In aquaculture, substituting costly fishmeal with less expensive plant-derived carbohydrate (CHO) reduces feed input costs. The provision of dietary CHO to carnivorous fish such as the European seabass (Dicentrarchus labrax L.) may also have environmental benefits since CHO utilisation can potentially spare the catabolism of dietary amino acids to glucose and nitrogenous waste( Reference Gatlin, Barrows and Brown 1 ). Dietary CHO influences growth, feed utilisation and deposition of nutrients according to species, quantity, origin and pre-treatment for improving digestibility( Reference Enes, Panserat and Kaushik 2 – Reference Stone 4 ). The effects of partial substitution of dietary protein by plant-derived CHO on food conversion efficiency and nutrient digestibility sources have been tested in seabass( Reference Adamidou, Nengas and Alexis 5 – Reference Moreira, Peres and Couto 7 ). In addition, its effects on energy retention and maintenance requirements( Reference Peres and Oliva-Teles 8 ) as well as the activity of key CHO-metabolising enzymes( Reference Enes, Panserat and Kaushik 6 , Reference Enes, Panserat and Kaushik 9 ) have been studied. These studies have suggested that, on the one hand, carnivorous fish are able to up-regulate their capacity for hepatic glucose utilisation through the increased expression of gateway enzymes such as glucokinase when fed with a high-CHO diet. However, on the other hand, supplementation of fish feed with up to 30 % starch has not been shown to alter the overall protein efficiency ratio or nitrogen retention in seabass( Reference Moreira, Peres and Couto 7 ). This is despite the high apparent digestibility coefficient of starch (especially gelatinised) for this species( Reference Enes, Panserat and Kaushik 2 ), suggesting that there is substantial absorption of glucose during feeding with starch-supplemented diets. Absorbed glucose can contribute to systemic glucose appearance, thereby potentially reducing the demand for endogenous glucose production via gluconeogenesis. Since amino acids are considered to be the principal source of gluconeogenic carbons in carnivorous fish( Reference Peres and Oliva-Teles 8 , Reference Lupatsch, Kissil and Sklan 10 ), a reduction in gluconeogenic activity would directly reduce the generation of nitrogenous waste.
A novel analysis of hepatic glucose and glycogen metabolism using 2H2O revealed that for seabass fed a conventional high-protein/low-CHO diet, essentially all blood glucose and hepatic glycogen synthesis were derived via gluconeogenesis( Reference Viegas, Rito and González 11 , Reference Viegas, Rito and Jarak 12 ). We hypothesised that when dietary fishmeal is partially substituted by digestible (gelatinised) starch, the contribution of dietary CHO to blood glucose and hepatic glycogen appearance would significantly increase while gluconeogenic contribution would correspondingly be reduced. So far, data on enzymes' activity and/or mRNA abundance have largely elucidated the response of CHO metabolism in fish( Reference Enes, Panserat and Kaushik 2 ). Therefore, we followed up the 2H2O analysis with the expression of key enzymes directly or indirectly involved with the utilisation of dietary CHO, in order to corroborate the hypothesised metabolic shift from a no-CHO to a high-CHO diet.
Materials and methods
Fish handling and sampling
For the present study, European seabass (D. labrax L.) provided by Tinamenor (Cantabria, Spain) were transported to the laboratory and randomly assigned to two different tanks (thirty-two fish per tank; mean initial length 10·8 (sem 0·2) cm and mean initial body weight 21·9 (sem 0·2) g). Fish were acclimatised in 200-litre polyethylene circular tanks supplied with well-aerated filtered seawater from a recirculated system set to 20 ± 1°C, 30 ± 1 % salinity and O2 levels above 80 % saturation under natural photoperiod. These conditions were maintained throughout the experiment. All experimental procedures complied with the Guidelines of the European Union Council (86/609/EU). The fish were fed two experimental diets that were formulated by Sparos Lda. (Table 1): a control diet (CTRL) with no CHO (except for an inert filler of cellulose without any nutritional value to maintain pellet integrity in water), and an experimental diet containing 30 % digestible starch (DS; gelatinised pea starch). All diets were formulated to fulfil the nutritional requirements of the species. During this feeding period, fish were fed twice per d ad libitum (6 d/week). Following this period, each group was transferred to a 5 % 2H-enriched seawater tank for 6 d. This tank was maintained with an independent closed filtering system, but had similar characteristics to the tanks used during the feeding period in terms of size, volume of water (200 litres), opacity, filtering material and water parameters. Seawater was enriched with 2H2O with the addition of 99 %-enriched 2H2O (Euriso-Top) as described previously by Viegas et al. ( Reference Viegas, Mendes and Leston 13 ). 2H enrichment was quantified throughout the experiment as described by Jones et al. ( Reference Jones, Merritt and Malloy 14 ). During the period in 2H-enriched seawater, fish were fed once per d (ad libitum) and killed 24 h after the last meal on day 5. Fish were anaesthetised in a 30-litre tank of 5 % 2H-enriched seawater containing 0·1 g/l of MS-222. Fish were measured and weighed, and blood was drawn from the caudal vein with heparinised syringes. Fish were then killed by cervical section. Liver was excised, weighed, freeze-clamped in liquid N2 and stored at − 80°C until further analysis.
