Fatty acid (FA) concentration in maternal plasma increases during pregnancy as a consequence of physiological hyperlipidaemia( Reference Al, van Houwelingen and Kester 1 ). Phospholipids (PL) are major sources of PUFA in serum( Reference Abbott, Else and Atkins 2 ). The increase in DHA (22 : 6n-3) concentration in both maternal plasma PL( Reference Al, van Houwelingen and Kester 1 , Reference Al, van Houwelingen and Hornstra 3 ) and erythrocyte PL( Reference Stewart, Rodie and Ramsay 4 ) is higher than for other PUFA. However, despite the increase in absolute concentrations, there is a steady decline from the first trimester until delivery in the percentage of DHA in plasma PL and erythrocyte PL( Reference Al, van Houwelingen and Kester 1 , Reference Matorras, Ruiz and Perteagudo 5 ), as well as in total plasma( Reference Matorras, Ruiz and Perteagudo 5 , Reference Montgomery, Speake and Cameron 6 ), with respect to the total FA content. The high prenatal accumulation of DHA in brain during the third trimester of pregnancy( Reference Clandinin, Chappell and Heim 7 , Reference Clandinin, Chappell and Leong 8 ), the selective and preferential maternal–fetal transfer of long-chain PUFA (LC-PUFA) with respect to other FA across the placenta( Reference Campbell, Gordon and Dutta-Roy 9 – Reference Haggarty, Page and Abramovich 11 ) and the low desaturase activity in both placenta( Reference Chambaz, Ravel and Manier 12 ) and fetal liver( Reference Szitanyi, Koletzko and Mydlilova 13 ) could explain this decline of LC-PUFA percentage in the maternal compartment. In recent years, great attention has turned to consider whether low DHA intakes in pregnant women may achieve an optimal central nervous system and visual development of the fetus( Reference Innis and Friesen 14 ). Moreover, DHA supplementation might slightly reduce the risk of preterm birth, especially before 34 weeks of gestation( Reference Makrides, Duley and Olsen 15 , Reference Salvig and Lamont 16 ), which highlights the potential role of DHA supplementation during pregnancy.
Several health organisations recommend an average dietary intake of at least 200 mg of DHA/d for pregnant women; intakes of up to 1 g/d DHA or 2·7 g/d n-3 LC-PUFA have been used in randomised clinical trials without significant adverse effects( Reference Koletzko, Cetin and Brenna 17 , 18 ). Several fat sources are currently available in the market for DHA supplements such as egg yolk extracts, single-cell microalgae oils, krill oils and fish oils. These sources contain DHA under different chemical forms, mainly PL or TAG, resulting in different gut digestion and absorption( Reference Ikeda, Sasaki and Yasunami 19 , Reference Christensen, Hoy and Becker 20 ). Moreover, the intake of DHA as PL or TAG may influence their distribution in plasma lipoproteins, as reported in piglets fed artificial formulas enriched with the same amount of DHA but from different fat sources (PL v. TAG)( Reference Amate, Gil and Ramirez 21 ). Valenzuela et al.( Reference Valenzuela, Nieto and Sanhueza 22 ) compared the bioavailability of natural PL or TAG DHA sources in pregnant rats, but data from placentas and fetal tissues were not reported. Pigs are the animal models that are more suitable to study lipid metabolism with respect to humans( Reference Miller and Ullrey 23 ), but no studies are available on the effects of DHA supplementation in the form of PL or TAG during gestation on lipoprotein metabolism, even when they are crucial for placental FA uptake and transfer.
In the present study, we examined the effects of DHA supplementation, from egg yolk (PL) or algae oil (TAG) during the last period of gestation in sows, on DHA availability in maternal plasma lipoproteins, placental distribution and accretion in fetal tissues, especially regarding fetal brain incorporation.
