Life expectancy in most developed countries has been rising over the past century. Data from the human mortality database suggest that children born during the 2000s will reach 100 years of age if the present life expectancy rate remains(1). In the UK, there are about 12 million people over 65 years old and centenarians have increased by 85 % in the past 15 years(Reference Storey, Coombs and Leib2). The population of developed countries is ageing as a result of the discovery of new drugs and treatments, improvements in public health, low fertility rates and changes in the lifestyle of the population(Reference Christensen, Doblhammer and Rau3). However, ageing is associated with the prevalence of pathological conditions, such as neurodegenerative disease(Reference Lafortune and Balestat4,Reference Meinow, Parker and Kareholt5) , type 2 diabetes (T2D)(Reference Wild, Roglic and Green6), CVD(Reference Crimmins and Saito7–Reference Ostchega, Dillon and Hughes9), many types of cancer(Reference Karim-Kos, de Vries and Soerjomataram10) and others. Statistics show that nearly 54 % of elderly people in the UK live with at least two chronic conditions, referred to as multi-morbidity(Reference Kingston, Robinson and Booth11), hence the urgency of identifying interventions that can prevent/treat such disorders and eventually promote the health span extension.
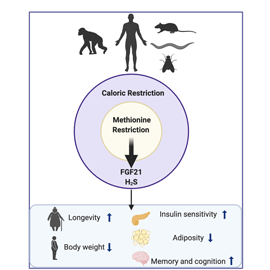
Several lifestyle modifications have been the focus of study as an approach to delay the onset of chronic diseases and the ageing process. Dietary interventions such as energetic restriction (ER) and, more specifically, methionine restriction (MR) show promising outcomes in increasing longevity. This improvement is associated with the prevention of ageing-associated disorders and cognitive decline. Thus, understanding the mechanisms involved in life span regulation, as well as control of the health span, with the prevention of development and progression of ageing-related diseases, is of utmost importance if we are to live longer lives.
Sulphur-containing amino acids and methionine metabolism
Amino acids that contain the element sulphur in its chemical structure are called sulphur-containing amino acids (SAA). Methionine, cysteine, homocysteine and taurine are the four amino acids included in this class, with the first two considered the main SAA because they are incorporated into proteins. They are known to play a significant role in protein synthesis, structure and function(Reference Brosnan and Brosnan12). Both methionine and cysteine are abundant in dietary protein sources, although only methionine is classified as an essential amino acid. However, cysteine can be endogenously produced by methionine and serine in the liver and other tissues(Reference Kalhan and Hanson13). The nitrogen balance in adults and the growth rate during childhood are parameters considered for the SAA nutritional requirements. In a study with rodents fed cereals (low protein diet), the animals restored nitrogen balance and lost body weight with the diet only after methionine and threonine supplementation, suggesting that both are the most rate-limiting amino acids in the maintenance of body nutrition(Reference Yoshida, Lásztity and Hidvégi14).
Methionine is an essential amino acid necessary for protein synthesis in prokaryotic and eukaryotic cells; it also plays a major role as an endogenous antioxidant and is involved in several physiological and biochemical processes(Reference Martínez, Li and Liu15). The methionine metabolism is responsible for the production of essential substances in many physiological pathways. The methionine cycle is the first step of methionine metabolism and includes the biosynthesis of S-adenosylmethionine (SAM) from methionine and ATP by the methionine adenosyltransferase (Fig. 1). SAM is a key intermediate in methionine metabolism and has many chemical roles. In mammals, the main function of SAM is to serve as a methyl donor in methyltransferase reactions(Reference Fontecave, Atta and Mulliez16). In the methionine cycle, SAM donates its methyl group to an acceptor metabolite, generating S-adenosylhomocysteine catalysed by methyltransferases. This product is converted to homocysteine by reversible hydrolysis. This sequence of reactions is known as transmethylation and is present in every cell.

Fig. 1. (Colour online) Methionine cycle and transsulphuration pathway (TSP). Methionine is converted to S-adenosylmethionine (SAM) by the methionine adenosyltransferase (MAT). Methyltransferases (MT) produce S-adenosylhomocysteine (SAH), which is converted to homocysteine by S-adenosyl-L-homocysteine hydrolase (SAHH). Homocysteine can synthesise methionine by methionine synthase (MS) and vitamin B12 or by betaine homocysteine methyltransferase (BHMT) and betaine. Homocysteine might also enter the TSP and be converted to cystathionine by cystathionine β-synthase (CBS), which can be processed to cysteine by the cystathionine γ-lyase (CGL), both reactions using vitamin B6 as a cofactor. Cysteine can be used to build proteins and in the synthesis of glutathione (GSH) and taurine.
Reviewed literature evidence from the past 20 years showed that high plasma levels of homocysteine is a risk factor for the development of neurodegenerative diseases in elderly people, and can be considered a biomarker for the Alzheimer's disease (AD) and dementia(Reference Smith, Refsum and Bottiglieri17). Also, hyperhomocysteine is associated with vascular disease and neurotoxicity(Reference Sachdev18). Once formed, homocysteine can be remethylated into methionine by methionine synthase or by betaine homocysteine methyltransferase, completing the methionine cycle. Methionine by methionine synthase uses 5-methyltetrahydrofolate (vitamin B12) as a cofactor for the donation of a methyl group, and betaine homocysteine methyltransferase requires betaine as the methyl donor(Reference Parkhitko, Jouandin and Mohr19). Endogenous methionine formation by methionine by methionine synthase occurs in most of the cells, otherwise, its synthesis using betaine occurs mainly in the liver and kidney(Reference Dong, Sinha and Richie20) (Fig. 1).