Table 1 Ingredients and proximate composition of the experimental diets provided to Dicentrarchus labrax
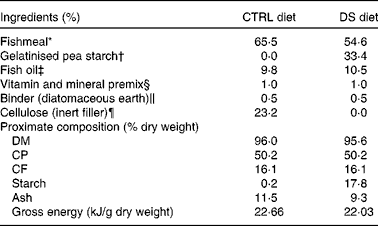
CTRL, control; DS, digestible starch; CP, crude protein; CF, crude fat.
* Peruvian fishmeal LT: 67 % CP, 9 % CF, EXALMAR.
† Aquatex 8071, gelatinised dehulled microground pea meal: 23 % CP, 50 % starch, SOTEXPRO.
‡ Marine oil omega 3: Henry Lamotte Oils GmbH.
§ PVO40·01 premix for marine fish, PREMIX Lda. Vitamins (IU or mg/kg diet): dl-α-tocopherol acetate, 100 mg; sodium menadione bisulphate, 25 mg; retinyl acetate, 20 000 IU; dl-cholecalciferol, 2000 IU; thiamin, 30 mg; riboflavin, 30 mg; pyridoxine, 20 mg; B12, 0·1 mg; nicotinic acid, 200 mg; folic acid, 15 mg; ascorbic acid, 1000 mg; inositol, 500 mg; biotin, 3 mg; calcium pantothenate, 100 mg; choline chloride, 1000 mg, betaine, 500 mg. Minerals (g or mg/kg diet): cobalt sulphate, 2·5 mg; copper sulphate, 1·1 mg; ferric citrate, 0·2 g; potassium iodide, 5 mg; manganese sulphate, 15 mg; sodium selenite, 0·2 mg; zinc sulphate, 40 mg; magnesium hydroxide, 0·6 g; potassium chloride 1·1 g; NaCl, 0·5 g; calcium carbonate, 4 g.
∥ Kielseguhr: LIGRANA GmbH.
¶ Microcrystalline cellulose, Blanver.
Metabolite preparation
Immediately after blood withdrawal, a 100 μl aliquot was kept on ice. After centrifugation (3000 g , 10 min), plasma samples were stored at − 20°C for 2H enrichments and glucose and TAG quantification with commercial kits (n 8, Cromatest®; Linear Chemicals). To obtain sufficient amounts of analytes for generating 2H NMR spectra of blood glucose derivatives with a high signal:noise ratio, each sample consisted of blood from three to seven fish (pooled analyses: n 6 per diet). Blood was deproteinised by the addition of 0·3 m-ZnSO4 and 0·15 m-Ba(OH)2 (1·5 ml of each solution per ml blood), followed by centrifugation at 3500 g for 15 min. The supernatant was desalted by passage through sequential columns containing Dowex® 50WX8 (hydrogen form; Sigma-Aldrich) and Amberlite® IRA-67 (free base, Fluka; Sigma-Aldrich), and lyophilised.
Pulverised liver tissue was used for enzyme assays (n 7), mRNA extraction (n 6), glycogen quantification (n 6), as described by Keppler & Decker( Reference Keppler, Decker and Bergmeyer 15 ). Liver samples from three to seven fish under each condition were pooled for glycogen extraction (pooled analyses: n 5 for the CTRL diet and n 6 for the DS diet). Hepatic glycogen was extracted by precipitation with 70 % ethanol followed by alkaline tissue hydrolysis, as described by Viegas et al. ( Reference Viegas, Rito and Jarak 12 ). To hydrolyse glycogen to glucose, glycogen was resuspended in 5 ml sodium acetate buffer (0·05 m, pH 4·5), and 20 μl of an aqueous solution containing sixteen units of amyloglucosidase from Aspergillus niger (glucose-free preparation; Sigma-Aldrich) were added. The samples were incubated overnight at 55°C and centrifuged at 3500 g for 10 min. The supernatant was collected and lyophilised. To optimise signal resolution in the 2H NMR spectra, both blood glucose and glucosyl units hydrolysed from hepatic glycogen were converted into monoacetone glucose, as described previously( Reference Viegas, Mendes and Leston 13 ).