Methods
Animals and diets
The protocol of this study was approved by the Animal Care Committee of the Murcia University (Murcia, Spain) and conforms to the ARRIVE guidelines for animal research. Animals received humane treatment in accordance with the European Union guidelines for the care and use of laboratory animals. In the present study, pigs were used as they are considered one of the best models to study nutrition issues in human( Reference Miller and Ullrey 23 ). Iberian sows of 12 months of age were supplied by Escamez S.L. Sows were randomly assigned to two groups of six animals per group. Experimental groups were blinded for the staff responsible for the care of animals. Animals consumed water and their assigned experimental diet ad libitum during the last third of gestation (40 d), from days 65 to 105 of gestation. In the present study, we tested two different fat sources of preformed DHA: egg yolk extract as DHA–PL source (OVOLIFE; Belovo) and algae oil as DHA–TAG source (DHASCO; Martek). OVOLIFE contained 88·1 % of PL (64–79 % phosphatidylcholine, 12–18 % phosphatidylethanolamine and <3 % lyso-phospholipids (Lyso-PL), analysed by the manufacturer using 31P-NMR PL analysis), 43·1 % SFA, 23·3 % MUFA, 33·5 % PUFA, 2·2 % arachidonic acid (AA) and 18·1 % DHA (99·2 % in PL form). DHASCO only contained TAG and was composed of 22·7 % SFA, 15·6 % MUFA, 61·6 % PUFA, 0·5 % AA and 59·0 % DHA (97·9 % in TAG form). Concentrated fungal oil (ARASCO; Martek) was used as a source of AA in the DHA–TAG diet in order to equilibrate AA content with respect to the DHA–PL diet.
The standard diet for pregnant sows did not contain DHA (Alimer S.Coop). The standard diet was enriched with a fat blend (10 g/kg) to obtain diets with 0·8 % DHA of total FA either as PL or TAG. In the DHA–PL diet, the total PL content was 3·68 % of total fat, whereas it was virtually 0 % in the DHA–TAG diet. Diets were finally composed (w/w) of 13·4 % protein, 3·4 % fat, 6·1 % ashes and 88·4 % DM. The total DHA intake for pregnant pigs was about 330 mg DHA/d. Ingredients and FA composition of experimental diets are summarised in Tables 1 and 2, respectively.
Table 1 Composition of experimental diets

DHA–TAG, diet supplemented with DHASCO in which DHA is in TAG form; DHA–PL, diet supplemented with OVOLIFE in which DHA is in PL form; ARASCO, fungal oil rich in AA (20 : 4n-6) provided by Martek; DHASCO; algae oil rich in DHA (22 : 6n-3) in the form of TAG provided by Martek; OVOLIFE, egg yolk extract rich in DHA in the form of PL provided by Belovo.
* Minerals (per kg of diet): NaCl, 4 g; CaCO3, 12·9 g; Ca3(PO4)2, 5·2 g; MnSO4, 35 mg; FeCO3, 50 mg; CuSO4.5H2O, 15 mg; ZnO, 90 mg; KI, 0·5 mg; 2CoCO3.3Co(OH)2.H2O, 0·45 mg and Na2SeO3, 0·06 mg. Vitamins (per kg of diet): vitamin A, 2·7 mg; vitamin D3, 0·04 mg and vitamin E, 30 mg.
Table 2 Fatty acid profile of experimental diets
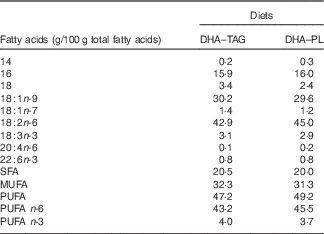
DHA–TAG, diet supplemented with algae oil in which DHA is in TAG form; DHA–PL, diet supplemented with egg yolk extract in which DHA is in PL form.
Collection of samples
On day 105 of gestation, animals were stunned through high CO2 atmosphere exposition. The maternal and fetal blood was extracted by jugular vein puncture in heparinised tubes and centrifuged at 1400 g for 10 min at 4°C to obtain plasma. Maternal liver, adipose tissue, brain, placentas, fetal liver and fetal brains (two fetuses and placentas were pooled per pig) were frozen in liquid N2 and stored at –80°C until analysis.
Isolation of plasma lipoproteins
Lipoproteins were isolated from 1 ml of fresh maternal plasma by ultracentrifugation using a discontinuous NaCl/KBr density gradient( Reference Chung, Wilkinson and Geer 24 ) in an Optima L-100 XP ultracentrifuge equipped with 100Ti rotor (Beckman Coulter). The rest of the plasma was frozen in liquid N2 and stored at –80°C until analysis.
