Globally, rice is a major agricultural commodity produced in lowland and upland cropping systems across a wide range of environments. Rice production in the United States is localized in two regions—California in the West and in the Midsouth. The Midsouth consists of four states: Arkansas, Mississippi, Louisiana, and Missouri. Collectively, these states produce 6.6 million metric tons of rice equating to 65% of U.S. rice produced and contributing US$1.9 billion to the world market (Workman Reference Workman2017; U.S. Department of Agriculture Economic Research Service 2016). Arkansas is the largest rice producer, consistently ranking 1st in overall production, and accounting for half the U.S. area and production. Arkansas producers can take advantage of several strategies to maximize production, including the adoption of ideal varieties, optimal location-specific fertilizer recommendations, and flooding as a primary means to reduce weed infestation. While rice variety selection and cultural management are critical to improve production, weed management is often considered the leading factor that limits productivity.
Weed species in rice are diverse, consisting of grasses, broadleaf weeds, and sedges that can survive in aerobic or anaerobic conditions or both. Among these, the Echinochloa genus is the most widespread and most damaging to rice yield (Danquah et al. Reference Danquah, Johnson, Riches, Arnold and Karp2002). Echinochloa and rice are morphologically and biologically similar. They tolerate flooded culture and coexist under similar environments. Members of this genus have been classified consistently as primary weed problems in U.S. rice fields. In California, early watergrass [Echinochloa oryzoides (Ard.) Fritsch], late watergrass [Echinochloa oryzicola (Vasinger) Vasinger], and barnyardgrass are the primary species; while in the Midsouth, barnyardgrass and junglerice are more prevalent (Fischer et al. Reference Fischer, Ateh, Bayer and Hill2000; Van Wychen Reference Van Wychen2015). Historically, barnyardgrass has been identified and ranked as the predominant weed species in Arkansas rice production fields. Season-long interference by barnyardgrass can result in up to a 70% loss in grain yield with a 50% yield reduction from a density of 52 plants m−2 (Smith Reference Smith1988). A recent study sought to assess the Echinochloa spp. present in Arkansas rice fields, identifying junglerice as the dominant species (Tahir et al. Reference Tahir, Burgos and Gentry2014). While this reclassification has not changed the recommendations for management, it does require updating the literature and the description of the impact of this species on rice production in the Midsouth.
In U.S. rice production, herbicides have been used since the 1950s to selectively manage Echinochloa and other major species including weedy rice (Oryza sativa L.), sprangletops (Leptochloa spp.), hemp sesbania [Sesbania herbacea (P. Mill) McVaugh], and northern jointvetch [Aeschynomene virginica (L.) B.S.P] (Talbert and Burgos Reference Talbert and Burgos2007). Propanil is a photosystem II inhibitor (WSSA Group 7) introduced in 1959 with excellent control of barnyardgrass and the added benefit of hemp sesbania control (Scott Reference Scott2017). In 1992, quinclorac, an auxinic herbicide (WSSA Group 4), was introduced specifically to mitigate propanil-resistant barnyardgrass with the added benefit of controlling other grasses. While these two herbicides have been the standard for rice weed control, clomazone (WSSA Group 13), cyhalofop (WSSA Group 1), and fenoxaprop (WSSA Group 1) also have been introduced for management of grasses in rice. Clearfield® technology was introduced in the early 2000s as the first non–genetically modified herbicide-resistant rice with resistance to acetolactate synthase (ALS) inhibitors (WSSA Group 2), specifically imidazolinones—imazethapyr, imazamox, and imazapic (not used in the United States). The Clearfield® rice technology improved the management of weedy rice throughout the Midsouth and also provided an additional mode of action for Echinochloa management. Despite crop rotation (primarily rice–soybean [Glycine max (L.) Merr.] in Arkansas) and the diversity of herbicides used to manage grass weeds across both crops (Hardke Reference Hardke2016), resistance to herbicides has evolved.
A survey of Arkansas and Mississippi rice crop consultants conducted in 2012 (Norsworthy et al. Reference Norsworthy, Bond and Scott2013) listed barnyardgrass as the most problematic (63% of respondents), with 58% and 52% of respondents, respectively, listing propanil- and quinclorac-resistant barnyardgrass as the common problem. It should be noted that Echinochloa species have been collectively called “barnyardgrass”; thus, the term includes junglerice. Barnyardgrass with resistance to propanil, quinclorac, clomazone, and imazethapyr has been reported and documented in Arkansas rice fields since the early 1990s (Heap Reference Heap2017). More recently, barnyardgrass populations with multiple resistance to propanil and quinclorac and a junglerice population with three-way resistance to propanil, quinclorac, and imazethapyr have been reported (Heap Reference Heap2017). Worldwide, barnyardgrass and junglerice have been documented with resistance to six modes of action in 34 countries throughout a variety cropping systems (Heap Reference Heap2017). The widespread distribution and ability of Echinochloa to evolve resistance to the diverse herbicides used for management is a great concern to both producers and researchers.
Surveys were conducted to (1) confirm the occurrence of herbicide resistance in Echinochloa, (2) assess the distribution and track the evolution of resistance patterns with time, and (3) improve demographic knowledge on the Echinochloa populations.
Materials and Methods
The surveys conducted from 2006 to 2016 with the goal of identifying and reporting herbicide resistance will be referred to as the “Echinochloa Herbicide Resistance Confirmation Survey.” The surveys conducted from 2010 to 2016 with the goal of characterizing the herbicide resistance profiles of common Echinochloa species in Arkansas, will be referred to as the “Echinochloa Herbicide Resistance Demographics Survey.” Bioassays conducted for both surveys followed similar methodologies unless otherwise described in the following sections.
Echinochloa Collection and Field Sampling
Rice field surveys and Echinochloa sampling were conducted according to Burgos (Reference Burgos2015) as weeds began maturing during the crop season until harvest. Sampling occurred in fields reported to crop consultants or university extension personnel as having populations that survived at least one herbicide application. For the Resistance Confirmation Survey, seeds were bulk sampled per field without discriminating among species. Samples were sent to the University of Arkansas by consultants and extension personnel. For the Resistance Demographics Survey, samples were bulked by site in the field and plant type. University of Arkansas faculty led the collection of most samples for this survey. Sample size ranged from panicles of a few plants (all that existed in a small patch) to about 200 g of seed (representing a large patch of one plant type); independent samples were collected within the same field and from separate fields. Samples were placed in paper bags and allowed to dry at room temperature. When possible, field history was obtained. The identity of species evaluated in the Resistance Demographics Survey was determined using taxonomic features, specifically the panicle structure and inflorescence features. Henceforth, each bulked sample from a field, or separate bulk samples from multiple sites in a field, will be referred to as “accessions.”