Homocysteine can also be processed to cysteine via the transsulphuration pathway (TSP). The cystathionine β-synthase is the first enzyme in the TSP and is responsible for cystathionine synthesis from the condensation of homocysteine and serine. The second key enzyme in this process is the cystathionine γ-lyase. This enzyme is responsible for the hydrolysis of cystathionine to cysteine. Furthermore, cysteine can be involved in the synthesis of proteins, glutathione and taurine (Fig. 1). The TSP and the full conversion of methionine to cysteine is an irreversible process and only occurs in a few tissues: liver, kidney, intestine and pancreas(Reference Brosnan and Brosnan12). Cysteine is considered the rate-limiting substrate for the synthesis of the antioxidant glutathione, which can act as a storage of cysteine and be broken down to favour cysteine formation when its levels are low in the cell(Reference Courtney-Martin, Moore and Ball21).
The TSP is also responsible for the production of hydrogen sulphide (H2S) from the catabolism of cysteine and homocysteine(Reference Parkhitko, Jouandin and Mohr19) (Fig. 2). H2S is a gas that was classified as toxic for many years. However, more recently, H2S has been considered as a potential therapeutic agent due to its role as a vasodilator, antioxidant molecule, anti-inflammatory and insulin release modulator(Reference Wallace and Wang22). Additionally, H2S provided by the TSP has been shown to be an essential molecule for the dietary restriction (DR) benefits, such as stress resistance and longevity(Reference Hine, Harputlugil and Zhang23).

Fig. 2. (Colour online) Hydrogen sulphide (H2S) production. H2S is produced during the methionine metabolism from the catabolism of homocysteine and cysteine by the enzymatic activity of cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL) and 3-mercaptopyruvate sulphurtransferase (MPST) alongside cysteine aminotransferase (CAT). The production of H2S might produce several cellular responses that cause stress resistance, vasodilation, antioxidant reactions, anti-inflammatory responses and insulin release.
The bioavailability of methionine in the organism regulates the rate of the methionine cycle, to maintain adequate levels of this amino acid in the tissues. The low consumption of proteins or SAA alters the activity of enzymes involved in the TSP, allowing for methionine to be preserved via protein degradation. Furthermore, the concentration of SAM and homocysteine regulates the methionine flux in its metabolic pathways(Reference Courtney-Martin, Moore and Ball21).
Dietary restriction
Dietary interventions have been used for decades as an approach to delay ageing and the development of diseases related to cell senescence. One of the most studied forms of delaying the onset of age-related diseases is by DR, which includes different nutritional interventions that can bring health benefits in a variety of species(Reference Fontana, Partridge and Longo24,Reference Piper, Partridge and Raubenheimer25) . Studies have shown that the extension of life span is associated with DR in many organisms, including yeast, Caenorhabditis elegans, Drosophila melanogaster and rodents. In mammals, different dietary regimens have been associated with health benefits, including intermittent fasting, a decrease in protein intake or reduction in daily food consumption. These interventions share some similar beneficial features, such as reduced adiposity, improved insulin sensitivity and glucose homeostasis. However, it is important to note that differences exist between these different approaches and each one has its singularity. In rodents, longevity is mainly attributed to a delay in ageing-related processes, associated with a lower incidence of the development of ageing-related diseases and neurodegeneration(Reference Fontana and Partridge26). A reduction in the activity of nutrient-sensing signalling is possibly one of the main pathways by which DR can increase life span(Reference Wu, Song and Liu27–Reference Edwards, Canfield and Copes29).
One of the most investigated DR interventions is the ER(Reference Speakman, Selman and Mclaren30–Reference Speakman and Mitchell32), which is defined as a reduction of 20–40 % of daily food intake with meal frequency being maintained, showing improvements in life and health span(Reference Masoro33). For many years, studies with rodents and primates have been providing evidence that the reduction of daily energy intake up to 40 % without malnutrition improves insulin resistance and prevents the development of several metabolic disorders associated with ageing, such as T2D, hypertension, obesity, chronic inflammation and cancer(Reference Weindruch, Walford and Fligiel34–Reference Mattison, Roth and Beasley38). ER diet is also associated with an overall decrease in mortality-related processes in primates(Reference Colman, Beasley and Kemnitz39). Furthermore, moderate ER with a decrease of only 10 % of energy intake daily was associated with protection against diabetes and decrease in intrahepatic lipid content in a rodent model of obesity(Reference Baumeier, Kaiser and Heeren40).
Clinical trials have been implemented during the years to assess the effects of ER on human health. Some of these studies revealed that ER in adult men and women improves glucose and insulin tolerance, as well as reducing the risk of T2D and CVD(Reference Weiss and Holloszy41). However, the energy intake and the levels of body fat mass that are associated with the health benefits and any possibility of an increase in the life span in human subjects is still to be determined. Furthermore, it is important to point out that excessive ER may be accompanied by malnutrition and brings harmful effects to the individuals' health(Reference Fontana and Klein42). Studies performed in obese children who were on a low-carbohydrate or a low-fat diet for 2 months suggested improvements in their body weight and lipid profiles. This effect was associated with low TAG serum levels, revealing that DR can improve metabolic parameters in obesity(Reference Ibarra-Reynoso, Pisarchyk and Pérez-Luque43). Additionally, a randomised, controlled clinical study was performed that assessed the effects of ER in non-obese adults, and revealed a significant weight loss accompanied with a decrease in systemic oxidative stress and ageing biomarkers, even 2 years after the dietary intervention(Reference Redman, Smith and Burton44).
For decades, the effects of ER in the ageing brain and the development of neurodegenerative diseases have been a topic of intense study. Longer-term clinical trials with ER (4 and 5 years) suggest that a decrease in energy intake over several years can decrease neuronal damage and delay the onset of symptoms related to AD and Parkinson's disease in elderly individuals(Reference Logroscino, Marder and Cote45,Reference Luchsinger, Ming-Xing and Shea46) . In agreement, studies examining neurodegeneration-associated behaviours and dietary interventions demonstrated that ER improved locomotor activity in aged rodents compared with mice fed an ad libitum diet(Reference Duffy, Leakey and Pipkin47). In the same approach, ER rats did not exhibit a decline in locomotor activity associated with the ageing process, as reported in animals with free access to food(Reference Yu, Masoro and McMahan48). In addition, mice were submitted to a long-term reduction in energy intake (during their entire life) protecting the animals from a decline in learning due to ageing, which raises the possibility that it may also protect rodents against neurodegeneration associated with AD mutations. Indeed, a decrease in dopaminergic neuron death was observed in animal models of Parkinson's disease following a 3-month ER regimen(Reference Ingram, Weindruch and Spangler49–Reference Zhu, Guo and Mattson51).