Fatty acid analyses
Total lipids were extracted from experimental diets (500 mg), 250 μl of plasma, the whole band of lipoproteins isolated from 1 ml of plasma and tissues (30–50 mg liver, 15–20 mg adipose tissue, 30–50 mg brain and 100 mg placenta) according to the method of Folch et al.( Reference Folch, Lees and Sloane Stanley 25 ). Before the extraction, an internal standard was added to the samples: 0·05 mg of pentadecanoic acid for total FA analyses and 0·01 mg FA each of pentadecanoic acid, tripentadecanoin, phosphatidylcholine dipentadecanoyl and cholesteryl pentadecanoate for lipid fraction analyses. Samples with internal standard were extracted into chloroform:methanol (2:1, v/v). The lipid extract was evaporated to dryness under nitrogen flux. The residue was taken up in 400 µl of chloroform–methanol (1:1, v/v) for analysis of lipid fractions. It was applied on silica gel plates (Merck), and the PL, TAG, cholesteryl esters and NEFA were isolated by development of the plates in n-heptane–diisopropylether–glacial acetic acid (60:40:3, by vol.)( Reference Carnielli, Wattimena and Luijendijk 26 ). Bands from different lipid fractions were detected with 0·2 % 2',7'-dichlorofluorescein in ethanol (w/v) under UV light and scrapped. The bands of the TLC and the dried residue for total FA analyses were both methylated according to Stoffel et al.( Reference Stoffel, Chu and Ahrens 27 ) by adding 1 ml of 3 N methanolic HCl (Supelco; Sigma-Aldrich) and heating at 90°C for 1 h. The derivatives were extracted into hexane and stored at −20°C until GC analysis.
FA methyl esters were analysed by GC using a SP-2560 capillary column (100 m×0·25 mm×20 µm) (Supelco) in a Hewlett-Packard 6890 gas chromatograph (Agilent Technologies) equipped with a flame ionisation detector( Reference Larque, Garcia-Ruiz and Perez-Llamas 28 ). The temperature of the detector and the injector was 240°C. The oven temperature was programmed at 175°C 30 min and increased at 5°C/min to 230°C and held at this temperature for 17 min. He was used as the carrier gas at a pressure of 45 psi. Peaks were identified by comparison of their retention times with appropriate FA methyl esters standards (Sigma-Aldrich) and FA concentrations determined in relation to peak area of internal standard.
Protein extracts for Western blotting
A measure of 30 mg of placental tissue and fetal brain were homogenised in 0·3 ml of ice-cold lysis buffer (20 mm TRIS-HCl pH 7·5, 150 mm NaCl, 5 mm Na2 EDTA, 1 mm ethylene glycol-bis(2-aminoethylether)-tetraacetic acid (EGTA), 1 % Triton, 2·5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin) from Cell Signaling Technology. Phenylmethanesulfonyl fluoride solution 1 mm was added to lysis buffer before homogenisation( Reference Ruiz-Alcaraz, Liu and Cuthbertson 29 ). Samples were homogenised using a Tissue Lyser LT device (Qiagen Iberia SL). Protein lysates were obtained from the supernatant after 15 min of centrifugation at 10 000 g at 4°C. Protein was quantified by Bradford assay( Reference Bradford 30 ) and samples were stored at −80°C until Western blot analysis.
Western blot analysis
The primary antibodies used were rabbit polyclonal antibody against the orphan transporter called ‘Major Facilitator Superfamily Domain Containing 2 A’ (MFSD2a; Abcam) and mouse monoclonal anti-β-actin (Sigma-Aldrich). Anti-mouse and anti-rabbit secondary antibodies conjugated with horseradish peroxidase were obtained from Santa Cruz Biotechnology. Protein extracts (15 μg protein) diluted in sample buffer were resolved on 10 % polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Merck Millipore). Membranes were then blocked in phosphate saline buffer with 0·05 % Tween-20 (PBS-T) containing 2 % bovine serum albumin for 1 h at room temperature. Thereafter, membranes were incubated with primary antibodies overnight at 4°C. Blots were then washed with PBS-T and probed for 1 h at room temperature with the corresponding secondary antibodies conjugated with horseradish peroxidase. Finally, membranes were stripped with Tris/HCl buffer pH 2·3 containing β-mercaptoethanol 0·1 m and re-probed with anti-β-actin to perform loading controls. Proteins were detected using a chemioluminescence kit according to the manufacturer’s instruction (Pierce ECL 2 Western Blotting Substrate; Thermo Fisher Scientific)( Reference Prieto-Sanchez, Ruiz-Palacios and Blanco-Carnero 31 ). Density of all bands was determined by densitometry using Image Quant LAS 500 software (GE Healthcare). Relative protein expression data were normalised for β-actin expression.