Herbicide Resistance Profiling
Major rice herbicides were used in the bioassays at field use rates, with recommended adjuvants (Tables 1 and 2). Herbicide resistance bioassays were conducted in the greenhouses at the University of Arkansas Altheimer Laboratory in Fayetteville. The greenhouses were set at 14-h day length with supplemental lighting and maintained at a temperature of 30 to 35 C. The bioassays occurred from January to March for initial reporting. For the demographic studies, a second run of the bioassays was conducted later in the year. Seeds were sown into pots containing a commercial potting mix with 75% to 85% peat (Sun Gro Horticulture, Seba Beach, Canada). Each experiment contained a nontreated control for each accession and a susceptible standard. For the Resistance Confirmation Survey of POST herbicides, seedlings were thinned to 5 plants pot−1 within 1 wk of emergence, with each pot serving as one experimental unit and replicated twice. The response to a PRE herbicide, clomazone, was evaluated by applying the herbicide to the surface of field soil (Captina silt loam, fine-silty, siliceous, active, mesic Typic Fragiudults) in which approximately 50 seeds were planted per replication. For the Resistance Demographics Survey involving POST herbicides, seedlings were thinned to 20 plants pot−1, with each pot serving as one experimental unit and replicated three times, with the experiment being conducted twice. All herbicide applications were made in an air-propelled, motorized spray chamber calibrated to deliver 187 L ha−1. Plants were sprayed when seedlings had 1 to 2 visible leaf collars. Following herbicide application, treated plants were left to dry before being returned to the greenhouse, and irrigated as necessary. Clomazone-treated pots were lightly misted following herbicide application to activate the herbicide and allow it to percolate to the seed zone.
Table 1 Herbicide common name, trade name, application rate, timing, and adjuvant (if necessary) used in the Echinochloa Herbicide Resistance Confirmation Survey from 2006 to 2016 and the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016.
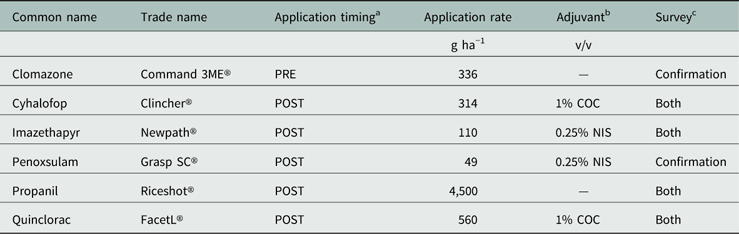
a Application timings: POST, 2- to 3-leaf Echinochloa; PRE, following planting.
b Adjuvant: COC, crop oil concentrate, Agridex®; NIS, nonionic surfactant, Induce®.
c Indicates the survey in which the herbicide was included for screening: Both, included in both surveys; Confirmation, included in the Echinochloa Herbicide Resistance Confirmation Survey.
Table 2 Herbicide-resistance profiles of Arkansas Echinochloa spp. accessions from the Echinochloa Herbicide Resistance Confirmation Survey from 2006 to 2016 treated with common rice herbicides.
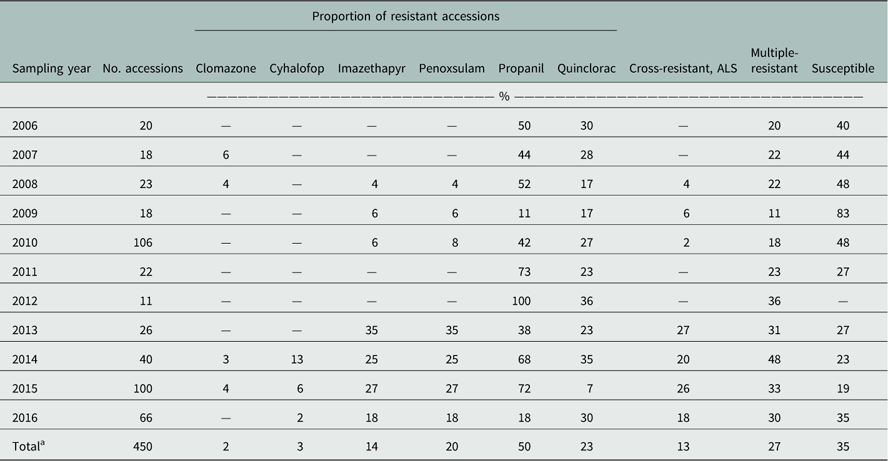
a Total percentage of accessions with resistance to the respective herbicide, based on the total number of accessions from 2006 to 2016.
Data Collection and Analysis
Treatment effects were evaluated 21 d after herbicide application. For the Resistance Confirmation Survey, injury/control (0%=no injury to 100%=complete plant death) was evaluated visually. Data were averaged across runs. Accessions showing less than 70% control were classified as resistant, thus generating a matrix of resistance confirmation across various herbicides. A description of the herbicide resistance profile is presented. For the Resistance Demographics Survey, the surviving plants were counted, and the level of visible injury on surviving plants was recorded (0%=no injury to 100%=plant death). Survivors (%) and injury data were averaged across replications and runs for analysis. Similar analysis was performed as described for the Resistance Confirmation Survey; further, cluster analysis was also performed to statistically delineate the accessions into different resistance groups, by herbicide, based on the injury (%) of survivors and frequency of surviving plants for the accession (%).
Results and Discussion
Echinochloa Herbicide Resistance Confirmation Survey
A total of 450 accessions from 27 counties were tested. The rice herbicides evaluated were clomazone, cyhalofop, imazethapyr, penoxsulam, propanil, and quinclorac. Resistance to propanil was confirmed in 50% of the accessions tested, and quinclorac resistance was confirmed in 23% of the accessions from 2006 to 2016 (Table 2). Resistance to clomazone or cyhalofop was rare, at 2% and 3%, respectively. Resistance to ALS inhibitors imazethapyr and penoxsulam occurred in 14% and 20% of the accessions, respectively. While both herbicides belong to Group 2, they are from different chemical families; 13% of the accessions were cross-resistant to these herbicides. From 2013 to 2016, cross-resistance to ALS inhibitors increased to 18% or more of the accessions. Multiple resistance was identified each year, totaling 27% of the accessions: 37% were resistant to a single herbicide and 28% were resistant to herbicides belonging to two or more modes of action (Figure 1). Resistance to propanil or quinclorac occurred at a higher frequency (57 or 12% of accessions) than resistance to other herbicides due to their long history of use in Arkansas (Figure 2). None of the accessions were resistant to only cyhalofop; rather, resistance to cyhalofop occurred along with resistance to other herbicides, indicating an excessive selection pressure by cyhalofop after failure of other herbicides to control Echinochloa. ALS inhibitor–resistant accessions were also resistant to propanil 5% of the time and, to a lesser extent, resistant to both propanil and quinclorac (2%). Only about one-third (35%) of accessions tested were susceptible to all herbicides evaluated.