Enzyme activity and mRNA expression levels
Several key enzymes were analysed due to their relevance in the metabolic network. Enzyme activity assays were carried out as described previously: glucokinase (GK, EC 2.7.1.2)( Reference Caseras, Metón and Fernández 16 ); glucose 6-phosphatase (G6Pase, EC 3.1.3.9)( Reference Caseras, Metón and Vives 17 ); pyruvate kinase (EC 2.7.1.40), 6-phosphofructo 1-kinase (PFK-1; EC 2.7.1.11), fructose-1,6-bisphosphatase (FBPase; EC 3.1.3.11)( Reference Metón, Mediavilla and Caseras 18 ); glutamate dehydrogenase (GDH; EC 1.4.1.2)( Reference Melo, Lundstedt and Metón 19 ). Alanine aminotransferase (cytosolic) (EC 2.6.1.2) was determined using a commercial kit (Cromatest; Linear Chemicals). All enzyme activity assays were carried out at 30°C and followed at 340 nm. Total protein content of the liver crude extracts was determined by the Bradford method (Bio-Rad) at 30°C using bovine serum albumin as a standard and followed at 600 nm. All assays for metabolites, enzyme activities and total protein were adapted for automated measurements using a Cobas Mira S spectrophotometric analyser (Hoffman-La Roche). Enzyme activities are expressed per mg of soluble protein (specific activity). One unit of enzyme activity was defined as the amount of enzyme necessary to transform 1 μmol of substrate per min. Total RNA extraction, reverse transcription, amplification by RT-PCR, RT-PCR product analysis and purification, transformation in competent cells, sequencing and design of specific oligonucleotides for quantitative real-time RT-PCR were conducted, as described previously, for GK and G6Pase( Reference Viegas, Rito and González 11 ), and cytosolic alanine aminotransferase( Reference Viegas, Caballero-Solares and Rito 20 ). To our knowledge, no sequence was available for GDH, so for its partial sequencing, we followed the procedure described by Viegas and colleagues( Reference Viegas, Rito and González 11 , Reference Viegas, Caballero-Solares and Rito 20 ). Amplification of GDH by RT-PCR was carried out using the pairs of primers designed from highly conserved regions of nucleotide sequences published in the GenBank for other fish species (Table 2). Quantitative real-time RT-PCR were carried out in a StepOnePlus™ Real-Time PCR System (Applied Biosystems®) using the pairs of oligonucleotides listed in Table 2. Variations in mRNA abundance and n-fold changes were calculated relative to the CTRL fish using the standard ΔΔC t method adjusted for PCR efficiency as proposed by Pfaffl( Reference Pfaffl 21 ).
Table 2 Primer pairs used for the partial complementary DNA amplification of glutamate dehydrogenase (GDH) by RT-PCR and expected band extension*

cALT, cytosolic alanine aminotransferase; G6Pase, glucose 6-phosphatase; GK, glucokinase.
* Primer pairs were used to assess the mRNA levels of cALT, GDH, G6Pase and GK by quantitative real-time RT-PCR analysis, and respective GenBank accession numbers.
2H NMR analysis
Plasma water with 2H enrichments were determined from 10 μl aliquots of plasma by 2H NMR, as described by Jones et al. ( Reference Jones, Merritt and Malloy 14 ). Plasma water was assumed to be 92 % of total plasma( Reference Krebs 22 ). Proton-decoupled 2H NMR spectra of monoacetone glucose samples were obtained as described previously for blood glucose( Reference Viegas, Rito and González 11 ) and liver glycogen( Reference Viegas, Rito and Jarak 12 ) using a Varian VNMRS 600 MHz NMR (Agilent) spectrometer. Spectra were analysed with the NUTS PC-based NMR spectral analysis software (Acorn NMR Inc.).
Quantification of blood glucose and hepatic glycogen sources
Sources of blood glucose were quantified from the 2H enrichment levels of blood glucose and plasma water (PW) as described previously( Reference Viegas, Rito and González 11 ). 2H enrichment levels of the two groups were normalised in order to directly compare the ratios of glucose and glycogen 2H enrichment levels relative to PW. It was assumed that the blood glucose pool had completely turned over during the 6 d period in 2H-enriched seawater. Contributions from glucose 6-phosphate (G6P) were calculated as follows:
Endogenous sources of glucose were resolved into gluconeogenic and non-gluconeogenic fractions based on the enrichment of positions 5 and 2. Under the feeding conditions, the fractional non-gluconeogenic contribution included G6P derived via glycogen phosphorylase as well as G6P synthesised by the futile cycling of glucose via GK and G6Pase:
Sources of hepatic glycogen synthesis were quantified from the 2H enrichment levels of hepatic glycogen and PW, as described previously( Reference Viegas, Rito and Jarak 12 ):
Unlike blood glucose, hepatic glycogen was only partially turned over during the 6 d period in 2H-enriched seawater. Thus, the unlabelled fraction consisted of pre-existing glycogen, whose provenance (i.e. synthesised via direct or indirect pathways) was unknown. From the labelled endogenous G6P, the contributions of direct and indirect pathways to glycogenic G6P flux were estimated from the ratio of glycogen position 5 and 2 enrichments:
Under the feeding conditions, hepatic glycogen levels were assumed to be constant over the 6 d period when the fish were in 2H-enriched water. Therefore, the fractional replacement rate was calculated from the fraction of endogenous G6P (equation 5) per h. The fractional replacement rate can be converted to the mean absolute rate by multiplying it by the mean hepatic glycogen level, expressed as μmol/kg fish. Indirect and direct pathway fluxes can be discriminated by multiplying the absolute replacement rates for equations 7 and 8, respectively.