Statistical analysis
Sample size was estimated based on DHA percentages in plasma PL of piglets published by Alessandri et al.( Reference Alessandri, Goustard and Guesnet 32 ). Type I error was set at α=0·05 and type II error at β=0·2 (power 80 %), obtaining a minimum sample size of three animals per group. The software used for this estimation was nQuery 7.0 (Statsols HQ).
The SPSS 15.0 software (SPSS, Inc.) was used for statistical analyses of data obtained. To evaluate the effects of DHA supplementation as PL v. TAG on FA profiles of maternal and fetal tissues, a t test was performed. Differences between plasma lipoprotein composition were assessed by one-way ANOVA followed by post hoc Bonferroni test. Statistically significant differences were established at P<0·05. Data are expressed as mean values with their standard errors.
Results
DHA supplementation as PL or TAG during the last third of gestation did not affect DHA percentage in total lipids of maternal plasma (Fig. 1(a)). In both experimental groups, DHA was preferentially incorporated in PL-rich lipoproteins (HDL and LDL), with the DHA percentage being significantly higher in LDL of animals fed DHA–PL with respect to the DHA–TAG diet (Fig. 1(b)). In fact, when we analysed plasma lipid fractions, maternal plasma showed a slight trend towards higher DHA percentage in PL fraction (P=0·13) of animals fed the DHA–PL diet compared with those fed the DHA–TAG diet (Table 3). No differences were observed for the rest of the plasma lipid fractions. Total DHA concentration in plasma was also similar between groups (DHA–PL group 0·04 (SEM 0·01) g/l v. DHA–TAG group: 0·03 (SEM 0·01) g/l; P=0·64). Therefore, the administration of 0·8 % DHA in the form of PL or TAG produced different lipoprotein incorporation in the mother, although the enrichment in maternal serum PL was moderated.
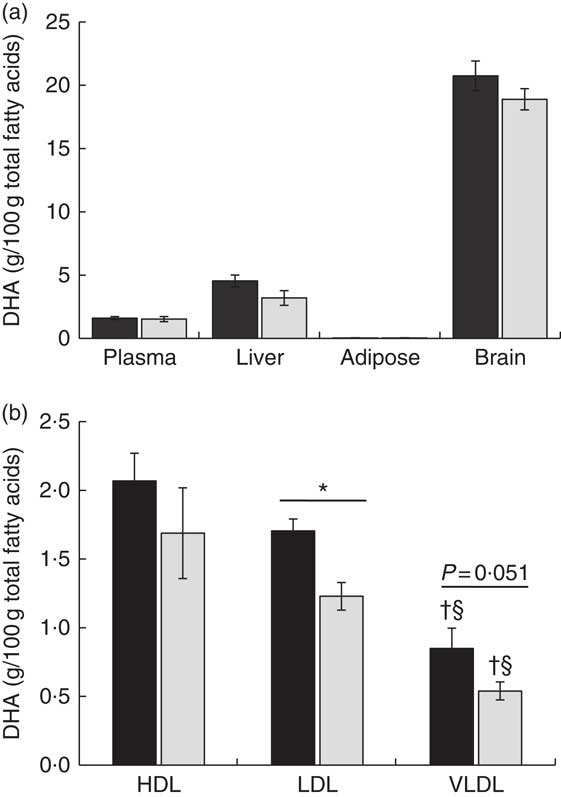
Fig. 1 DHA percentage at delivery in maternal plasma, liver, adipose tissue, brain (a) and lipoproteins (b) of pigs fed during the last third of gestation with DHA (0·8 % of total fatty acids) as phospholipid (DHA–PL, ![]() ) or TAG (DHA–TAG,
) or TAG (DHA–TAG, ![]() ). Values are means (n 6/group), with their standard errors represented by vertical bars. * Significant differences between PL and TAG groups (P<0·05). † Significant differences between HDL and VLDL lipoproteins within the same PL or TAG group (P<0·05). § Significant differences between LDL and VLDL lipoproteins within the same PL or TAG group (P<0·05).
). Values are means (n 6/group), with their standard errors represented by vertical bars. * Significant differences between PL and TAG groups (P<0·05). † Significant differences between HDL and VLDL lipoproteins within the same PL or TAG group (P<0·05). § Significant differences between LDL and VLDL lipoproteins within the same PL or TAG group (P<0·05).
Table 3 Percentage of DHA and arachidonic acid (AA) in lipid fractions of maternal plasma, maternal liver and placenta of sows after DHA supplementation (0·8 % of total fatty acids) as phospholipid (DHA–PL) or TAG (DHA–TAG) during the last third of gestation (Mean values with their standard errors; n 6/group)

ND, non-detectable.