Methionine restriction
The primary way of modulating the rate of the TSP is by altering the dietary consumption of methionine. Dietary MR is considered a dietary intervention that mimics DR, without ER. Dietary MR can alter enzymatic activity in the methionine cycle and consequently, the synthesis of its metabolites. This nutritional intervention is widely associated with the benefits observed in DR but without malnutrition; reducing adiposity but at the same time increasing both food intake and energy expenditure(Reference Orgeron, Stone and Wanders52).
One of the earliest pieces of evidence that MR could increase longevity in rodents was demonstrated by Orentreich et al.(Reference Orentreich, Matias and DeFelice53). In this study, a reduction of the SAA methionine from 0⋅86 to 0⋅17 % was able to extend the life span in rodents about 30 %, despite the higher food intake promoted by the diet(Reference Orentreich, Matias and DeFelice53). In another study, control pair-fed animals, consuming the same amount of food as rats on MR diet, did not exhibit an extension of life span, promoting the idea that methionine itself is the key player behind life span extension and not necessarily the alteration in total energy consumed. Moreover, blood levels of glutathione, a well-known antioxidant molecule, were maintained during ageing in animals on MR diet, and different rodent strains submitted to this dietary intervention revealed slowing in the ageing process, suggesting that MR may modify the rate of ageing(Reference Zimmerman, Malloy and Krajcik54) without alterations in reactive oxygen species. Furthermore, studies with C. elegans and rodents have shown that the deletion of antioxidant enzymes, e.g. superoxide dismutase and glutathione peroxidase 1, did not alter animal life span and was not crucial for the ageing process(Reference Zhang, Shao and Zhai55,Reference Van Raamsdonk and Hekimi56) , confirming a separate role for MR in longevity.
The effects of dietary MR in mice were reported by Miller et al. (Reference Miller, Buehner and Chang57), who presented evidence that MR diet is capable of increasing longevity alongside lower hepatic oxidative stress. These mice exhibited low serum levels of insulin, insulin-like growth factor 1, glucose and thyroid hormones after a long-life MR diet intake (from age 6 weeks until natural death)(Reference Miller, Buehner and Chang57). The modulation of rodents' metabolism by the decrease in methionine intake was supported by the observation that rats fed MR diet for 80 weeks had higher insulin sensitivity and lower visceral fat content than animals fed a control chow diet(Reference Malloy, Krajcik and Bailey58). In vitro studies revealed that the decrease in adiposity observed in MR-fed rodents was due to a disruption of lipogenesis and lipolysis cycle, with a high rate of both lipid catabolism and lipid synthesis(Reference Perrone, Mattocks and Hristopoulos59).
A clinical trial including twenty-six adults (six male and twenty female) with metabolic syndrome reported that individuals provided with the MR diet for 16 weeks, or a control diet, decreased body weight and fasting glycaemia, irrespective of the diet. Interestingly however, a specific effect only observed in the MR group of volunteers was a decrease in the intrahepatic lipid content and increased fatty acid oxidation. However, this study presented elevated levels of non-compliance in human participants due to poor palatability of the diet. In order to achieve better responses in human subjects during clinical trials, it is necessary to develop more palatable tasting food in which methionine is selectively decreased(Reference Plaisance, Greenway and Boudreau60).
To understand the physiological mechanisms triggered by dietary MR, a hyperinsulinemic–euglycemic clamp was performed in mice after MR treatment. The mice exhibited a decrease in hepatic gluconeogenesis, followed by higher insulin sensitivity in the liver and high serum levels of the fibroblast growth factor 1 (FGF21), providing evidence of a direct effect of methionine in liver metabolism and FGF21 availability(Reference Stone, Wanders and Orgeron61). Increased levels of FGF21 are associated with positive metabolic outcomes, as it has been shown to reduce insulin resistance and hepatic lipid levels in obese and diabetic mice(Reference Inagaki, Lin and Goetz62,Reference Jimenez, Jambrina and Casana63) . FGF21 is a growth factor released mainly in response to fasting by the liver, being shown to regulate important metabolic pathways(Reference Badman, Pissios and Kennedy64). In human subjects, FGF21 is highly expressed after 7 d of fasting and regulates the energy balance during this period by adapting metabolic signalling to the reduction of nutrients(Reference Fazeli, Patwari and Steinhauser65).
Furthermore, MR was able to decrease lipogenic genes in the liver of aged mice and increase insulin sensitivity in white adipose tissue (WAT) and skeletal muscle. Alongside these findings, aged mice had higher serum and hepatic levels of FGF21, associated with lower circulating leptin levels after 8 weeks of MR. Furthermore, increased FGF21 levels were seen in a short-term 48 h MR regimen, together with improved whole-body glucose homeostasis. These improvements occurred prior to alterations in animals' body weight/adiposity, adding evidence that MR itself drives the improvements in whole body metabolism. The authors suggested that the MR effects observed were most likely driven by FGF21(Reference Lees, Krói and Grant66). Similar increase in FGF21 levels was observed after only 12 h of MR diet switch in the serum and liver of mice(Reference Stone, Wanders and Orgeron61). High FGF21 levels were maintained after 1, 2 and 4 weeks of MR intake(Reference Stone, Wanders and Orgeron61). Recently, a clinical trial with overweight and obese women on a low methionine and cysteine diet for 1 week revealed a significant increase in FGF21 plasma levels. However, the role of each specific amino acid restriction in the modulation of FGF21 content could not be separated, which is a limitation of this study(Reference Olsen, Øvrebø and Yasein67).