Statistical analysis
Student's two-tailed unpaired t test was used to compare means between the CTRL and DS diets for several parameters. Analyses were performed in GraphPad Prism® 6 software (GraphPad Software, Inc.), and differences were considered statistically significant at P <0·05.
Results
Growth rates and plasma metabolite levels
Biochemical parameters and growth performance in fish fed with a no-CHO (CTRL) and a high-CHO (DS) diet are summarised in Table 3. Blood glucose values were not significantly different. Hepatic glycogen levels were significantly higher in the DS fish than in the CTRL fish, although the hepatosomatic index did not reflect such difference. Growth performance was not affected by diet composition as similar daily growth indices were observed.
Table 3 Biochemical parameters and growth performance in seabass (Dicentrarchus labrax) fed with a no-carbohydrate diet as the control (CTRL) and a 30 % digestible starch (DS) diet (Mean values with their pooled standard errors)

DGI, daily growth index; HSI, hepatosomatic index.
* Mean value was significantly different from that of the CTRL diet (P< 0·05; t test).
† DGI = 100 × (final body weight1/3− initial body weight1/3)/d.
‡ HSI = 100 (liver weight/body weight).
Plasma water and metabolite enrichments from 2H2O
PW enrichment for the CTRL and DS fish was 5·0 (sem 0·2) %, which was consistent with the 2H-enriched tank water. Moreover, during the 6 d period, tank water 2H enrichments did not change significantly (data not shown). Since fish PW and tank water enrichments became equivalent within less than 6 h( Reference Viegas, Mendes and Leston 13 ), the PW enrichment level measured at the end of the study was considered to reflect that of a constant 2H-precursor enrichment level over the preceding 6 d. As a result, synthesis and/or cycling from G6P and gluconeogenic precursors led to the incorporation of 2H into specific positions of both blood glucose( Reference Viegas, Mendes and Leston 13 ) and hepatic glycogen( Reference Viegas, Rito and Jarak 12 ). Figure 1 shows the representative 2H NMR spectra of monoacetone glucose derived from glucose and glycogen obtained from fish fed the CTRL and DS diets. The positional 2H enrichment levels estimated from the analysis of their respective 2H NMR signals are listed in Table 4. The high signal:noise ratio of 2H resonances allowed precise quantification of all carbon positional 2H enrichments, where equivalent intensities indicate equivalent enrichment for a certain position. Glucose enrichment in position 2 calculated from the spectra approached that of PW, confirming that the blood glucose pool fully turned over during the 6 d period in 2H-enriched seawater for the CTRL and DS fish. In contrast, the hepatic glycogen turnover rate was slow for both diets as revealed by glycogen position 2 enrichment, which was lower than PW. The CTRL fish turned over about one-half and the DS fish turned over one-third of their hepatic glycogen pool during the 6 d period in 2H-enriched seawater, reflecting a relatively static hepatic glycogen pool under the daily feeding regimen. Except for position 2, the 2H enrichment distribution of blood glucose showed significant differences between the CTRL and DS fish. While the enrichment of position 2 approached that of PW for both the CTRL and DS conditions, the enrichment of position 5 was significantly lower for fish fed with the DS diet. From the enrichment distribution (Table 4), the fractional sources of blood glucose were calculated, and data are shown in Fig. 2. The results reveal a significant shift from gluconeogenesis towards contributions of unlabelled glucose that had undergone futile cycling via hepatic G6P. This cycled glucose was presumably originated from digested dietary starch. In the DS fish, the fraction of blood glucose derived directly by absorption (therefore unlabelled in any position by 2H) almost doubled compared with the CTRL fish, accounting for approximately 20 % of blood glucose appearance. Contributions from absorbed glucose that had undergone futile cycling also increased significantly compared with the CTRL fish, accounting for 27 % of blood glucose appearance. Thus, 24 h after the last meal, almost half of the circulating glucose was derived from dietary CHO in the DS fish (20 % unlabelled +27 % non-gluconeogenic), while gluconeogenesis accounted for 85 % of circulating glucose in the CTRL fish. For hepatic glycogen, the CTRL fish exhibited equivalent 2H enrichments of positions 2 and 5. This is consistent with a substantial contribution of the indirect pathway to glycogen synthesis flux (Tables 4 and 5). For the DS fish, there was a significant direct pathway contribution to glycogen synthesis, indicating the recruitment of dietary glucose for glycogenesis. Meanwhile, indirect pathway fluxes were significantly smaller, consistent with a sparing of gluconeogenic carbon flow for hepatic glycogen synthesis.

