Introduction
Stenotrophomonas maltophilia is now a prevalent carbapenem-resistant gram-negative bacteria causing bloodstream infections (BSI) in the United States. Reference Kullar, Wenzler, Alexander and Goldstein1,Reference Cai, Tillotson, Benjumea, Callahan and Echols2 While S. maltophilia is generally considered a pathogen with low virulence, opportunistic infections in susceptible patient populations can be problematic due to its intrinsic resistance to a wide range of antibiotics. Reference Brooke3 Moreover, the incidence of S. maltophilia is thought to be increasing especially in immunocompromised patients and those exposed to broad-spectrum antibiotics. Reference Brooke3,Reference Safdar and Rolston4 S. maltophilia, along with other gram-negative organisms, pose a threat to the Veteran population who often have comorbidities predisposing them to resistant pathogens. Risk factors for mortality in hospitalized patients with S. maltophilia include common chronic conditions such as arterial hypertension, type 2 diabetes, acute myocardial infarction, presence of a urinary catheter, hemodialysis, or peritoneal dialysis. Reference Cai, Tillotson, Benjumea, Callahan and Echols2
Despite the potential for the pathogenicity of S. maltophilia, a paucity of scientific evidence on antimicrobial treatment options and effectiveness exists. As with many multidrug-resistant organisms, the treatment for S. maltophilia is not clearly defined. Traditionally, trimethoprim-sulfamethoxazole (TMP-SMX) has been used most frequently to treat infections associated with S. maltophilia. Reference Brooke3,Reference Anđelković, Janković and Kostić5 However, this practice is largely based on in vitro studies, retrospective non-randomized clinical trials, or expert opinion, and there is limited evidence to support TMP-SMX as the preferred therapy along with optimal dosing to maximize its benefits. Reference Cai, Tillotson, Benjumea, Callahan and Echols2,Reference Anđelković, Janković and Kostić5,Reference Nicodemo and Paez6 There have also been increasing concerns for TMP-SMX resistance, leading to questions about whether TMP-SMX would continue to be as effective against S. maltophilia as it was once thought to be. 7–Reference Sader and Jones9
The Infectious Diseases Society of America’s (IDSA) suggested approach for the management of S. maltophilia infections has evolved since its initial release in 2021. Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy10 Recent guidance focuses on moderate to severe cases of S. maltophilia infections with two main suggested approaches to management: either the combination of two potentially active agents, with options including TMP-SMX, minocycline or tigecycline, levofloxacin, or cefiderocol; or the combination of ceftazidime-avibactam plus aztreonam in the setting of significant clinical instability and no other viable options. Transitioning to monotherapy can be considered with appropriate clinical response, except for levofloxacin which is advised to be only used as a component of combination therapy due to concerns for resistance. At this time, the authors of the guidance document acknowledge that there is insufficient data to recommend a “standard-of-care” therapy, and there is a need for more robust clinical trials that compare the effectiveness of different treatment strategies and their impact on patient outcomes.
Little data exists on treatment trends for S. maltophilia infections, especially in the Veteran patient population, where older age and existence of multiple comorbidities increase this population’s vulnerability for infection and poor outcomes. The goal of this study is to describe antibiotic treatment strategies utilized to treat S. maltophilia BSI in the Veteran patient population to gain a better understanding of treatment approaches.
Methods
This retrospective cohort study was conducted across all Department of Veterans Affairs (VA) healthcare facilities during a 10-year timeframe from January 2012 to December 2021. Data were extracted from the VA Corporate Data Warehouse (CDW), a national repository that includes clinical and administrative data from the Veterans Health Administration. These data were used to obtain microbiology, pharmacy, and encounter data, including facility characteristics, culture data, and antimicrobial treatment information. Additionally, patient demographics, characteristics, and comorbidities were also collected. Comorbidities were identified by ICD-10 codes in the last 365 days prior to an S. maltophilia culture and used to calculate the Charlson comorbidity index. Reference Charlson, Pompei, Ales and MacKenzie11 Chart reviews were conducted to verify antibiotic treatment and microbiologic results and obtain additional patient demographic and medical data, including past medical history and allergy information.