Previous work in aged male rats had shown that MR feeding improved oral glucose tolerance maintenance(Reference Malloy, Krajcik and Bailey58). Our own work compared young (2 months) v. aged mice (12 months) and presented the idea that MR could improve glucose homeostasis after longer (8 weeks) as well as short-term (2 d) restriction, supporting the hypothesis that MR can reverse the age-induced deterioration in glucose and lipid metabolism and handling(Reference Lees, Krói and Grant66). These pieces of evidence can be associated with findings that MR increases energy expenditure in young and aged mice together with elevated heat production, which is mainly due to increased brown adipose tissue activation and higher uncoupling protein 1 (UCP1) expression in this tissue(Reference Hasek, Stewart and Henagan68). Knowing that UCP1 expression is also high in WAT during MR, Ucp1−/− mice were subjected to MR. The findings revealed that the uncoupling respiration in cells is essential for the effects of MR in increasing energy expenditure, but not for improving insulin sensitivity in this tissue. The remodelling of metabolic function in MR animals is integrated with a lower metabolic efficiency as observed with the behaviour of hyperphagia, suggesting the involvement of a nutrient-sensing mechanism that could compensate for the reduction in methionine by alterations in the body's energy homeostasis(Reference Wanders, Burk and Cortez69). Moreover, the increase in energy expenditure, energy intake, brown adipose tissue and WAT thermogenesis is abolished in Fgf21−/− mice fed MR diet, which also showed lower insulin sensitivity when compared with wild-type mice on MR. These data demonstrated that FGF21 is an essential mediator of the MR effects observed in rodents(Reference Wanders, Forney and Stone70). Additionally, a more recent study, where rats were introduced to MR diet postweaning or at mature age, resulted in different hyperphagia outcomes. In young animals, the hyperphagic effect of MR resulted in an increase in energy intake that overcomes the higher energy expenditure; an effect not observed in ageing rats, indicating that MR could have different outcomes depending on age(Reference Wanders, Forney and Stone71).
Methionine restriction and obesity
Obesity and diabetes are the major metabolic disorders of public health relevance that have an urgent need for effective interventions. MR promotes loss of body weight and adiposity, increases glucose tolerance, insulin sensitivity and overall fatty acid oxidation, which makes it a promising lifestyle intervention to tackle these disorders(Reference Miller, Buehner and Chang57,Reference Plaisance, Greenway and Boudreau60,Reference Stone, Wanders and Orgeron61,Reference Lees, Krói and Grant66) . To investigate if MR could ameliorate obesity, ob/ob mice were placed on the diet for 12 weeks; this improved their hepatic lipid profile, with no changes in insulin sensitivity, body weight and/or adiposity(Reference Stone, Wanders and Calderon72). However, this animal model has an impaired β-adrenergic input, which may correlate with the lack of adipose tissue response to MR. In addition, ob/ob mice on MR failed to increase adiponectin serum levels, suggesting a possible role for this hormone in insulin sensitising effects mediated by MR(Reference Stone, Wanders and Calderon72). Interestingly, the metabolic effects of MR had been investigated previously in the same ob/ob mouse model, resulting in an improvement of hepatic steatosis that developed after 14 weeks of treatment. This effect was accompanied by a reduction in hepatic TAG levels, a high rate of fatty acid oxidation and down-regulation of inflammatory markers. Insulin levels were also decreased in this study together with increased adiponectin levels(Reference Malloy, Perrone and Mattocks73). The mechanism by which MR regulates liver metabolism could be related to the modulation of micro RNA expression. MR in young and diet-induced obese mice promotes repression and up-regulation of several micro RNA that control synthesis and transport of cholesterol, fatty acids and insulin, suggesting that the hepatic benefits of MR in rodents occur through multiple mechanisms to prevent the accumulation of lipids(Reference Park, Cooke and Plummer74).
MR diet also appears to improve cardiovascular function in obesity. In diet-induced obese mice, submitted to MR, the dietary intervention led to improved systolic function in middle age (28 weeks old), and was accompanied by a decrease in cardiac inflammation and oxidative stress(Reference Han, Wu and Feng75). This overall improvement in cardiac function was associated with increased levels of H2S in the heart promoted by the diet(Reference Han, Wu and Feng75). MR seems to improve cardiovascular function despite the elevated heart: body weight ratio and hyperhomocysteinemia, which are features associated with a high risk of CVD(Reference Han, Wu and Feng75). Ables et al.(Reference Ables, Ouattara and Hampton76) reported that mice with high plasma levels of homocysteine did not have their cardiac function altered following an MR intake, due to the up-regulation of cardioprotective hormones, FGF21 and adiponectin by the diet(Reference Ables, Ouattara and Hampton76). Indeed, there is evidence to suggest that high methionine intake could be associated with aortic plaque formation. APOE−/− mice fed methionine supplementation exhibited high homocysteine levels and increased total aortic lesion area, indicating that methionine levels, and not homocysteine itself, are related to CVD(Reference Troen, Lutgens and Smith77). A recently published clinical trial in North America (11 567 people) assessed the association between the cardiometabolic disease risk and the content of SAA intake in their diet. The study reported that a high intake of SAA, methionine and cysteine was closely associated with a CVD risk score, high serum cholesterol, glucose, uric acid, insulin and glycated Hb levels(Reference Dong, Gao and Chinchilli78). These findings suggest that low SAA intake, including MR diet, could be a potential intervention to reduce the risk of CVD.
