG (on the 30th and 37th day, respectively). This fact is supported by the shorter TPOP and mean generation time (T), as well as the slightly higher intrinsic (r) and finite (λ) rates of increase. However, this faster development contrasts with a higher total production of eggs displayed by female adults reared with BSG. In fact, this population maintains a reproductive value above 200 d−1 between the 34th and 57th day (table 4).
Conclusions
In general, the population reared with SOG displayed a faster preadult development and a higher survival rate. However, these differences were compensated for by a larger number of eggs laid when BSG was chosen as the main larval medium. The relationship between larval diet and adult longevity/fecundity is still unclear in this species, since the results are variable and not significantly different, although the greater number of eggs and oviposition, as well as the more compact fecundity range and shorter APOP could point to some nutrients present in the BSG as being responsible of these noteworthy differences. However, more studies are required to accurately establish the effect of this rearing medium on the adult performance and fecundity of this species.
Considering all these biological and population parameters, it must be decided which aspect is preferable in the artificial rearing of this species: a faster development or a higher egg production. We believe that the BSG medium provides much better prospects since this medium has not been used before with eristalines and the methodology still has ample room for improvement; for instance, by adjusting the quantity of the product and the environmental conditions, or by analysing the medium to find which supplementary nutrients are required and how the fermentation process modifies the medium.
From an economic and ecological point of view, the use of BSG allows the inclusion of the larval rearing process as part of a circular economy, using by-products from a different industry, in contrast with the use of SOG, which implies the generation of a high volume of waste, since the oat grains are not directly consumed by the larvae. Additionally, BSG is a much cheaper option, which clearly benefits the artificial production of commercial pollinators. Further studies are required to obtain better parameters for using BSG or alternative by-products from other industries.
Acknowledgements
The current manuscript is part of a PhD, written by Andrés Campoy Pomares at the University of Alicante (Spain). This project is funded by a FPU grant (FPU16-01985), provided by the Spanish Ministry of Universities (MIU).