* Mean value was significantly different from that of the DHA–PL group (P<0·05).
Concerning the other maternal tissues, we did not find any statistically significant difference in DHA composition between the experimental groups either in liver, adipose tissue or brain (Fig. 1(a)). However, placental tissue showed significantly higher DHA percentage (Fig. 2(a)) and also higher DHA concentration in total lipids of the DHA–PL group (DHA–PL group: 0·07 (sem 0·01) mg/g v. DHA–TAG group: 0·05 (sem 0·01) mg/g, P=0·03). This DHA increase in placenta was exclusively due to the incorporation of DHA in PL fraction (Table 3). Placenta is a tissue with a high amount of PL in its structure, and this could be related with the fact that only placenta, and not other maternal tissues, was comparatively more enriched in DHA after feeding the animals with the DHA–PL diet compared with the DHA–TAG-fed group. Despite enhanced DHA accumulation in placentas of DHA–PL-fed animals, MFSD2a protein expression, which carries up Lyso-PL, was not higher in the placenta of this group (DHA–PL: 1·54 (sem 0·53) v. DHA–TAG: 1·75 (sem 0·27) arbitrary units, P=0·72). Concerning AA percentage, no differences were found in lipid fractions of maternal plasma. However, the DHA–TAG group presented lower AA percentage in total lipids of placenta (DHA–PL: 10·75 (sem 0·67) % v. DHA–TAG: 8·28 (sem 0·78) %, P=0·025) and also in the PL fraction (Table 3).
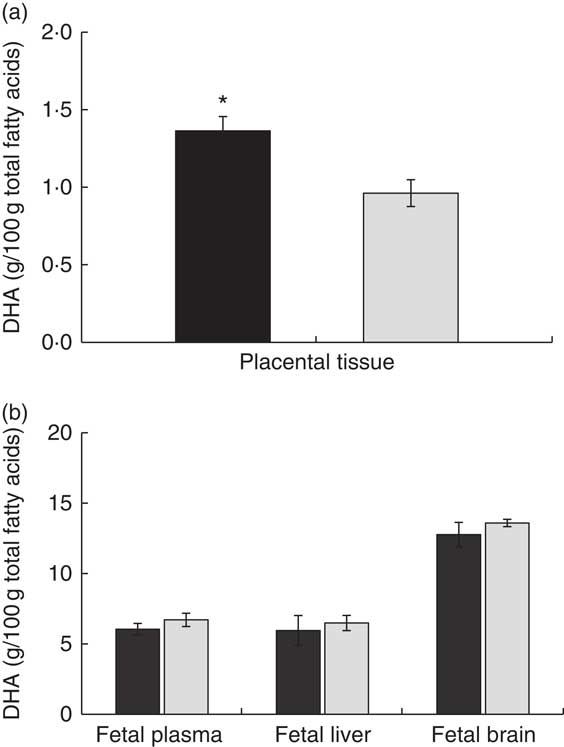
Fig. 2 DHA percentage at delivery in placenta (a) and fetal plasma, fetal liver and fetal brain (b) after maternal DHA supplementation (0·8 % of total fatty acids) as phospholipid (DHA–PL, ![]() ) or TAG (DHA–TAG,
) or TAG (DHA–TAG, ![]() ) during the last third of gestation. Values are means (n 6/group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the DHA–TAG group (P<0·05).
) during the last third of gestation. Values are means (n 6/group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the DHA–TAG group (P<0·05).
Despite the higher incorporation of DHA in placenta, maternal DHA–PL supplementation did not enhance DHA accretion in fetal structures, either in fetal plasma, liver or brain with respect to fetus from mothers fed the DHA–TAG diet (Fig. 2(b)). The fetus again re-distributes DHA in plasma lipids, and no differences were found in the PL fraction between groups (Table 4). In fact, only DHA percentage in plasma cholesteryl esters was higher in the offspring of the DHA–PL group (Table 4), which could be because of the link between PL and cholesteryl ester formation. More importantly, no differences were found in DHA percentage either in total lipids or PL fraction of fetal brain (Fig. 2(b), Table 4) or DHA content in the whole brain (DHA–PL group: 2·23 (sem 0·30) mg/g v. DHA–TAG group: 2·41 (sem 0·19) mg/g, P=0·59). Fetus from the DHA–TAG group presented higher values of AA in total lipids of plasma than in the DHA–PL-fed group (DHA–PL: 12·55 (sem 0·75) % v. DHA–TAG: 15·32 (sem 0·62) %, P=0·011), and this was also observed in fetal plasma PL fraction (Table 4), in contrast to placental tissue of these animals. The DHA–PL group tended to have higher MFSD2a expression in fetal brain (DHA–PL: 1·38 (sem 0·15) v. DHA–TAG: 0·96 (sem 0·25) arbitrary units, P=0·14). MFSD2a band pattern in the fetal brain was completely different from that observed in the placenta, in which only a predominant band of approximately 100 kDa was detected (Fig. 3), which corresponded to the expected size of the protein.