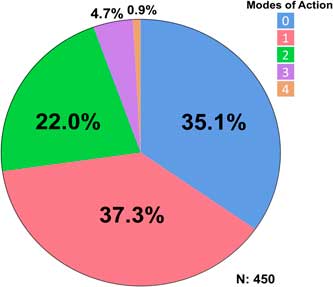
Figure 1 Frequency (%) of Echinochloa accessions showing different resistance profile categories, collected from Arkansas rice fields, and tested in the Echinochloa Herbicide Resistance Confirmation Survey from 2006 to 2016.
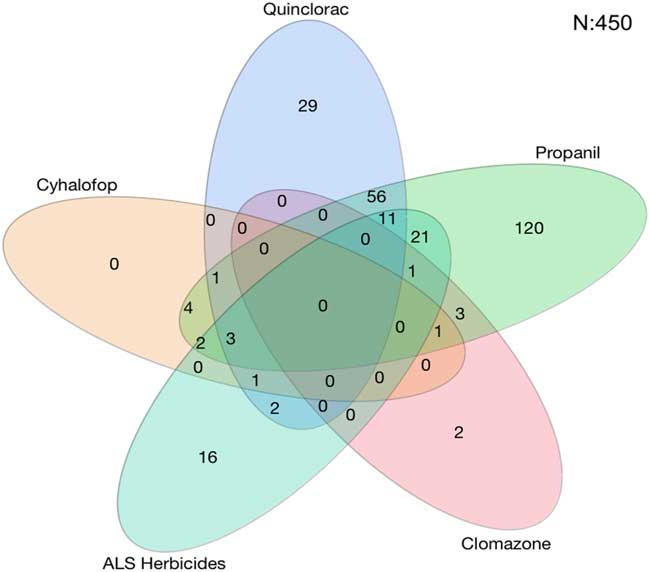
Figure 2 Number of Echinochloa accessions with resistance to common rice herbicides used in Arkansas, collected from Arkansas rice fields, and tested in the Echinochloa Herbicide Resistance Confirmation Survey from 2006 to 2016. Each oval represents one herbicide. Overlapping ovals indicate that the accessions within a given group are multiply resistant to the respective herbicides. The oval for acetolactate synthase (ALS) herbicides contains the number of accessions with cross-resistance to both imazethapyr and penoxsulam.
Sampling of fields was nonrandom, as the accessions were submitted by growers, extension personnel, or independent consultants who observed remaining Echinochloa infestations in the field after herbicide applications. However, important information can be gleaned from the distribution and characterization of these accessions (Figure 3). Sixty-five of the 450 accessions submitted did not have county information and therefore could not be shown on the maps. Herbicide resistance occurs throughout the major rice-producing areas of eastern Arkansas. The highest number of accessions submitted were from Arkansas (45), Cross (23), Greene (49), Jefferson (20), Lawrence (42), Poinsett (22), and Prairie (44) counties (unpublished data). Greene and Lawrence counties, located at the northeast corner of the state, had the highest number of confirmed resistance cases. Approximately 50% of the accessions in these two counties were multiple-resistant. Another area with high frequency of resistance is in the central part of the state, along the I-40 corridor, in what is collectively referred to as the Grand Prairie region. Monroe County, which had only 14 accessions submitted for testing, consistently had a higher number of accessions with cyhalofop, propanil, quinclorac, and multiple resistance. To better develop an integrated and community-driven herbicide resistance management approach, it is necessary to identify the locations with high frequencies of resistance to improve the strategies used in these areas while reinforcing effective management strategies in low-resistance areas to prevent the spread of resistance.
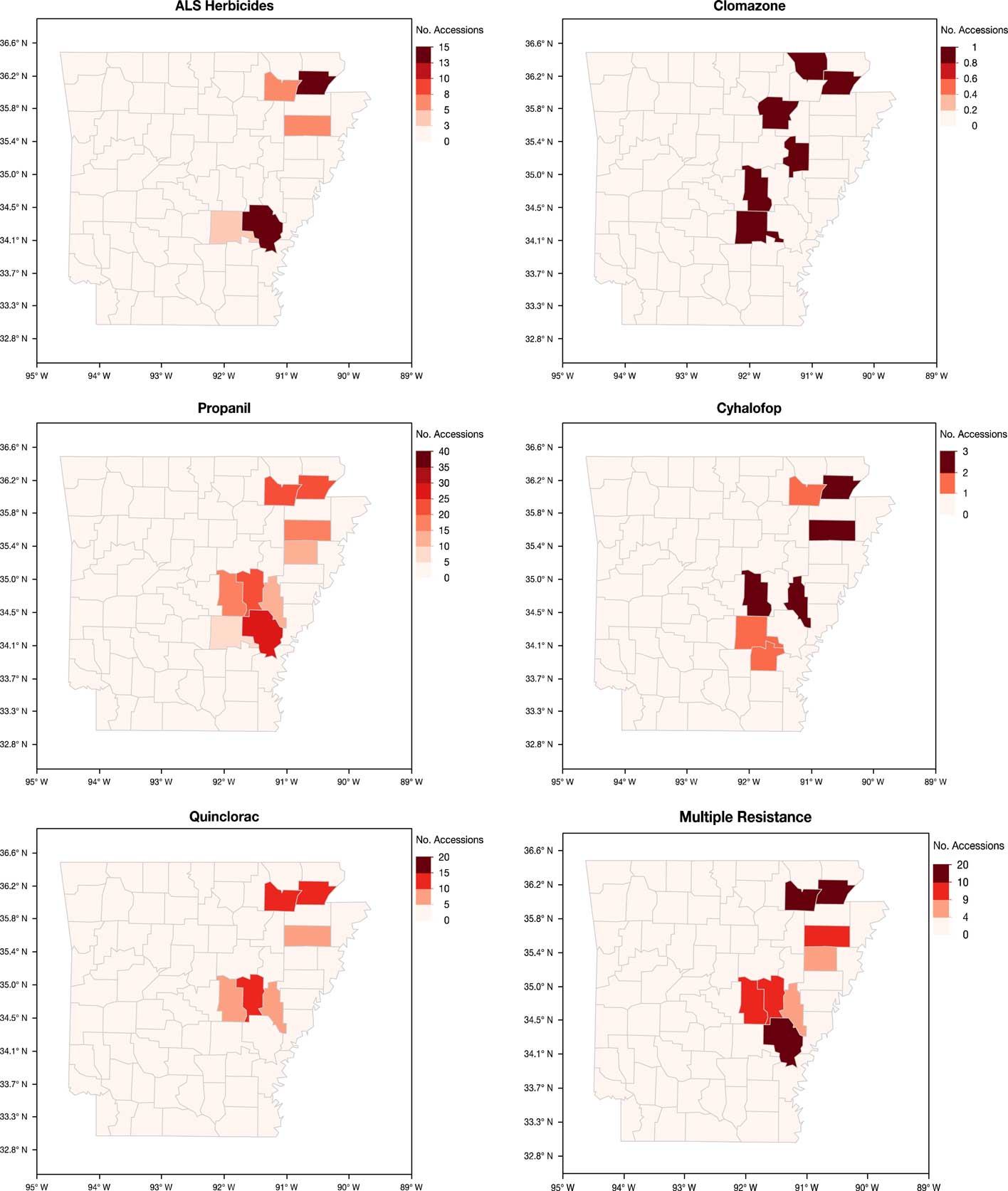
Figure 3 Arkansas maps showing the distribution of the accessions of Echinochloa spp. resistant to five common rice herbicides from the Echinochloa Herbicide Resistance Confirmation Survey from 2006 to 2016.