Methionine restriction and diabetes
The development of insulin resistance and T2D has been associated with increased serum levels of methionine and cysteine in many clinical trials, usually before the onset of clinically diagnosed T2D(Reference Felig, Errol and Cahill79–Reference Elshorbagy, Refsum and Smith81). A large cross-sectional study with more than 16 000 individuals showed that the plasma concentration of cysteine was correlated with BMI and these levels were specifically related to body mass and not lean mass(Reference El-Khairy, Ueland and Nygârd80,Reference Elshorbagy, Refsum and Smith81) . Moreover, metabolite profile studies indicated that alterations in methionine concentration in the plasma may be indicative of insulin resistance and the risk of T2D. Non-diabetic obese adults had increased circulating levels of methionine if compared with non-obese patients(Reference Felig, Errol and Cahill79). Also, male patients with T2D show high levels of homocysteine in the blood with lower methionine transmethylation and homocysteine clearance, suggesting an impaired methionine metabolism in this condition(Reference Tessari, Kiwanuka and Coracina82). Taken together, these studies propose that changes in metabolism and glucose homeostasis alter SAA metabolism, ultimately resulting in alterations in methionine and cysteine circulating levels.
MR diet has been shown to ameliorate glucose tolerance and insulin sensitivity in several experimental models, preventing the development of T2D. Insulin resistance-prone C57Bl/6J mice fed a high-fat MR diet were found to be more glucose tolerant, with increased insulin sensitivity and decreased intrahepatic lipids, in comparison to high-fat control diet animals. This was associated with high levels of FGF21 and adiponectin, and low circulating levels of leptin and insulin-like growth factor 1(Reference Ables, Perrone and Orentreich83). Dietary MR was shown to increases overall insulin sensitivity and tissue-specific insulin sensitivity (liver, skeletal muscle, heart and adipose tissue), by an enhanced insulin-dependent protein kinase B phosphorylation(Reference Stone, Wanders and Orgeron61). More recently, New Zealand obese mice, a model for polygenic obesity and T2D, were fed a high-fat diet on MR for 9 weeks. MR diet prevented the onset of hyperglycaemia in New Zealand obese mice and increased FGF21 levels, as well as adiponectin and thermogenic genes in WAT. The same study compared both vegan and vegetarian diet with an omnivore diet in adults, with evidence that a low protein diet increased FGF21 levels in human subjects. These hormones were also increased after omnivore individuals switched their diet to a vegetarian diet for 4 d, suggesting a short-term metabolic beneficial effect of reducing protein intake(Reference Castaño-Martinez, Schumacher and Schumacher84).
The improvement in glucose homeostasis and insulin sensitivity due to MR may be related to improved insulin signalling in the tissues and insulin secretion by the pancreas. In vitro studies demonstrated that the limitation of methionine concentration in HepG2 cell media promotes higher insulin-dependent protein kinase B phosphorylation, with a similar pattern occurring in skeletal muscle and WAT of mice fed an MR diet(Reference Stone, Wanders and Orgeron61). Similar observations were made in the kidneys of mice on MR for 8 weeks. Aged mice on MR had enhanced insulin-stimulated phosphorylation of insulin-dependent protein kinase B and ribosomal protein S6. MR diet also induced up-regulation of UCP1, Srt1, FGF21, klotho, and B-klotho gene expression, suggesting resistance or reversal to the ageing process in this tissue(Reference Grant, Lees and Forney85). Corroborating these findings, the supplementation of methionine in a low-protein diet eliminated the beneficial effects observed in diabetic kidneys by the reduction of protein intake in diabetic rats. The specific effects of low methionine provided by the low-protein diet were regarding anti-oxidative stress, anti-inflammation and anti-fibrosis features in the diabetic kidney, possibly via the mechanistic target of rapamycin complex 1 in this tissue(Reference Kitada, Ogura and Monno86). Investigating further the effects of MR on the insulin signalling pathway, a mouse model of hepatic protein tyrosine phosphatase 1B knockout was fed with MR for 8 weeks. The results suggested no additional synergetic effect of protein tyrosine phosphatase 1B knockout and MR in insulin sensitivity and lipid metabolism, suggesting that the hepatic MR effects are either not mediated by protein tyrosine phosphatase 1B pathway or that there is a ceiling level to which either/both interventions can improve glucose homeostasis(Reference Lees, Krói and Grant66).
There is currently no evidence that MR can directly modulate insulin secretion by the pancreas. However, H2S levels may be an insulin-release modulator in pancreatic β-cells. It was demonstrated that H2S inhibits insulin secretion stimulated by glucose and decreases the insulin-stimulated glucose uptake by adipocytes(Reference Feng, Chen and Zhao87). Nonetheless, the administration of a cystathionine γ-lyase inhibitor can enhance glucose uptake in adipocytes, which suggests that H2S might be a novel insulin resistance regulator. Also, in diabetic rats, the cystathionine γ-lyase pathway is enhanced, confirming the H2S role in insulin sensitivity in adipose tissue(Reference Feng, Chen and Zhao87). However, the H2S effect may differ depending on tissue type, as in the liver, H2S has been reported to stimulate gluconeogenesis and glycogenolysis and inhibit glucose catabolism and glycogen storage(Reference Zhang, Yang and Untereiner88). In addition, high-fat diet and the development of diabetes stimulate a reduction of cystathionine γ-lyase and H2S production in the rat livers(Reference Bravo, Palleschi and Aspichueta89,Reference Peh, Anwar and Ng90) . Thus, the modulation of the TSP and H2S production by MR might indirectly intervene with insulin secretion and glucose uptake by the tissues.
Recent evidence suggests that the effects of MR in metabolic health and insulin sensitivity may also differ between sexes. A short-term MR dietary regimen (1 week) was introduced in male and female diet-induced obese mice, showing an improvement in glucose tolerance in both sexes, as expected. However, MR was able to increase energy expenditure and induce the FGF21–UCP1 axis only in males(Reference Yu, Yang and Miller91). These findings were corroborated by evidence that only male mice had their lean mass preserved after MR, while the female mice had a preference to maintain their fat mass, suggesting a sexually dimorphic effect of MR in young mice(Reference Forney, Stone and Gibson92). However, the underlying mechanisms in MR responsiveness related to glucose homeostasis and insulin sensitivity in males v. females still need to be investigated.