Fig. 1 Representative 2H NMR spectra of monoacetone glucose samples derived from (a, c) hepatic glycogen and (b, d) blood glucose of seabass (Dicentrarchus labrax) fed with (a, b) a no-carbohydrate diet as the control and (c, d) a 30 % digestible starch diet, and sampled after a 6 d residence in a tank with 5 % 2H-enriched water. The numbers above each signal represents its position within the original glucose molecule. The signals at positions 2 and 5 for each spectrum are highlighted (in red and blue, respectively). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Table 4 Blood glucose and hepatic glycogen positional 2H enrichments‡ and plasma water (PW) 2H enrichment as quantified by 2H NMR in seabass (Dicentrarchus labrax) fed with a no-carbohydrate diet as the control (CTRL) and a 30 % digestible starch (DS) diet and sampled after a 6-d residence in a tank with 5 % 2H-enriched water (Mean values with their standard errors)
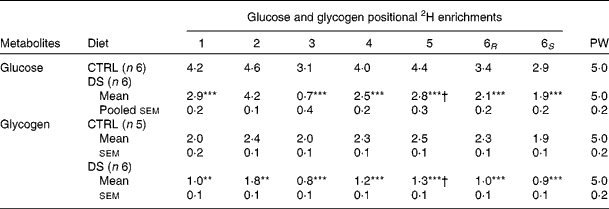
Mean value was significantly different from that of the CTRL group: **P< 0·01, ***P< 0·001 (t test).
† Mean value was significantly different from that at position 2 (P< 0·05; t test). Note that means were tested only between the positions of interest (2 and 5).
‡ Enrichments adjusted for tank water at 5·0 % 2H2O.

Fig. 2 Sources of blood glucose and hepatic glycogen for seabass (Dicentrarchus labrax) fed with a control (CTRL) diet containing no carbohydrate and with a diet containing 30 % digestible starch (DS). Contributions are represented as percentage of total (%) and are resolved into unlabelled (dietary absorption, ![]() ), non-gluconeogenic glucose 6-phosphate (G6P; direct pathway in glycogen,
), non-gluconeogenic glucose 6-phosphate (G6P; direct pathway in glycogen, ![]() ) and gluconeogenic G6P (indirect pathway in glycogen,
) and gluconeogenic G6P (indirect pathway in glycogen, ![]() ). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the CTRL diet: *P< 0·05, ***P< 0·001 (t test).
). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the CTRL diet: *P< 0·05, ***P< 0·001 (t test).
Table 5 Hepatic glycogen synthesis fluxes in seabass (Dicentrarchus labrax) fed daily with a no-carbohydrate diet as the control (CTRL) and a 30 % digestible starch (DS) diet and sampled after a 6-d residence in a tank with 5 % 2H-enriched water (Mean values with their standard errors)
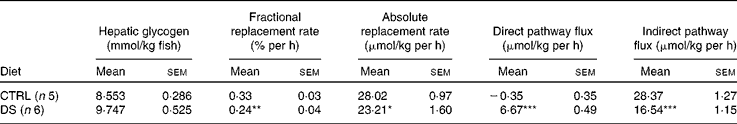
Mean value was significantly different from that of the CTRL diet: *P< 0·05, **P< 0·01, ***P< 0·001 (t test).
Enzyme activity and mRNA expression levels
To determine whether hepatic nutrient fluxes were associated with changes in the mobilisation of amino acids and glucose, activity and/or expression levels of enzymes involved in amino acid and CHO metabolism were determined (Fig. 3). Liver transaminase activity (alanine aminotransferase) was similar in fish subjected to both diets, while GDH activity was significantly higher in the DS fish than in the CTRL fish. A 603 bp complementary DNA sequence encoding GDH was isolated and cloned from the liver of D. labrax. This fragment was approximately 90 % identical to Monopterus albus (GenBank accession no. JF694445) and approximately 85 % identical to Salmo salar (AJ532825) and Oncorhynchus mykiss (AF427344). The mRNA expression levels of GDH showed no statistical differences. The activity of hepatic enzymes responsible for the uptake and disposal of glucose, GK and G6Pase, respectively, did not show any modulation resulting from increased CHO ingestion. However, mRNA expression levels of GK were significantly increased in the DS fish. In contrast to the observations on GK v. G6Pase activity, increased CHO ingestion resulted in an expected reciprocation between PFK-1 v. FBPase activities. The DS fish exhibited an increase in PFK-1 activity, but a decrease in FBPase activity.

Fig. 3 Specific activity (![]() ) and mRNA abundance (
) and mRNA abundance (![]() ) for amino acid metabolism, glycolysis and gluconeogenesis enzymes in the liver of seabass (Dicentrarchus labrax) fed with a no-carbohydrate diet as the control (CTRL) and a 30 % digestible starch (DS) diet: (a) glucokinase; (b) glucose 6-phosphatase; (c) 6-phosphofructo 1-kinase; (d) fructose-1,6-bisphosphatase; (e) pyruvate kinase; (f) cytosolic alanine aminotransferase; (g) glutamate dehydrogenase. Activity is expressed as mU/mg protein and mRNA abundance as fold change with respect to the CTRL fish. mRNA abundances were normalised with mRNA abundance from ribosomal subunit 18 of D. labrax. Values are means, with their standard errors represented by vertical bars (n 6). Mean value was significantly different from that of the CTRL diet: *P< 0·05, ***P< 0·001 (t test).