Echinochloa Herbicide Resistance Demographics Survey
For the Resistance Demographics Survey, 258 accessions from 28 counties were collected (Table 3). Testing for resistance to cyhalofop, imazethapyr, propanil, and quinclorac were prioritized in this survey because of their widespread use in rice production. Resistance to propanil and quinclorac data were similar to data from the Herbicide Resistance Survey, with propanil and quinclorac resistance confirmed in 53% and 28% of accessions, respectively. A higher proportion of cyhalofop-resistant accessions (12%) and a lower proportion of imazethapyr-resistant accessions (6%) were detected in this survey relative to the data from the Resistance Confirmation Survey. Multiple resistance was confirmed in 28% of the accessions, almost identical to that of the Resistance Confirmation Survey. Resistance to propanil and quinclorac was the dominant multiple-resistance profile, observed in 16% of the accessions (Figure 4A). This was followed by multiple resistance to propanil, quinclorac, and cyhalofop, which was confirmed in 5% of the accessions. Only 36% of accessions were deemed susceptible to the herbicides tested, similar to the Resistance Confirmation Survey.
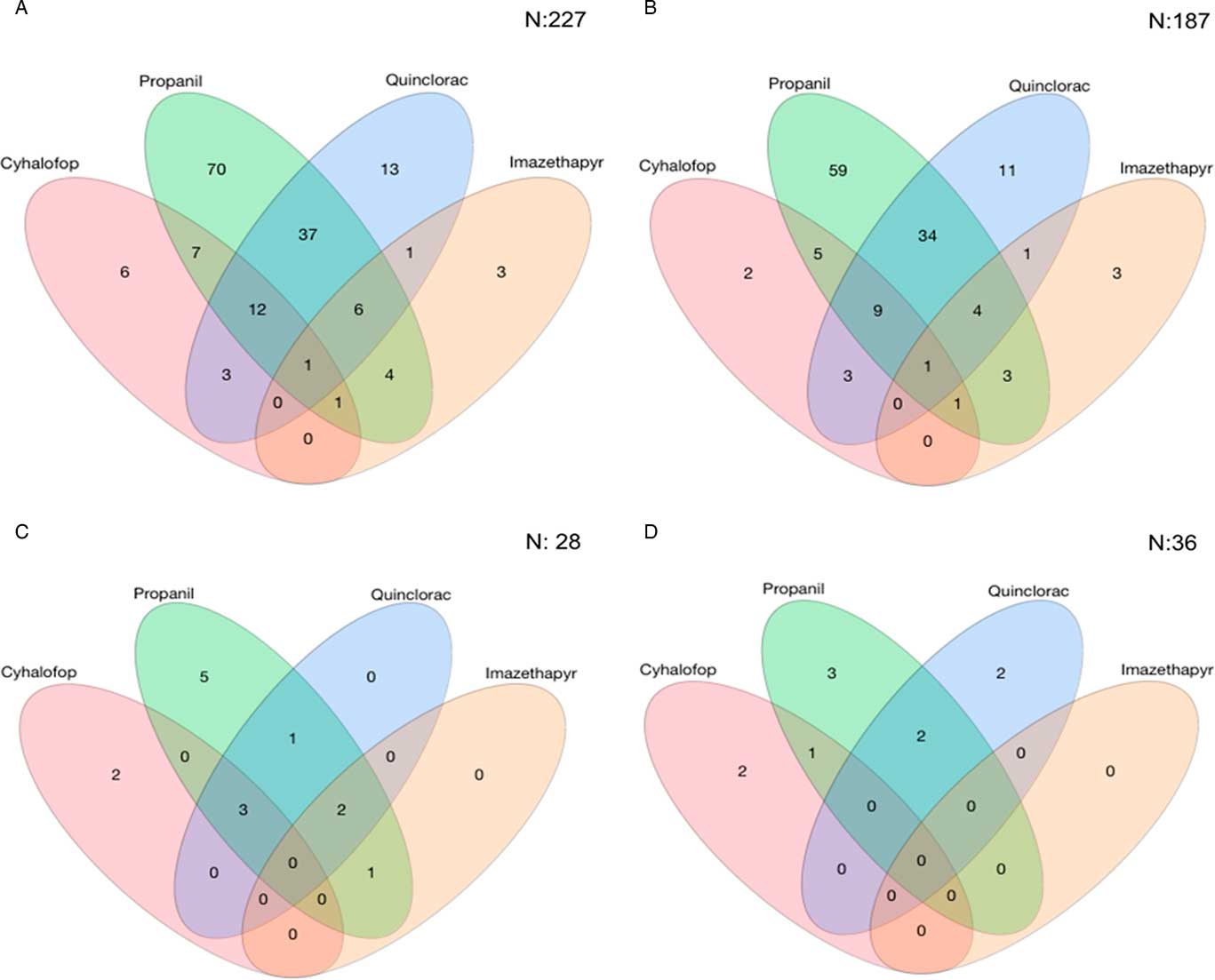
Figure 4 Number of Echinochloa spp. accessions with resistance to the four most common rice herbicides used in Arkansas tested in the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016. (A) All Echinochloa spp. accessions; (B) junglerice; (C) barnyardgrass; and (D) rough barnyardgrass.
Table 3 Herbicide resistance to common rice herbicides of Arkansas Echinochloa spp. accessions profiled in the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016.