An alternative, pharmacological approach has also been employed to simulate dietary MR. In vivo studies with an oral recombinant methioninase, which catabolised methionine to α-ketobutyrate and ammonia, have been shown to prevent diet-induced obesity, increase glucose tolerance and decrease fat mass in mice fed a high-fat diet(Reference Tashiro, Han and Tan93). Hepatic lipids were also reduced in male mice after recombinant methioninase treatment, suggesting a role for this intervention in preventing fatty liver and obesity in rodents(Reference Tashiro, Han and Tan93). However, no clear evidence has been offered regarding the duration of effects caused by this intervention, and what side effects there may be, bringing attention to the need for more studies in this area. It is nevertheless confirmation that decreasing the levels of methionine, either through dietary or pharmacological interventions, may be a promising and achievable way of preventing the onset of diabetes and obesity, and improving overall metabolic health, thus health span.
Methionine restriction and cognitive function
The influence of different dietary intervention and prevention, to improve memory or delay the onset of neurodegenerative diseases, has been a topic of great interest. During ageing, several functional and structural alterations occur in the brain that impair neuroplasticity and memory(Reference Alexander, Ryan and Bowers94–Reference Mattson and Arumugam96). Initial studies demonstrated that ER can enhance spatial memory in rodents, with age-related motor impairment and learning being prevented following ER for 4 months(Reference Ingram, Weindruch and Spangler49,Reference Stewart, Mitchell and Kalant97) . ER has also been associated with improving synaptic activity and stimulation of neuroprotective signalling in the brain(Reference Mattson98–Reference Park and Prolla100). In a state of ER, the brain can produce more brain-derived neurotrophic factor, offering neuroprotection(Reference Murphy, Dias and Thuret99). Experimental evidence revealed previously that ER-induced neurogenesis in the dentate gyrus of the hippocampus is associated with higher brain-derived neurotrophic factor expression(Reference Lee, Duan and Mattson101). Cognitive impairments exacerbated by obesity were attenuated by ER due to higher levels of N-methyl-D-aspartate receptor subunits, essential for long-term potentiation and synaptic plasticity in the hippocampus after 10 weeks(Reference Yilmaz, Vural and Yilmaz102). However, some studies have demonstrated that ER could act in increasing neuronal stem cells via N-methyl-D-aspartate-independent mechanisms, for example, via brain-derived neurotrophic factor. Altogether, improvement in the levels of N-methyl-D-aspartate receptor and synaptophysin levels in the CA3 region of the hippocampus were observed due to ER and associated with better performance in a spatial memory task(Reference Adams, Shi and Linville103).
Long-term ER can also improve working spatial memory in mice(Reference Kuhla, Lange and Holzmann104). However, some studies associated short-term ER, at later stages of life, with the modulation of biochemical markers in the brain related to cognitive decline. The neural cell adhesion molecule and the astrocytic marker glial fibrillary acidic protein were significantly elevated in 24-month-old mice after ER(Reference Kaur, Sharma and Kaur105). Late-onset short-term ER regimen in rodents was also shown to prevent age-related neurodegeneration in the hippocampus and cortex of rats by decreasing oxidative stress in these regions(Reference Sharma, Singh and Kaur106). In addition, only 7 weeks of ER in old mice (17 months old) reversed changes observed in glutathione redox state in the cortex, hippocampus, striatum and cerebellum, preventing loss of function in these areas(Reference Rebrin, Forster and Sohal107). These studies suggest that the introduction of ER in older animals may benefit brain health, preventing the tissue from ageing-related damages.
Preservation of neuronal function within the ageing process is correlated with an increase in life span. The effect of ER in the maintenance of brain integrity seems to be associated with an early shift from glucose to ketone bodies' metabolism in ageing mice(Reference Guo, Bakshi and Lin108). These findings were recently confirmed by another study revealing that ER induced high levels of neurotransmitters and neuronal integrity markers in a postprandial response(Reference Yanckello, Young and Hoffman109). Moreover, a low glycolysis activation pathway was observed following ER; these effects were not noticed in ad libitum mice. This indicates that an essential role for neuroprotection in ageing may be related to early changes in brain metabolism and glucose utilisation(Reference Yanckello, Young and Hoffman109).
The beneficial effects of ER in the brain have been being investigated in primates and human studies. Analysis with primates exposed to a chronic, moderate ER revealed an overall reduction in the development of ageing-associated diseases and significant preservation of the white matter in different brain regions. However, the authors observed a faster loss of grey matter without affecting cognitive performances(Reference Pifferi, Terrien and Marchal110). The impact of 40 % ER was also evaluated in small primates (Microcebus murinus) for 19 d, demonstrating reduced learning performance. No differences in locomotor capability were detected in the Rotarod tests(Reference Villain, Picq and Aujard111). In human subjects, a clinical trial with healthy elderly individuals reported a significant improvement in memory performance after 3 months of ER regimen, compared to increased unsaturated fatty acids intake group and ad libitum controls(Reference Witte, Fobker and Gellner112). The results were correlated with improved insulin sensitivity and reduced inflammatory markers, supporting corresponding animal studies, and the concept of conserved brain integrity(Reference Witte, Fobker and Gellner112).
The effects of MR on cognitive performance have also been investigated in recent years. Evidence suggests that obesity is not only a risk factor for the development of T2D and CVD, but also has been correlated with the prevalence of AD and cognitive decline(Reference Mcneilly, Williamson and Sutherland113). High-fat diet-induced obese mice exhibit impaired learning and memory, accompanied by a reduction in H2S production in the hippocampus, cortex and plasma(Reference Xu, Yang and Sun114). Higher hippocampal inflammation was also observed. However, obese animals fed high-fat and low methionine diet for the same period improved in all behavioural tasks, alongside decreased brain inflammation and normalisation of H2S levels(Reference Xu, Yang and Sun114). Dietary alterations might alter the methionine cycle, producing chronically elevated levels of homocysteine. The increased plasma concentration of homocysteine has been linked with cognitive decline, dementia and AD(Reference Nurk, Refsum and Tell115), being shown to induce alterations in the hippocampal plasticity and a slow-onset reduction of synaptic transmission, what confirms its possible role in the pathology of neurodegenerative diseases(Reference Christie, Riedel and Algaidi116).