Veterans were included if they were aged ≥ 18 years with ≥ 1 positive blood culture for S. maltophilia in an inpatient setting. Index date was defined as the collection date of the blood culture positive for S. maltophilia. Antimicrobial treatment was defined as administration of at least one agent with possible activity against S. maltophilia between days –2 and +5 of the index date. Antibiotics with possible activity against S. maltophilia for which data was collected included cefiderocol, ceftazidime, ceftazidime-avibactam plus aztreonam, ciprofloxacin, eravacycline, levofloxacin, minocycline, moxifloxacin, polymyxins, tigecycline, and TMP-SMX. Given broad-spectrum antibiotic exposure is a potential risk factor for S. maltophilia infections, data for antibiotic history was obtained by collecting documented administration of common broad-spectrum antibiotics 90 days prior to the index date. Microbiologic data collected included susceptibility results, if reported, identification of S. maltophilia in specimens other than blood, and whether the positive blood culture was monomicrobial or polymicrobial. Susceptibility results for blood cultures were collected based on textual interpretations (ie, resistant, intermediate, or susceptible) of the reported site.
Descriptive statistics were used to summarize patient demographics, medical characteristics, previous healthcare exposure, facility characteristics, microbiologic data, and antibiotics received. Poisson regression was applied as a trend test to assess changes in antibiotic usage over years. Two-sided p-values <0.05 were considered significant. Statistical analyses were conducted using SAS, version 9.2 (SAS Institute).
Results
A total of 374 blood cultures positive for S. maltophilia were identified across 75 of the 171 VA facilities. Of these, 282 unique BSI cases were identified (Table 1) with 14% being polymicrobial. Among the 282 unique patients, the majority were male (93.6%), white (67.4%), with a mean age of 64 ± 13.1 years. Severity of comorbidities was assessed using the Charlson comorbidity index. The mean and median score of the 282 patients was 4, indicating a moderate to severe burden of comorbidities and increased risk of short-term mortality. Apart from older age, end-stage renal disease was a common comorbidity identified with 25.5% patients diagnosed with this at the time of S. maltophilia BSI. The most commonly administered antibiotics within 90 days prior to culture date were beta-lactam/beta-lactamase inhibitors (40.8%), third- or fourth-generation cephalosporins (38.9%), and fluoroquinolones (34%). The majority of the cases received care in Southern locations (46.1%) and close to 20% of all case encounters occurred in rural areas. Most patients were managed at higher complexity VA facilities.
Table 1. Patient and facility characteristics associated with S. maltophilia bacteremia

SD, Standard deviation; IQR, Interquartile range.
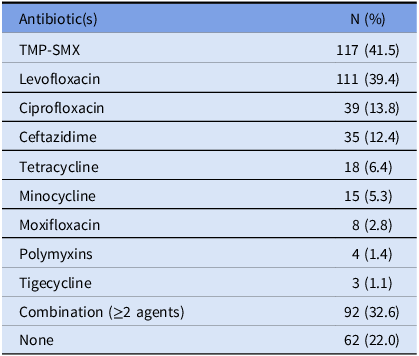
Out of the 374 blood cultures positive for S. maltophilia, the most tested antibiotics for susceptibility were TMP-SMX (n = 356 [95.2%]), levofloxacin (n = 254 [67.9%]), and ceftazidime (n = 153 [40.9%]), and resistant isolates were reported for these antibiotics 4.5%, 4.3%, and 44.4% of the time, respectively (Figure 1). For the 282 unique patients with S. maltophilia BSI, the most frequently used antibiotics with possible activity against the pathogen were TMP-SMX (41.5%) and levofloxacin (39.4%), followed by ciprofloxacin (13.8%) and ceftazidime (12.4%). Combination therapy, with at least 2 active agents, was given in 32.6% of the cases (Table 2). A total of 62 patients (22%) did not receive any antibiotic with possible activity within the prespecified window of days –2 to +5 of index date. Among these patients, 15 (24%) did not receive treatment due to mortality prior to therapy or withdrawal of care. Other reported reasons for not initiating antibiotics active against S. maltophilia included administration of therapy outside of the prespecified time frame of study protocol, and no treatment per clinician judgment based on symptoms, source control, or other microbiologic data.

Figure 1. Frequency of resistant S. maltophilia isolates to commonly tested antibiotics.