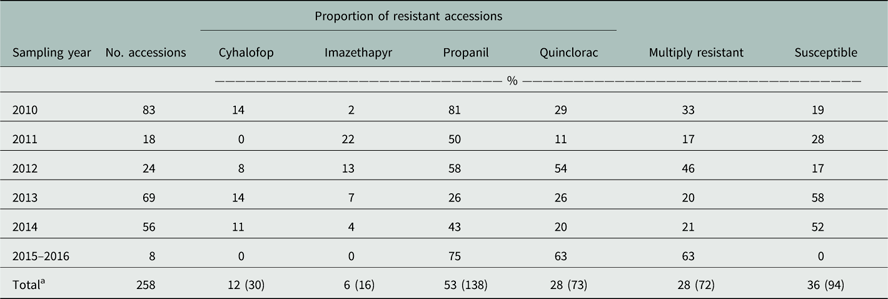
a Total percentage of the accessions with resistance to the respective herbicide from the total number of collections from 2006 to 2016; numbers in parentheses indicate the number of accessions with resistance to the respective herbicides.
Three primary species characterized in the Resistance Demographics Survey were junglerice (N=187), barnyardgrass (N=28), and rough barnyardgrass (N=36) (Figure 4). A fourth grouping is also included in the analysis (n=7) that could not be identified unequivocally and is signified as “ECH.” The presence of multiple Echinochloa species in Arkansas was reported previously, but the resistance profiling had not been done by species (Bryson and Reddy Reference Bryson and Reddy2012; Burgos et al. Reference Burgos, Rouse, Tseng, Abugho, Hussain, Salas, Singh and Singh2015; Tahir et al. Reference Tahir, Burgos and Gentry2014). The survey could not determine, without bias, whether rough barnyardgrass was more common than barnyardgrass because of the relatively small sample size of these species. A more extensive survey is needed to answer this question. The resistance profile of junglerice aligned with the whole collection, showing high resistance frequency to propanil only (32%), followed by resistance to quinclorac only (6%), and multiple resistance to both herbicides being prevalent (18%) (Figure 4B). Considering that junglerice comprised 73% of the total collection, it should dictate the overall resistance pattern. Resistance to only propanil is higher in barnyardgrass (18%) and rough barnyardgrass (8%) than to the other herbicides evaluated (Figure 4C and D). This is expected, since propanil was the primary selector for resistance. Resistance to imazethapyr was not observed among the rough barnyardgrass accessions. Approximately 40% of the junglerice accessions were resistant only to a single herbicide, while 33% were resistant to two or more herbicides (Figure 5). Barnyardgrass accessions had similar frequencies of single resistance (29%) and multiple resistance (25%). The frequency of three-way resistance in barnyardgrass (18%) was higher than that in other species. Only three accessions of rough barnyardgrass (8%) were confirmed as multiple-resistant, which was substantially lower than in the other species. Both barnyardgrass and rough barnyardgrass had a higher frequency of susceptible individuals than junglerice.
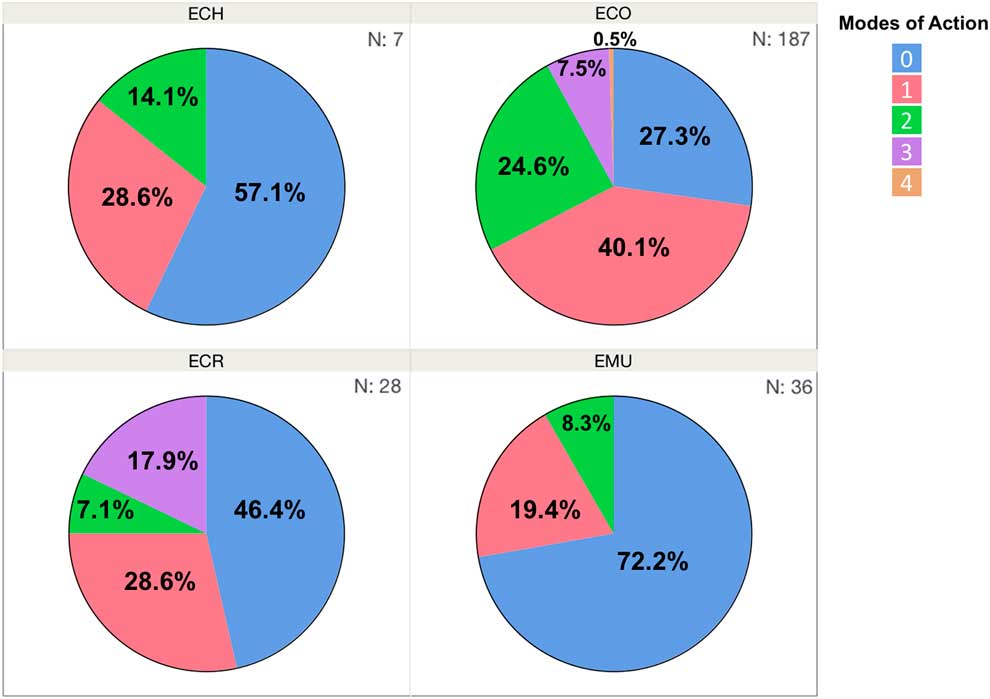
Figure 5 Frequency (%) of Echinochloa accessions in each resistance profile category, from Arkansas rice fields, tested in the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016. ECH, unknown Echinochloa spp.; ECO, junglerice; ECR, barnyardgrass; EMU, rough barnyardgrass.
The occurrence of resistance was concentrated in the northeast and Grand Prairie regions of the state (Figure 6). Greene (56%) and Lawrence (55%) counties had higher proportions of accessions with resistance to the four herbicides tested. Prairie County in the Grand Prairie region had more accessions with resistance to these herbicides, with propanil- (75%) and quinclorac- (35%) resistant individuals being predominant. Multiple-resistant populations were distributed across the rice-producing regions of the state, with the top four counties being Greene, Lawrence, Jackson, and Prairie (Figure 7A). In the southern region of the state, multiple resistance was detected in Ashley and Chicot counties. Junglerice was distributed evenly throughout the rice-producing regions of Arkansas, with the occurrence of multiple resistance following a similar distribution as the whole collection (Figure 7B). Higher frequencies of multiple resistance in junglerice were observed in the northeast and Grand Prairie. Barnyardgrass and rough barnyardgrass appeared to be mostly present in the northeast corner of the state, except for a few barnyardgrass observed in Ashley County. Again, the highest proportion of accessions with multiple resistance in both species was in Greene and Lawrence counties. The data represent a relatively small nonrandom sampling of Echinochloa spp. populations in the state of Arkansas; thus, data should be interpreted within these limits.