Recent work using C57BL/6J mice fed a high-fat diet for 4 weeks, followed by MR diet for 8 weeks, reported that MR protected the animals against overall inflammation and the brain dysfunction by potentially altering the circadian homeostasis of gut microbiota and the brain(Reference Wang, Ren and Hui117). Additionally, behavioural tests performed in older mice (12 and 15 months old) fed MR for 3 months revealed improved performance in spatial memory tasks, associated with less neuronal damage and synapse damage in the hippocampus. FGF21 levels were significantly elevated after MR; furthermore, FGF21 knockdown severely blunted the MR's effects on the ageing brain(Reference Ren, Wang and Liu118). These studies suggest that MR may offer promising therapeutic intervention or even prevention of cognitive decline during ageing and in associated disorders, such as AD.
In support of this idea, a dietary protein restriction that includes reduced intake of methionine, isoleucine, leucine, phenylalanine, threonine, tryptophan, valine and arginine improved behavioural performance in an AD mouse model. The authors found a decrease in phosphorylated tau protein in the hippocampus of 9-month-old 3XTgAD mice, suggesting that protein restriction may partially protect the brain against age-related pathologies(Reference Parrella, Maxim and Maialetti119). Additionally, Tg2576 mice placed on a methionine supplementation in the diet presented higher levels of homocysteine, which was associated with increased amyloid-β peptide (Aβ) deposition and behavioural impairments(Reference Zhuo, Portugal and Kruger120). Moreover, chronic treatment with a methionine-enriched diet promoted increased levels of phosphorylated tau and Aβ plaques, as well as higher inflammation and oxidative stress in the hippocampus of healthy mice. Memory impairments were also observed following methionine supplementation, giving rise to a neurotoxic effect of high circulating levels of methionine, however homocysteine levels were not evaluated in this study(Reference Tapia-rojas, Lindsay and Montecinos-oliva121).
Interestingly, nutritional deficits in B vitamins might lead to hyperhomocysteinemia and the development of AD pathology. High levels of homocysteine were previously demonstrated to have a bi-directional effect on long-term potentiation in hippocampal slices of rats exposed acutely to this amino acid, showing an impairment in neuronal communication what might contribute to cognitive decline(Reference Christie, Riedel and Platt122). Moreover, rats exposed to long-term homocysteine daily injections (14 weeks) showed alterations in synaptic activity and long-term potentiation in the hippocampus, together with changes in spatial learning(Reference Algaidi, Christie and Jenkinson123). Furthermore, vitamin B12 deficiency is associated with poor cognition and the onset of AD(Reference Mohajeri, Troesch and Weber124) and was shown to stimulate presenilin (PS) 11 and β-site amyloid precursor protein (APP) cleaving enzyme expression, causing more Aβ plaques deposition(Reference Marques and Outeiro125). Vitamin B12 is associated with the methionine cycle, as mentioned previously, as well as folate and vitamin B6. Folate concentration is also a factor that could be associated with neurodegenerative diseases and neurodevelopmental disorders. Mild-cognitive impairment observed in T2D patients was correlated with low folate and SAM circulating levels(Reference Zheng, Zhang and Yang126). Also, low levels of folate and vitamin B12 have been widely correlated with women who gave birth to children with spina bifida(Reference Shields, Kirke and Mills127). These findings support the idea that the modulation of the methionine cycle and its components may serve as an important tool to prevent neuronal damage and subsequent neurodegenerative diseases.
Dietary restriction and Alzheimer's disease
Dietary interventions such as ER not only seem to improve cognition and prevent memory loss during the ageing process, but have also been associated with delayed progression of neurodegeneration(Reference Bruce-Keller, Umberger and Mcfall128–Reference Youssef, Ramchandani and Manswell132). Due to the rising global number of elderly people, AD is one of the most prevalent diseases of our time, thus far without effective treatment. AD is considered a multi-factorial syndrome, and its causes are still widely debated. Two types of AD are commonly recognised: sporadic and inherited (familial) AD(Reference Bekris, Yu and Bird133). The sporadic type is the most common form, accounting for >90 % of the cases, and usually leads to the late onset of the disease. Environmental and lifestyle factors contribute to the development of sporadic AD, including diabetes, hypertension, CVD, hypercholesterolemia, hyperhomocysteinemia, smoking and others. A small number of cases (<1 %) are causally directly inherited AD(Reference Campion, Dumanchin and Hannequin134–Reference Reitz, Brayne and Mayeux136). Usually, this form occurs earlier in life (from about 45 years), and results from mutations in genes for APP, PS1 or PS2, often also categorised by a more aggressive disease progression. The early symptoms of AD include memory impairments, mood and sleep disturbances, and anxiety. With the progression of the disease, deterioration of cognitive functions can be clinically diagnosed(Reference Masters, Bateman and Blennow137,Reference Chakrabarti, Khemka and Banerjee138) , yet ultimately requires post-mortem confirmation.