Table 2. Antimicrobial treatment for S. maltophilia BSI
From 2012 to 2021, the frequency of S. maltophilia blood cultures were noted to decrease over time, with 14.4% in 2012 to 3.5% in 2021 (Figure 2). No significant trends were observed in TMP-SMX (IIR = 1.00, 95% CI 0.94–1.06, p = 0.99), fluoroquinolones (IRR = 0.99, 95% CI 0.94–1.05, p = 0.81), or ceftazidime (IIR = 0.94, 95% CI 0.83–1.05, p = 0.28) prescribing over this time frame (Figure 3).

Figure 2. Frequencies of S. maltophilia positive blood cultures by year across 75 VA facilities.

Figure 3. Trends in prescribing of the most commonly used agents for S. maltophilia BSI in Veterans from 2012 to 2021.
Discussion
TMP-SMX and levofloxacin were the most commonly prescribed antibiotics for S. maltophilia BSI, with no discernible trends in utilization over time. Despite TMP-SMX historically being considered the preferred therapy for S. maltophilia, the utilization of fluoroquinolones was similar to that of TMP-SMX. The demographics of the patients were predominantly male, white, and older. These characteristics were consistent with the national VA population statistics. 12 Although 34% of patients had documentation of fluroquinolone use 90 days prior to the index date, resistance rates were similar in fluoroquinolones compared to TMP-SMX. The utilization of other potentially effective agents was low. The low usage of minocycline in this study (5.4% of patients) has been described elsewhere in a similar database study, possibly due to tetracyclines not being preferred in practice for the treatment of BSI. Reference Charlson, Pompei, Ales and MacKenzie11 Although only a few isolates were tested for minocycline susceptibility, only 2.6% were reported to be resistant. No cases received cefiderocol, eravacycline, or the combination of ceftazidime-avibactam plus aztreonam, which is likely due to the relatively recent approval of these newer agents as well as the time frame of this study predating the IDSA Antimicrobial Resistance (AMR) guideline release. An overall downward trend was observed in the frequency of S. maltophilia BSI in the 10-year time frame studied with the majority of cases observed in southern geographical regions.
As specimens other than blood isolates of S. maltophilia may sometimes indicate colonization rather than true infection, this study exclusively focused on patients with S. maltophilia in the blood. Although clinical outcomes were not evaluated, the results were remarkable in that 22% of the patients studied did not receive antimicrobial treatment within the evaluated time frame active against the S. maltophilia. While about a quarter of these cases did not receive culture directed therapy due to mortality prior to therapy or withdrawal of care, there were also instances where patients, if asymptomatic, were monitored without receiving treatment specific for the S. maltophila. While the presence of gram-negative pathogens in the blood is not typically considered contamination, it is conceivable that the S. maltophilia was not treated in the setting of absence of symptoms.
Prior studies have also suggested that appropriate treatment is not consistently provided after S. maltophilia is detected in blood cultures. If treatment is provided, fluoroquinolones have been found to be prescribed more often than TMP-SMX. A retrospective observational study of Japanese medical facilities aimed to describe clinical characteristics of S. maltophilia BSI cases between 2007 and 2013. Reference Ebara, Hagiya, Haruki, Kondo and Otsuka13 Out of a total of 44 cases, 36% were reported to have not received appropriate antimicrobial therapy, yet 69% of these patients survived. The most frequently administered antimicrobial class was fluoroquinolones, followed by minocycline and TMP-SMX (15, 10, and 3 cases, respectively). A more recent retrospective database study similarly evaluated patients with S. maltophilia BSI between 2010 and 2015 to describe patient, microbiologic, and treatment characteristics. Reference Cai, Tillotson, Benjumea, Callahan and Echols2 Out of 486 unique cases, 95% and 84% of cultures were susceptible to TMP-SMX and fluoroquinolones, respectively, and utilization of levofloxacin was higher (48.9%) when compared to TMP-SMX (38.3%). In this study, 15% of patients did not receive appropriate definitive therapy.