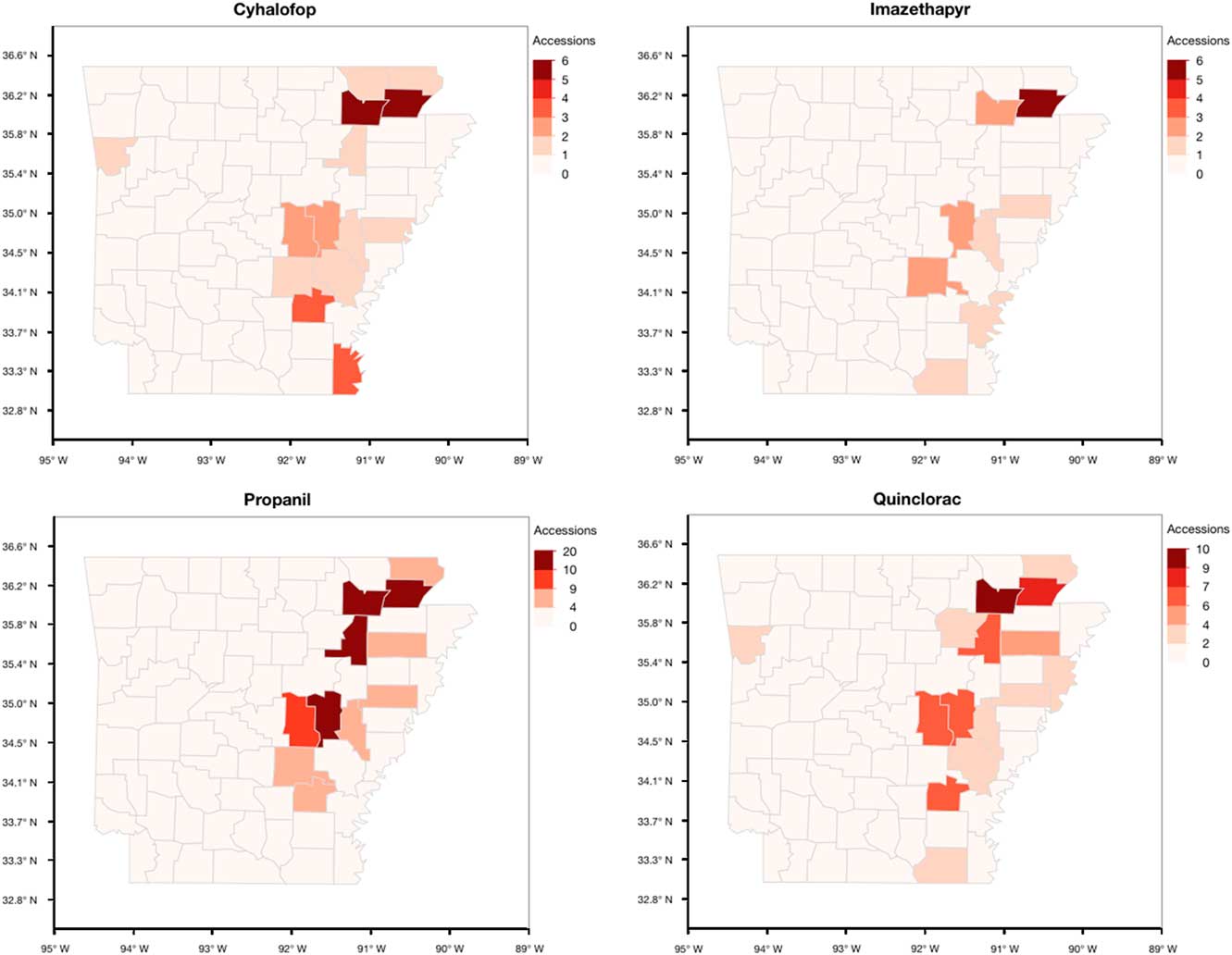
Figure 6 Arkansas maps showing the distribution of the accessions of Echinochloa spp. resistant to the four common rice herbicides tested in the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016.
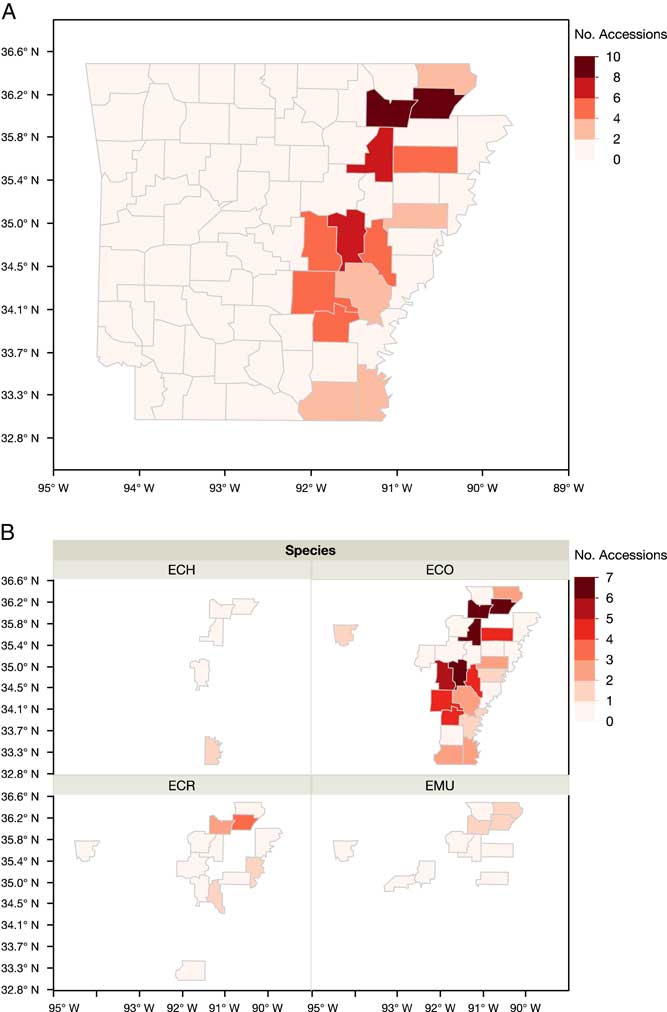
Figure 7 Arkansas maps showing the occurrence of multiple resistance from the accessions of Echinochloa spp. evaluated in the Echinochloa Herbicide Resistance Demographics Survey from 2010 to 2016. (A) Distribution of multiply resistant accessions of Echinochloa spp. (B) Distribution of the multiple resistance of the accessions by species: ECH, species not identified; ECO, junglerice; ECR, barnyardgrass; EMU, rough barnyardgrass.