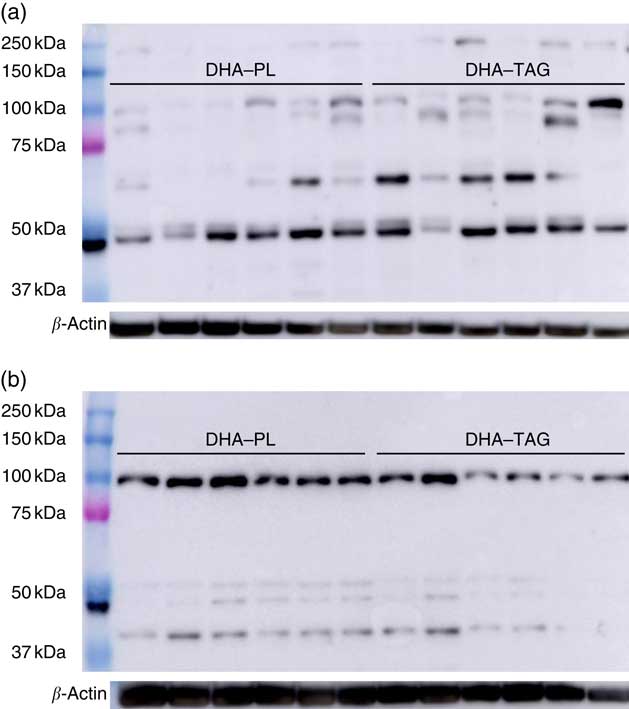
Fig. 3 Western blotting membranes of Major Facilitator Superfamily Domain Containing 2a (MFSD2a) in placenta (a) and fetal brain (b) of pigs after maternal DHA supplementation (0·8 % of total fatty acids) as phospholipid (DHA–PL) or TAG (DHA–TAG) during the last third of gestation. Three different bands are present in placental tissue (approximately 100, 65 and 50 kDa), which might correspond with different glycosylation patterns, whereas only a predominant band (approximately 100 kDa) appeared in the fetal brain.
Table 4 Percentage of DHA and arachidonic acid (AA) in lipid fractions of fetal plasma, fetal liver and fetal brain after maternal DHA supplementation (0·8 % of total fatty acids) as phospholipid (DHA–PL) or TAG (DHA–TAG) during the last third of gestation (Mean values with their standard errors; n 6/group)

* Mean value was significantly different from that of the DHA–PL group (P<0·05).
Therefore, maternal lipid supplementation with DHA in the form of PL during the last third of gestation positively affected DHA accumulation in placenta, but it did not enhance fetal DHA accretion compared with the administration of DHA in TAG form. Fetal brain DHA accretion seems to be strongly regulated.
Discussion
It is uncertain whether DHA consumption during pregnancy in PL form could be a source with higher placental bioavailability and fetal brain accretion than DHA in the form of TAG. In the present study, sows fed a diet supplemented with 0·8 % DHA in the form of PL or TAG during the last third of gestation (40 d) showed similar DHA accumulation in total lipids of maternal plasma. However, there was a slight trend towards higher DHA incorporation in maternal plasma PL, which indicates that maternal metabolism modulates in a certain degree the effect of dietary DHA, modifying its incorporation in maternal serum lipid fractions. Some reports in piglets, full-term infants and children also indicated that the plasma lipid fraction in which DHA is incorporated after gut absorption is not always related with the chemical form of DHA consumed( Reference Goustard-Langelier, Guesnet and Durand 33 – Reference Vaisman, Kaysar and Zaruk-Adasha 35 ). Jiménez et al.( Reference Jiménez, Boza and Suárez 36 ) showed higher DHA incorporation in plasma PL of newborn piglets fed a formula supplemented with LC-PUFA PL from pig brain extract compared with the control group fed sow milk or control formula without DHA, whereas no differences were observed in brain composition of piglets. In case of pig brain concentrates, other lipid components apart from PL, for example cerebrosides, gansliosides, sphingolipids and lyso-phosphatidylcholine (Lyso-PC), can be present and might alter the absorptive process and later metabolism of FA.