Studies on S. maltophilia infections in Veterans are scarce overall, and even more limited specifically for BSI. National VA data from 2010 to 2018 demonstrated a significant decrease in S. maltophilia prevalence (by 5.4% per year) as well as a decrease in resistance to TMP-SMX over time. Reference Ebara, Hagiya, Haruki, Kondo and Otsuka13 The authors theorized that the decreasing trend in cultures may be related to efforts in inpatient settings to improve infection control and antimicrobial stewardship across the health system. Although less than 10% of the hospital cultures (n = 10,513) were from the blood, the downtrend in prevalence of S. maltophilia was consistent with the results of our study, even when cultures from other body sites were included in the analysis (ie, urine, skin and soft tissue, or respiratory). The same authors evaluated treatment of S. maltophilia infections in the Veteran population and found that the most common antibiotic agents used over a 9-year period were piperacillin/tazobactam (39.7%), TMP-SMX (23.3%), and levofloxacin (23.2%), however these antimicrobials may have been directed toward other pathogens in the case of a polymicrobial culture. Reference Charlson, Pompei, Ales and MacKenzie11 Interestingly, combination therapy was used in 16.6% of patients, 50% less than findings from the current study which evaluated more recent data.
Limitations to the current study include the relatively small sample size, despite the wide time frame reviewed and the inclusion of a large number of VA facilities, and the mostly male sample minimizing generalizability to females. Despite this, the current study adds to the limited data in the literature evaluating treatment trends specifically for S. maltophilia BSI. Our study did not consider variability in susceptibility testing across different VA facilities nor the potential advancements in laboratory methods that could have enhanced the detection and identification of organisms in blood cultures over the 10-year period, however, frequency of positive S. maltophilia blood cultures in the current study decreased over time. Considering the historical practice of evaluating blood culture results and antibiotic therapy for suspected bacteremia over a period of up to 5 days, along with the average time-to-positivity of S. maltophilia observed in previous studies, we established the inclusion criteria as antibiotic administration during −2 to +5 days from the index date. Reference Pollack and Srinivasan14–Reference Reimer, Wilson and Weinstein20 This time frame was chosen to encompass the majority of patients with positive cultures who received pathogen-directed therapy, however the incidence of those who had delays in culture positivity and treatment administration is unclear. Finally, due to the time frame evaluated, this study did not capture any patients who were treated with newly approved antimicrobials, as currently recommended in the IDSA guidelines, thus more recent data is needed to describe the uptake of these agents.
In conclusion, TMP-SMX and levofloxacin were the most commonly used antibiotics for the treatment of S. maltophilia BSI in Veterans during 2012–2021. Despite TMP-SMX being the preferred agent historically, its use was comparable to fluoroquinolones. The utilization of tetracyclines was low, and there was no utilization of newer antibiotic agents. Combination therapy was prescribed in only 33% patients contrary to the recent proposed approach of initiating combination therapy to increase the likelihood that at least one agent is effective against the organism.
As newer antimicrobial agents showing efficacy against S. maltophilia are introduced and guidance for managing S. maltophilia infections expands, we anticipate future treatment trends to be more diverse, incorporating a broader range of agents and favoring combination therapy over the traditional reliance on TMP-SMX and fluoroquinolones.
The selection of agents was appropriate in majority of cases as both TMP-SMX and levofloxacin can be effective for S. maltophilia infections. As the management of S. maltophilia evolves, future trends are expected to incorporate newer antimicrobials, such as cefiderocol, or higher usage of combination therapy. TMP-SMX and levofloxacin are anticipated to remain as viable options for S. maltophilia infections given limited effective therapies.
Author contribution
Clara H. Lee: Conceptualization, investigation, writing—original draft, and writing—review and editing. Ursula C. Patel: Conceptualization, investigation, and writing—review and editing. Amanda Vivo: Formal analysis, investigation, methodology, resources, and writing—review and editing. Lishan Cao: Data curation. Charlesnika T. Evans: Conceptualization, funding acquisition, investigation, methodology, supervision, and writing—review and editing.
Financial support
This work was supported by The Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development IIR 16-028 HX002169 to C.T.E. and Research Career Scientist Award (RCS 20-192) to C.T.E.
Competing interests
All authors report no conflicts of interest or financial disclosures relevant to this article.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Research transparency and reproducibility
We are committed to collaborating and sharing these data to maximize their value to improve Veterans and others’ health and health care, to the greatest degree consistent with current Veterans Administration regulations and policy. We can provide access to the programming code used to identify the sample and conduct analyses. We cannot provide a link to the database directly as it would compromise patients’ anonymity, and permissions from VA are needed to obtain the data.