For the four herbicides, the accessions separated into five distinct clusters (Table 4). Within each herbicide, the clusters were tabulated from lowest to highest mean injury. With respect to propanil, the majority of accessions (55%) fell into Clusters 1 to 3, in which the average injury of survivors ranged from 4% to 51%. Cluster 1 included accessions with 21% survivors but with negligible injury from the field use rate of propanil. Cluster 2 had the highest frequency of survivors (83%), which also had barely perceptible injury. This cluster was highly resistant to the field use rate of propanil. Twenty-six percent of accessions belonged to Cluster 3, which was characterized by having few survivors (4%) that incurred substantial (50%) injury. Low frequency of resistant plants in a population usually indicates an early phase of selection (Salas et al. 2016). This indicates continuing evolution of resistance to propanil because it is still being used in combination with other herbicide modes of action. Propanil resistance was first reported in 1994 among populations evaluated between 1991 and 1992 in Poinsett County, AR (Baltazar and Smith Reference Baltazar and Smith1994). Following this initial discovery, the first statewide survey revealed 16 counties with at least one propanil-resistant population (Carey et al. Reference Carey, Hoagland and Talbert1995). In 20 yr since this initial description, the evolution of resistance to propanil has occurred in 28 counties within Arkansas.
Table 4 Cluster analysis summary for the four common rice herbicides evaluated in the Echinochloa Herbicide Resistance Demographics Survey 2010 to 2016.
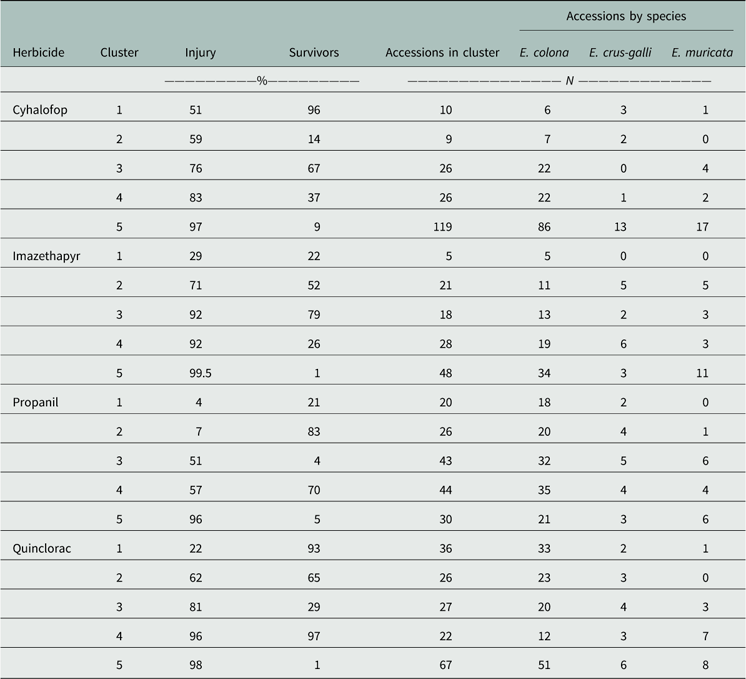
Treatment with a field use rate of quinclorac placed the largest group of accessions (67/178) in Cluster 5 (Table 4). This was the susceptible group, with few survivors (3%) and high injury (97%). Accessions in Cluster 4 (22/178) were still susceptible, as indicated by having high frequency of live plants (97%) at 21 DAT, but with high injury (96%). Cluster 1 contained the most resistant accessions, with 93% of plants remaining, but with 22% injury. Resistance to quinclorac in Arkansas was first characterized from a single population collected in 1999 from Craighead County (Lovelace et al. Reference Lovelace, Talbert, Hoagland and Scherder2007; Malik et al. Reference Malik, Burgos and Talbert2010). Accessions from 22 counties were confirmed resistant to quinclorac, this being the second most common resistance problem in Arkansas. The high frequency of accessions with multiple resistance to propanil and quinclorac is a concern, but not unexpected. These surveys indicated that 127 accessions were resistant to both herbicides and, while propanil resistance was high, there were more accessions resistant only to propanil than there were accessions resistant only to quinclorac. The historic use of these herbicides has undoubtedly resulted in the evolution of multiple-resistant populations (Talbert and Burgos Reference Talbert and Burgos2007). Based on the current literature, the mechanisms of resistance to each herbicide in barnyardgrass or junglerice appear to be independent, with propanil resistance being metabolism based and quinclorac resistance being yet undetermined (Carey et al. Reference Carey, Hoagland and Talbert1997; Lovelace et al. Reference Lovelace, Talbert, Hoagland and Scherder2007). However, new technologies have arisen since the early characterization of these populations, allowing for better investigation into the molecular basis of resistance. Hence, more research is needed to elucidate the causal mechanisms.
The activity of cyhalofop, overall, was lower than for most other herbicides, because cyhalofop is comparatively weaker, or inconsistent, on Echinochloa than propanil or quinclorac. One issue often noted by university extension personnel, and documented by Jha et al. (Reference Jha, Norsworthy and Scott2010), is the poor activity of cyhalofop under adverse environmental conditions. Pre-flood applications generally result in poor control (<50%). Cluster 5 was the largest group (119/190) composed of the most susceptible accessions (9% survivors, 97% injury). Both surveys detected low resistance frequency to cyhalofop, but the occurrence of several survivors from 10% of accessions (Clusters 1 and 2) is a concern. Cyhalofop-resistant Echinochloa spp. had not been reported previously in the state of Arkansas. The data indicate that it is an increasing problem in the rice-producing regions, having been confirmed in 13 counties.
Echinochloa species responded similarly to imazethapyr as they did to cyhalofop, with the majority of accessions falling into Cluster 5, which showed fewer survivors that were highly injured. Imazethapyr-resistant accessions with a high number of survivors in Cluster 1 were less frequent, indicating that this herbicide is still effective in most fields. Echinochloa populations with cross-resistance to ALS herbicides in Arkansas were first reported in 2012 from Greene and Prairie counties (Riar et al. Reference Riar, Norsworthy, Bond, Bararpour, Wilson and Scott2012). Accessions evaluated in both surveys exhibited single or cross-resistance to ALS-inhibiting herbicides; 114 accessions across 20 counties were confirmed with ALS resistance in Arkansas. The evolution of resistance to imazethapyr coincided with the adoption and use of Clearfield® technology in rice. Peak Clearfield® rice production occurred in 2011 with approximately 70% of production hectarage in Clearfield® production, which declined by 5% each subsequent year (Hardke Reference Hardke2016). Before 2011, less than 10% of Arkansas Echinochloa submitted for testing were classified as resistant to one or both ALS herbicides. From 2013 to 2016, more than 20% were identified with resistance to one of these two herbicides. In the Resistance Demographics Survey, most of the imazethapyr-resistant accessions were among those collected in 2011 and 2012. It is possible that in these years, selection of fields to accession was biased toward those with a history of ALS herbicide use in anticipation of resistance evolution in these fields.