In non-pregnant humans, Ramprasath et al.( Reference Ramprasath, Eyal and Zchut 37 ) reported an increase of Omega-3 index in healthy individuals receiving 4-week n-3 FA supplements from krill oil v. fish oil. However, the magnitude of DHA change in plasma was minimal and they introduced an inappropriate variable into their study owing to fish oil capsules that contained over 32 % linoleic acid (18 : 2 n-6) of total FA, which is an antagonist of n-3 incorporation in mammalian tissues( Reference Nichols, Kitessa and Abeywardena 38 ). Other authors did not report differences in humans after PL or TAG DHA supplementation( Reference Sala-Vila, Castellote and Campoy 34 , Reference Vaisman, Kaysar and Zaruk-Adasha 35 , Reference Maki, Reeves and Farmer 39 ).
There is only one study conducted in pregnant animals but developed in rats, in which animals were fed during gestation with DHA as PL or TAG, resulting in similar DHA levels in total lipids of maternal plasma( Reference Valenzuela, Nieto and Sanhueza 22 ); these authors reported only higher levels of DHA in erythrocyte PL when using the PL source. With respect to maternal tissues, liver PL and adipose tissue had higher DHA in the PL-DHA group before mating than in the DHA–TAG group, but these differences were not significant at the time of delivery( Reference Valenzuela, Nieto and Sanhueza 22 ). These results are in agreement with our data, and there is no evidence of enhancement of DHA content in maternal tissues with DHA supplementation as PL v. TAG at the same dose during pregnancy. Maybe it is more efficient to increase the DHA dosage administered to pregnant mothers than change the lipid source used to improve DHA availability.
Despite similar DHA incorporation in total lipids of maternal plasma, we found higher incorporation of DHA in PL-rich lipoproteins (HDL and LDL), probably linked to the observed trend towards higher DHA percentage in plasma PL. This higher DHA incorporation in LDL of the DHA–PL group could have promoted the higher placental uptake detected in our study. Amate et al.( Reference Amate, Gil and Ramirez 21 ) also reported different DHA incorporation in serum LDL PL and HDL PL of piglets when using PL v. TAG sources, whereas no differences were reported in DHA percentage in plasma PL fraction. We did not analyse FA composition of lipoproteins per lipid fractions.
Placenta displays HDL-receptors and LDL-receptors in its membranes, as well as lipases to release FA from plasma lipoproteins. Endothelial lipase mainly releases the FA from the sn-1 position of the PL, producing a Lyso-PL( Reference Campbell, Gordon and Dutta-Roy 9 ). Nevertheless, with time, this enzyme releases also the FA esterified in the sn-2 position of the Lyso-PL( Reference Chen and Subbaiah 40 ). These Lyso-PL could be an additional source of FA for the placenta and other tissues( Reference Lagarde, Bernoud and Brossard 41 , Reference Thies, Delachambre and Bentejac 42 ). In the present study, we describe for the first time a higher incorporation of DHA in placentas from animals fed DHA from the PL source. As placenta is a tissue with more than 85 % of PL in its structure( Reference Klingler, Demmelmair and Larque 43 ), this could facilitate a higher DHA uptake from PL-rich lipoproteins. This finding also supports, at least in part, the hypothesis that endothelial lipase could release DHA from maternal circulating PL, especially in the DHA–PL group, which might have been preferentially taken up by placental tissue.
DHA Lyso-PC has been proposed as a preferred physiological carrier of DHA to the brain( Reference Lagarde, Bernoud and Brossard 41 , Reference Thies, Delachambre and Bentejac 42 ), probably via MFSD2a transporter( Reference Nguyen, Ma and Shui 44 ). Recently, we have demonstrated that lower MFSD2a expression was related to disturbed DHA placental transfer in the offspring of gestational diabetes mothers( Reference Prieto-Sanchez, Ruiz-Palacios and Blanco-Carnero 31 ). Inactivation of MFSD2a protein has been also linked to severe outcomes such as microcephaly syndrome( Reference Alakbarzade, Hameed and Quek 45 , Reference Guemez-Gamboa, Nguyen and Yang 46 ). However, in the present study, placental MFSD2a did not change among groups, whereas fetal brain expression tended to reach higher values in the DHA–PL group.