) for amino acid metabolism, glycolysis and gluconeogenesis enzymes in the liver of seabass (Dicentrarchus labrax) fed with a no-carbohydrate diet as the control (CTRL) and a 30 % digestible starch (DS) diet: (a) glucokinase; (b) glucose 6-phosphatase; (c) 6-phosphofructo 1-kinase; (d) fructose-1,6-bisphosphatase; (e) pyruvate kinase; (f) cytosolic alanine aminotransferase; (g) glutamate dehydrogenase. Activity is expressed as mU/mg protein and mRNA abundance as fold change with respect to the CTRL fish. mRNA abundances were normalised with mRNA abundance from ribosomal subunit 18 of D. labrax. Values are means, with their standard errors represented by vertical bars (n 6). Mean value was significantly different from that of the CTRL diet: *P< 0·05, ***P< 0·001 (t test).
Discussion
Hepatic enzyme regulation from increased carbohydrate ingestion
In regularly fed fish, changes in transaminase activity are mainly caused by dietary protein, but not dietary CHO. This is thought to reflect the use of excess carbon backbones from amino acids to supply energy demands( Reference Enes, Panserat and Kaushik 23 – Reference Pérez-Jiménez, Hidalgo and Morales 26 ). However, in agreement with the present study, modifying the amount and type of CHO under constant dietary protein levels did not cause any significant alteration in alanine aminotransferase activity, as has been already observed in seabass( Reference Enes, Panserat and Kaushik 9 ). GDH activity, quantified in the direction of glutamate production, increased with ingestion of dietary CHO. It is known that this enzyme plays a role in directing glutamate carbon entry into the Krebs cycle. However, GDH activity is highly influenced by the cellular energy state, being activated when the mitochondrial energetic status is poor( Reference Stanley 27 ). We speculate that elevated hepatic GDH activity may reflect a suboptimal hepatocellular energy status, possibly reflecting a limited adaptation of these carnivorous fish towards energy generation from CHO-rich diets.
Feeds with high protein:CHO ratios, that mimic the seabass natural diet, result in residual mRNA and activity levels for hepatic GK. Stimulation of GK expression by dietary CHO has been reported in several fish species( Reference Caseras, Metón and Vives 17 , Reference Enes, Panserat and Kaushik 28 – Reference Skiba-Cassy, Panserat and Larquier 32 ) as that observed in the DS fish. However, GK activity seems to be differently influenced by dietary CHO depending on the species, CHO level and time of sampling after feeding. Postprandial GK activity is relatively dynamic, with maximal activities reaching after 6–8 h post-feeding( Reference Caseras, Metón and Fernández 16 , Reference Panserat, Medale and Blin 30 , Reference Kirchner, Kaushik and Panserat 33 – Reference Wade, Skiba-Cassy and Dias 35 ). In most studies where sampling was performed during peak response, GK activity has been found to be highly induced by dietary CHO in species such as rainbow trout (O. mykiss)( Reference Panserat, Medale and Blin 30 , Reference Kirchner, Kaushik and Panserat 33 , Reference Capilla, Medale and Navarro 36 ), gilthead seabream( Reference Caseras, Metón and Vives 17 , Reference Enes, Panserat and Kaushik 23 , Reference Enes, Panserat and Kaushik 28 , Reference Panserat, Medale and Blin 30 , Reference Enes, Panserat and Kaushik 37 ) or common dentex (Dentex dentex)( Reference Pérez-Jiménez, Hidalgo and Morales 26 ). However, other studies with gilthead seabream( Reference Couto, Enes and Peres 38 ) and seabass( Reference Moreira, Peres and Couto 7 , Reference Enes, Panserat and Kaushik 9 ) have reported otherwise. This raises doubts regarding the responsiveness of this enzyme's activity to a high-CHO diet, at least in these species. In studies where sampling was performed 20–24 h after the meal, where transition from the post-absorptive to the basal metabolic state takes place, enhanced GK activity has been found in rainbow trout( Reference Panserat, Medale and Blin 30 ) and gilthead seabream( Reference Metón, Caseras and Fernandez 29 , Reference González, Caballero and Viegas 39 ). Studies with Senegalese sole (Solea senegalensis)( Reference Dias, Rueda-Jasso and Panserat 40 ), blackspot seabream (Pagellus bogaraveo)( Reference Figueiredo-Silva, Corraze and Rema 41 ), common carp (Cyprinus carpio) and gilthead seabream( Reference Panserat, Medale and Blin 30 ) have reported no such