Multiple-resistance evolution may occur via simple accumulation of independent target-site (TSR) or non–target site resistance (NTSR) mechanisms as exemplified by the occurrence of Echinochloa spp. with multiple NTSR mechanisms to propanil and quinclorac (Malik et al. Reference Malik, Burgos and Talbert2010). During field sampling in 2001 and 2002, Malik et al. (Reference Malik, Burgos and Talbert2010) reported that 76% of farmers in the counties where fields were sampled had been using propanil for more than 20 yr and quinclorac for around 5 yr. In that sampling period, two Echinochloa samples were confirmed to be multiply resistant to propanil and quinclorac. Resistance to propanil was documented in the early 1990s (Carey et al. Reference Carey, Hoagland and Talbert1995) and is due to enhanced detoxification by arylacylamidase (Carey et al. Reference Carey, Hoagland and Talbert1997). Many populations were already resistant to propanil when farmers started using quinclorac. Resistance to quinclorac is due to enhanced activity of another enzyme, β-cyanoalanine synthase (β-CAS) as observed by Burgos et al. in E. colona (MC Batoy, NRB, CER, unpublished data) and Yasour et al. (Reference Yasuor, Milan, Eckert and Fischer2011) in E. phyllopogon. However, induction of β-CAS accounted only for low-level resistance to quinclorac; extremely high resistance is facilitated by other cytochrome P450 enzymes (Yasour et al. Reference Yasuor, Milan, Eckert and Fischer2011) or other mechanisms yet unknown. Because resistance to quinclorac did not evolve until after about 8 yr of use (Talbert and Burgos Reference Talbert and Burgos2007), resistance to quinclorac is independent from resistance to propanil. However, multiple resistance may also occur if the resistance mechanism to the first selector is mediated by NTSR genes, which endow broad resistance to abiotic stressors, including herbicides. If this was the case for the resistance mechanism to propanil, then quinclorac would not have been effective on the propanil-resistant populations from the beginning. Similarly, multiple resistance involving TSR+NTSR mechanisms can occur via successive or simultaneous selection. Another means of acquiring stacked resistance traits is via gene flow. This occurs quickly and is a major avenue for spread of resistance.
A predictive model was developed to estimate the potential time frame for resistance evolution to occur among Arkansas Echinochloa populations, given the increased adoption of ALS herbicides (Group 2) with Clearfield® rice and the use of acetyl-CoA carboxylase (ACCase) herbicides (Group 1), including cyhalofop (Bagavathiannan et al. Reference Bagavathiannan, Norsworthy, Smith and Neve2014). The assumption was that resistance to each group would be by a different mechanism. With the parameters used, the model predicted multiple resistance to ACCase (Group 1) and ALS (Group 2) herbicides by year 16 of adoption. Given that Clearfield® rice was commercialized in 2002 and has since been widely adopted, the surveys showed that multiple resistance to ACCase and ALS herbicides occurred several years earlier than the model predicted. While multiple resistance to ALS and ACCase inhibitors was identified in this research, it often occurred with other resistance traits and represented <1% of the total accessions evaluated. Coevolution of resistance to ALS and ACCase herbicides in barnyardgrass was documented by Panozzo et al. (Reference Panozzo, Scarabel, Tranel and Sattin2013) in rice production, where multiple resistance to both herbicides was noted in low frequencies. The populations resistant to the ACCase herbicides showed low-level resistance, indicating a non–target site, polygenic mechanism, which was not included in the model by Bagavathiannan et al. (Reference Bagavathiannan, Norsworthy, Smith and Neve2014). Given the criterion of the surveys at 70% injury as an indicator of resistance, it is possible that some accessions with low-level resistance to ACCase herbicides were excluded from the analysis. This evolutionary process also has been characterized in Australian populations of rigid ryegrass (Lolium rigidum Gaudin), in which resistance to as many as three modes of action were endowed by similar xenobiotic-detoxifying enzymes (Owen et al. Reference Owen, Walsh, Llewellyn and Powles2007; Preston et al. Reference Preston, Tardif, Christopher and Powles1996). A multiple-resistant population of prostrate pigweed (Amaranthus blitoides S. Wats.) has a mutation in two herbicide target sites, one in the ALS gene and one in the psbA gene for the photosystem I complex, endowing resistance to ALS-inhibiting herbicides and atrazine, respectively (Sibony and Rubin Reference Sibony and Rubin2003). The selection of these mutations in two target sites could occur simultaneously if both herbicides are used sequentially in a cropping season or in tank mixes. More research needs to be done to understand the process of coevolution of resistance traits, because it poses a much larger threat to crop production than independent evolution of single resistance traits.
Herbicide resistance frequency and distribution provides insight into management of problematic weed species and the evolution of resistance within a species. This research presents the trend in resistance evolution to multiple herbicides and characterization of multiple resistance in Arkansas Echinochloa populations. The Weed Science Society of America has outlined best management practices that focus on reducing the evolution of resistance and recommend effective strategies for improving sustainable weed control (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). Among these recommendations is the use of alternate effective modes of action to extend the efficacy of herbicides and reduce or delay the evolution of resistance. Given that Arkansas rice producers have at least five modes of action to integrate in weed management programs, the potential for herbicide resistance evolution should be minimized. However, Echinochloa spp. in Arkansas have evolved resistance to all major herbicides and modes of action currently used in rice production. The distribution of resistance is widespread and appears to be concentrated heavily in the northeast and Grand Prairie regions of the state, which have been the leading rice production areas. Given the presence of single, multiple, and cross-resistance, growers can still manage problematic species by using a combination of herbicides and increasing rotation to other crops such as soybean. While this research provides information on the status of resistance, it provides no information on the genetic or physiological mechanisms that endow resistance. Further research is required to improve our understanding of the underlying mechanisms that allow Echinochloa spp. to adapt to diverse abiotic stressors such as herbicide application.
Acknowledgments
The authors thank the county extension personnel, independent consultants, and rice farmers who submitted samples or facilitated collecting samples. Specifically, the authors also thank: Seth Abugho, Mohammed Bararpour, Mariccor Batoy, Caroline Bevilacqua, Leopoldo “Jun” Estorninos, Fernando Martini, Jeremy Green, Kevin Mills, Clark Moore, Travis Jones, Nicholas Korres, Teal Penka, Ana Carolina Roso, Reiofeli Salas-Perez, Vijay Singh, Shilpa Singh, and Hussain Tahir. Funding for this research was provided by the Arkansas Rice Research and Promotion Board, BASF Corporation, and Bayer Crop Science.