Human placenta is a discoidal endotheliochoreal placenta, which means that the distance between maternal blood and the fetal capillaries is minimal (sometimes just a single layer of throphoblast cells), whereas pigs have a diffuse epitheliochoreal placenta with intact layers of epithelial cells between both blood circulations. These histological differences in placenta of humans and pigs could affect or modulate the FA uptake and transport across the tissue in a different way. Moreover, a complex pattern of bands appeared for MFSD2a by Western blot analysis in sow placentas and fetal brain. Placenta expressed MFSD2a with three different molecular weights – approximately 100, 65 and 50 kDa – whereas fetal brain only presented a clear band at approximately 100 kDa. Previous studies in mice and several human cell lines have shown different levels of protein glycosylation( Reference Alakbarzade, Hameed and Quek 45 , Reference Berger, Charron and Silver 47 , Reference Reiling, Clish and Carette 48 ). Berger et al.( Reference Berger, Charron and Silver 47 ) also reported different MFSD2a glycosylations in liver and brown adipose tissue in mice. Different glycosylation patterns or others post-translational changes of MFSD2a between different tissues of the same animal, or the same tissue between different species, could imply different functions. A regulatory effect of fasting/refeeding on liver MFSD2a expression and lipid metabolism has been even described in mice by the same authors( Reference Berger, Charron and Silver 47 ). More studies are needed to fully understand the role of MFSD2a in body growth, lipid metabolism and brain integrity.
Surprisingly, the major accumulation of DHA in placenta of these animals did not lead to a higher DHA status in the offspring. Valenzuela et al.( Reference Valenzuela, Nieto and Sanhueza 49 ) showed increased DHA levels in PL fraction of cerebellum and hippocampus of pups at 2 months of age after maternal supplementation during pregnancy with DHA as Lyso-PC in rats. In our study, the level of Lyso-PC was minimal in the diets as Ovolife product contained <3 % of total fat as Lyso-PL, with phosphatidylcholine being the major PL source (64–79 % of total PL). The DHA–TAG diet was not supplemented with free choline to compensate the choline provided by phosphatidylcholine in the DHA–PL diet, which should be mentioned. We did not find statistically significant differences in DHA content in total FA analysis or lipid fractions of fetal brain between both experimental groups. Placenta releases FA as NEFA( Reference Gil-Sanchez, Koletzko and Larque 50 ) and the re-esterification by the fetal liver limits the selective accretion of DHA in PL of fetal plasma and hence tissue accretion.
One of the most remarkable strengths of this study is the use of sows as an experimental model, which is difficult to handle, as it is one of the species with a lipid metabolism closest to the humans and allows us to collect very important tissues such as maternal or fetal brain. Among the limitations of this study, it should be mentioned that pigs and humans have different placenta structures. Pigs have a diffuse epitheliochoreal placenta whereas humans have a discoidal endotheliochoreal placenta in which maternal blood is directly in contact with trophoblast cells. It is not known whether these structural differences of placentas might imply differences in lipid placental transfer. Only total PL were considered, and it would be interesting to measure Lyso-PC even when they were administered in low doses in this study.
In conclusion, this study provides evidence that DHA as PL from egg yolk extract and DHA as TAG from microalgae oil have similar availability for fetal tissues. The maternal supplementation during the last third of gestation with DHA as PL v. TAG resulted in small differences in maternal plasma, with higher incorporation in maternal LDL that could explain higher placental DHA uptake. Nevertheless, this higher placental uptake was not linked to higher DHA levels in fetal brain, which seems to be well-regulated.
Acknowledgements
The authors thank Juan García and Ismael Martínez for their assistance with animal care and sample collection.
This work was supported by Séneca Foundation, Murcia, Spain (11921/PI/09); the Excellence Network for Maternal and Child Health and Development, Instituto de Salud Carlos III, Madrid, Spain (RED SAMID II, RD 12/0026/0015); and the Research Group of Excellence CHRONOHEALTH, Murcia, Spain (19899/GERM/15). Funders had no role in the design, analysis or writing of this article.
A. G. and E. L. designed the research and wrote the manuscript. A. G. and M. R.-P. conducted the research and sample determination. A. G. performed statistical analysis. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