differences and better align with our findings. While GK is responsive to increased dietary CHO, glycaemic control in this setting also requires a reciprocal suppression of G6Pase activity. However, we did not observe significant changes in either G6Pase activity or mRNA abundance by dietary CHO. Moreover, no differences in glycaemia were observed between conditions as previously reported for seabass( Reference Moreira, Peres and Couto 7 , Reference Enes, Panserat and Kaushik 9 , Reference Peres and Oliva-Teles 42 ). Studies with gilthead seabream( Reference Enes, Panserat and Kaushik 28 , Reference Enes, Panserat and Kaushik 37 , Reference Panserat, Medale and Breque 43 ) as well as seabass( Reference Enes, Panserat and Kaushik 6 , Reference Moreira, Peres and Couto 7 , Reference Enes, Panserat and Kaushik 9 ) have also reported no effect of dietary CHO on G6Pase activity, 6 h after the last meal. Other similar reports have even revealed an increase in G6Pase activity in seabass( Reference Enes, Panserat and Kaushik 28 ) and increased activity and mRNA abundance in rainbow trout( Reference Kirchner, Kaushik and Panserat 33 ) with increasing dietary CHO ingestion. Similar to our observations, no alterations in G6Pase mRNA were induced 24 h after the last meal by dietary CHO in rainbow trout( Reference Skiba-Cassy, Panserat and Larquier 32 , Reference Panserat, Medale and Breque 43 ), gilthead seabream and common carp( Reference Metón, Caseras and Fernandez 29 , Reference Panserat, Plagnes-Juan and Kaushik 44 ). The lack of G6Pase suppression by CHO has been suggested as contributing to the inability of carnivorous fish to control glycaemia when fed with CHO-enriched diets. It also explains the high rates of futile glucose–G6P cycling observed under feeding conditions since both GK and G6Pase are active at the same time( Reference Viegas, Mendes and Leston 13 , Reference Skiba-Cassy, Panserat and Larquier 32 , Reference Kamalam, Medale and Kaushik 45 ). Metabolic fluxes obtained from 2H NMR in the present study and other studies( Reference Martins, Rito and Jarak 46 ) associated with the lack of response observed in pyruvate kinase (present study), and PFK-1( Reference Kamalam, Medale and Kaushik 45 , Reference Dai, Panserat and Mennigen 47 ), located downstream of G6P in the glycolytic pathway, seem to support this assumption. Along with this futile cycling of glucose, the contribution of glycogen conversion to glucose via glycosidases (EC 3.2.1, includes glucosidases, amylases and glycogen-debranching enzymes) is yet to be evaluated. These alternative glucose-producing pathways do not involve in the exchange of glucose and water hydrogens generating unlabelled glucose in the presence of 2H2O( Reference Viegas, Rito and González 11 ). Therefore, the present methodology is unable to estimate their roles in glucose fluxes.
The interconversion between fructose 6-phosphate and fructose 1,6-bisphosphate by the PFK-1/FBPase substrate cycle is another important regulatory locus for glucose metabolism, and unlike GK v. G6Pase, reciprocal changes in their activities between the CTRL and DS feeding conditions were observed. These findings were consistent with the expected shift towards glycolysis over gluconeogenesis in response to the more abundant dietary CHO for the DS fish as has previously been observed elsewhere for activity( Reference Metón, Caseras and Fernandez 29 , Reference Kirchner, Kaushik and Panserat 33 , Reference Fernández, Miquel and Córdoba 48 ) and mRNA abundance( Reference Skiba-Cassy, Panserat and Larquier 32 , Reference Kirchner, Seixas and Kaushik 34 , Reference Panserat, Plagnes-Juan and Kaushik 44 ).
Analysis and comparison of 2H2O with other tracer studies
While enzyme activity and expression show robust responses to dietary CHO supplementation, supported by an extensive body of literature, how these translate to alterations in nutrient fluxes remains poorly understood. Recently, elegant analyses based on dietary 13C-enriched starch( Reference Ekmann, Dalsgaard and Holm 49 , Reference Felip, Ibarz and Fernández-Borràs 50 ), 13C-enriched protein( Reference Ekmann, Dalsgaard and Holm 51 ) and 15N-enriched protein( Reference Felip, Ibarz and Fernández-Borràs 50 , Reference Felip, Blasco and Ibarz 52 ) precursors coupled with mass spectrometric analysis of different tissues allowed carbon and nitrogen assimilation to be followed into protein, glycogen and lipids.