End-stage AD is characterised by two main pathological marks that include the extracellular deposition of Aβ plaques, and the formation of neurofibrillary tangles containing hyperphosphorylated tau protein(Reference Hardy and Selkoe139). Importantly, recent evidence indicates that soluble, non-fibrillar forms of Aβ and tau play a more significant causal role compared to the final, aggregated species(Reference Koss, Jones and Cranston140,Reference Koss, Dubini and Buchanan141) . An early study investigating the effects of dietary modifications to ameliorate neurodegeneration associated with AD-linked mutation was published in 1999. The investigators found that DR for 3 months in PS1 knock-in mice (which exhibit spatial memory deficits at 6 months of age)(Reference Elder, Gustave and Place142) resulted in less damage to hippocampal CA1 and CA3 neurons when compared with ad libitum fed animals(Reference Zhu, Guo and Mattson51). In the following years, studies using AD transgenic mice revealed that a short-term ER (4 weeks) can reduce Aβ accumulation(Reference Patel, Gordon and Connor130); a similar pattern was detected in APP/PS1 mutated mice in long-term ER (18 weeks) resulting in a decrease in neuritic plaque deposition(Reference Mouton, Chachich and Quigley143). Female Tg2576 mice carrying a double APP mutation(Reference Hsiao, Chapman and Nilsen144) also presented a decrease in Aβ plaque formation after 9 months of an ER diet. The authors reported that ER may promote anti-amyloidogenic α-secretase activity and decrease components of the pro-amyloidogenic γ-secretase complex(Reference Wang, Ho and Qin131,Reference Schafer, Alldred and Lee145) . Furthermore, ER improved age-related behavioural deficits in a triple-transgenic rodent model of AD (3xTgAD, overexpressing mutated PS1, APP and Tau)(Reference Oddo, Caccamo and Shepherd146). At 17 months of age, 3XTgAD mice on ER diet for 14 months performed better in the water maze task and displayed higher exploratory behaviour than animals on the ad libitum diet. Hippocampal levels of Aβ40, Aβ42 and phospho-tau were also decreased after ER(Reference Halagappa, Guo and Pearson147). Elderly human subjects free of dementia were followed for 4 years. Between the individuals who carried the ApoE e4 allele and those whose daily energy intake was elevated showed a higher risk of AD(Reference Luchsinger, Ming-Xing and Shea46), supporting the idea that the reduction in energy intake could improve AD-like symptoms.
Further studies have been conducted to understand the mechanism(s) by which ER may improve memory and cognition in several animal models. C57/BL6J mice, receiving ER diet for 10 months, presented with enhanced learning and memory capacity in the water maze, associated with a decrease in inflammatory and insulin signalling markers and activation of autophagy(Reference Dong, Wang and Ma148). Furthermore, the modulation of apoptosis seems to be regulated by ER. Ma et al.(Reference Ma, Wang and Ph149) observed a reduction in apoptosis markers in the hippocampus of C57/BL6J mice in 10 months of ER, which was associated with improved memory in behavioural tests(Reference Ma, Wang and Ph149). Finally, the same authors reported improvements in hippocampus-dependent spatial learning associated with higher AMP-activated protein kinase and GLUT4 levels in the hippocampus, suggesting a possible role of AMP-activated protein kinase in this process(Reference Ma, Wang and Dong150). Another study demonstrated a correlation between the neuroprotective effects of ER in PDAPP-J20 mice for 6 weeks with the modulation of glial cells and the autophagy processes(Reference Gregosa, Vinuesa and Florencia151). Moreover, ApoE-deficient mice on ER exhibited increased post-synaptic (PSD95)-positive neurons and elevated levels of FGF21 in both plasma and brain, associated with improved performance in the water maze. This evidence suggested that the neuroprotection of ER may also be dependent on FGF21 signalling(Reference Rühlmann, Wölk and Blümel152), similar to evidence presented earlier for metabolic disorders.
Conclusion
Dietary disease prevention and interventions that extend the life span and ameliorate the impact of ageing have received attention in recent years as a method of extending the period free of disease; i.e. the health span. ER (without causing malnutrition and deficiencies) is one of the most studied forms of prevention and/or reversal of age-related disorders. The reduction in energy intake can improve brain health and may be a good candidate for reducing the risk of dementia, especially in midlife(Reference Floud, Simpson and Balkwill153). However, caution is warranted here, as the controversies surrounding underweight and extreme weight changes and dementia remain unresolved(Reference Emmerzaal, Kiliaan and Gustafson154).
Ultimately, present evidence suggests that the total amount of energy is not the key parameter responsible for health benefits, but rather the reduction of specific macronutrients in the diet(Reference Solon-Biet, Mcmahon and Ballard155). Even though ER can decrease body weight/adiposity and increase insulin sensitivity, the underlying mechanism(s) are still not well understood. In addition, long-term ER in human subjects may not be achievable and cause a range of deficiencies along the way. Therefore, DR related to specific nutrients, without ER, offers an attractive alternative, achievable in human subjects.
One such dietary intervention is MR, which can mimic the positive health span effects of ER without the associated reduction in food intake. Decreasing the amount of methionine in the diet has been suggested as a promising strategy to extend longevity, prevent and/or reverse obesity and metabolic disorders, with many of the effects being driven by its ability to induce FGF21 secretion and production. However, palatability of the MR-manipulated diets should be improved to gain more compliance from human subjects. Vegan diets and foods naturally low in methionine such as green leafy vegetables, nuts, fruit and beans could possibly recapitulate the positive effects of MR on metabolism; however, these may not be appropriate for children, pregnant women or elderly. Positive outcomes of MR were also reported for cognitive processes, thus opening an opportunity of developing MR mimetics for the prevention of AD and other neurodegenerative diseases. To achieve this, further studies are necessary to identify cellular mechanisms, pathways and targets underpinning the neuropathology of the disease and the role of methionine therein.
Financial Support
M. S. M. is a recipient of the Elphinstone Scholarship of the University of Aberdeen as well as Institute of Medical Sciences postdoctoral studentship. Work in B. P. and M. D. laboratories is funded by Alzheimer's Research UK, British Heart Foundation and Diabetes UK. Tenovus Scotland and BBSRC DTP studentship funded the published work on methionine restriction in M. D. laboratory.
Conflict of Interest
None.
Authorship
M. S. M. wrote the review; B. P. and M. D. edited the review; all authors approved the final version. Figures in this review were created using BioRender.com.