As observed in the present study, hepatic glycogen increases consistently with dietary CHO as reported previously( Reference Moreira, Peres and Couto 7 , Reference Enes, Panserat and Kaushik 9 , Reference Enes, Panserat and Kaushik 23 , Reference Peres and Oliva-Teles 42 , Reference Enes, Sanchez-Gurmaches and Navarro 53 ). In the study of Felip et al. ( Reference Felip, Blasco and Ibarz 52 ) with gilthead seabream fed with a single meal of 13C-enriched starch, hepatic glycogen enrichment from 13C-enriched starch was presumed to occur mainly via the direct pathway, hence the fact that 13C enrichment was maximal at 6 h suggests that direct pathway activity was most active at this time( Reference Felip, Blasco and Ibarz 52 ). The depletion of glycogen 13C enrichment between 6 and 24 h in the face of static hepatic glycogen levels may be explained by a switch from the direct to the indirect pathway (i.e. unlabelled) sources for glycogen turnover over this interval. For DS-fed seabass in the present study, we measured a direct pathway contribution of approximately 30 % to hepatic glycogen turnover at 24 h post-feeding. Based on these results, it is conceivable that the direct pathway contribution could have been higher at earlier times post-feeding but because of the sluggish hepatic glycogen turnover rate, it is, nevertheless, not expected to have a dominating role in postprandial hepatic glycogen levels. In contrast, for juvenile gilthead seabream that were fed over 10 d with 24 % dietary wheat starch enriched with 13C, over two-thirds of hepatic glycogen were estimated to originate from this source( Reference Ekmann, Dalsgaard and Holm 49 ), suggesting a stronger recruitment of dietary glucose for hepatic glycogen synthesis. Above and beyond systematic methodological differences between the 13C-enriched starch and 2H2O methods, these data may also reflect a more dynamic role of hepatic glycogen turnover and its synthesis from dietary glucose in seabream v. seabass.
The quantification of blood glucose and hepatic glycogen sources with 2H2O is based on the assumption that enrichment of hexose position 2 and PW are equivalent (i.e. H2/PW = 1·0). For glycogen position 2 enrichment, this assumption may not be correct. Since hepatic glycogen was only partially turned over during 2H2O administration, the observed glycogen H2/PW of 0·3–0·5 is in part explained by pre-existing unlabelled glycogen. Hepatic glycogen synthesised from [U-2H7]glucose during a glucose tolerance test retained a significant amount of 2H in position 2, indicating incomplete exchange with PW( Reference Martins, Rito and Jarak 46 ). In fasted fish refed with standard fish feed in the presence of 2H2O, the glycogen H2:PW ratio was significantly less than 1·0. Under these study conditions, pre-existing hepatic glycogen was depleted and, therefore, could not account for the H2/PW ratio < 1·0( Reference Viegas, Rito and Jarak 12 ). In contrast to hepatic glycogen, blood glucose was completely turned over during the 2H2O-labelling period, and the H2/PW ratio approached unity under the conditions of endogenous glucose production (CTRL). Therefore, the assumptions that underpin the estimates of blood glucose sources with the 2H2O method are well supported.
Conclusions
We demonstrated that incorporation of digestible starch into fish feed results in a significant contribution of absorbed CHO to endogenous glucose and glycogen fluxes under standard feeding conditions. Hepatic glycogen synthesis is significantly shifted from indirect to direct pathway fluxes; however, these changes seem quantitatively unimportant in terms of overall carbon and nitrogen fluxes. A significant shift in systemic glucose appearance from gluconeogenesis to dietary CHO absorption can potentially attenuate gluconeogenic amino acid utilisation and NH3 synthesis. However, uncertainties in glucose turnover rates preclude a more precise evaluation of the extent to which amino acid gluconeogenesis is spared by dietary CHO.
Acknowledgements
The authors acknowledge financial support from Fundação para a Ciência e Tecnologia (FCT) in the form of a fellowship to I. V. (no. SFRH/BPD/90032/2012), a research grant to J. G. J. (No. PTDC/EBB-BIO/098111/2008), and structural funding for the Center for Neurosciences and Cell Biology (no. PEst-C/SAU/LA0001/2011), also co-funded by the European Regional Development Fund (FEDER) through the programme COMPETE – Operational Competitiveness Programme. The authors also acknowledge financial support in the form of research grants from MCI (Spain) (no. BIO2009-07589) and MEC (Spain) (no. AGL2012-33305; co-funded by the European Regional Development Fund, FEDER). The Varian VNMRS 600 MHz spectrometer is part of the National NMR Network (PTNMR) and was purchased in the framework of the National Programme for Scientific Re-equipment (contract REDE/1517/RMN/2005), with funds from POCI 2010, European Regional Development Fund, and FCT.
The authors' contributions were as follows: I. V., M. A. P., I. V. B. and J. G. J. designed the research; I. V., J. R., I. J., S. L. and A. C.-S. performed the experiments; I. V., I. M., I. V. B. and J. G. J. analysed the data; I. V., I. M., I. V. B. and J. G. J. interpreted the results of the experiments; I. V. and J. G. J. prepared the figures; I. V. and J. G. J. drafted the manuscript; I. V., M. A. P., I. V. B. and J. G. J. edited and revised the manuscript; I. V., I. V. B. and J. G. J. approved the final version of the manuscript.
The authors declare that they have no conflicts of interest, financial or otherwise.










