Introduction
Salmonellosis is an infection caused by Salmonella species. Humans with salmonellosis usually develop gastroenteritis with symptoms such as nausea, vomiting, diarrhoea and abdominal pain and sometimes have bacteremia [Reference Chen1, Reference Dhanoa and Fatt2]. Generally, salmonellosis is a self-limited illness lasting for 4–7 days. However, the elderly, infants and immunocompromised patients may have more severe symptoms that require hospitalisation and occasionally even lead to death [Reference Eng3]. An estimated 93.8 million cases of salmonellosis with 155,000 deaths occurred worldwide each year [Reference Majowicz4]. The high disease burden of salmonellosis raises public health concerns globally.
People can be infected with Salmonella by ingesting contaminated foods or water or through contact with infected animals [Reference Heymann, Barton Behravesh and Griffin5–Reference Marus7]. The primary transmission route of salmonellosis is the consumption of Salmonella-contaminated foods. A previous study estimated that approximately 80.3 million cases of salmonellosis globally were foodborne [Reference Majowicz4]. Salmonella contamination can occur at any point on the farm-to-plate continuum, during production, processing, handling and storage of foods. Foods such as eggs, milk products, poultry and meat products, vegetables, seafood and even spices have been identified as vehicles in Salmonella outbreaks [Reference Inns8–Reference Venkat14].
In Taiwan, Salmonella species are the third most common bacteria, accounting for 19% (97/500) of bacteria-related foodborne outbreaks from 2013 to 2018, following Vibrio parahaemolyticus (29%; 147/500) and Staphylococcus aureus (28%; 141/500) [15]. In some of these salmonellosis outbreaks, investigations identified foods containing eggs as risk factors; in most, however, the vehicles and contamination routes remained unknown [Reference Lu16–Reference Lee19].
On 27 April 2018, 19 ill persons associated with a foodborne outbreak in southern Taiwan were reported to the Taiwan Centers for Disease Control (TCDC). Of these ill persons, 12 were hospitalised and one (a 24-year-old male) died. A preliminary investigation by a local health authority found that all of the ill persons had eaten at a local restaurant. The Taiwan Field Epidemiology Training Program of the TCDC initiated an investigation on 1 May 2018 to identify the infection sources and contamination routes and to recommend preventive measures.
Methods
Our investigation sought epidemiological, food and environmental, and microbiological data.
Epidemiological investigations
We conducted an unmatched case-control study to determine whether certain food items were associated with disease occurrence. We defined a case-patient as a customer who developed diarrhoea within 72 h after eating at the restaurant during 16–27 April 2018. We defined a control as a customer who did not develop any symptoms within 72 h after eating at the restaurant during that same period.
Despite the notifications of ill persons from hospitals and the Institute of Forensic Medicine, we conducted active case finding to discover possible outbreak-related patients who were diagnosed with acute gastroenteritis (AGE) during 18–27 April 2018 at the emergency departments of local hospitals, by selecting for ICD-10 codes 009.0, 9.1, 9.2, 787.01, 787.02, 787.03, 787.3 and 787.91. Local health authorities set up a hotline for customer self-reports and we obtained the list as part of our active case finding. According to the Communicable Disease Control Act [20], the public shall cooperate with competent authorities and follow disease control measures. Therefore, we required the restaurant to provide customers' contact information which was left on the orders for meal reservation during the outbreak period. We also got the consent of these customers before further interviews. We gathered data on demographics, consumption of foods and drinks, and clinical symptoms by telephone interviews using a standard questionnaire.
We conducted a univariate analysis to compare data on demographics and food exposure between case-patients and controls. Continuous variables were analysed with the Mann–Whitney U test and a P value <0.05 was considered statistically significant. Categorical variables were analysed with a chi-square test or Fisher's exact test and odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. Data were analysed with Epi-InfoTM version 7.1.
Food and environmental investigations
On 27 April 2018, local health authorities collected two food items – soy milk tea and a vegetable salad sandwich – that were consumed by the decedent before disease onset. The authorities also collected environmental samples, including water and swabs of the cooking counter, chopping board, egg crates and utensil surfaces, on 1 May 2018. The food and environmental samples were examined for enteric bacteria, norovirus and rotavirus at the Taiwan Food and Drug Administration (TFDA) laboratory. We conducted a walk-through inspection of the restaurant on 1 May 2018 to assess environmental sanitation and food hygiene practices, as prescribed in the Good Hygiene Practice (GHP) guidelines [21].
Once food items were identified to be risk factors associated with the outbreak, on the basis of our epidemiological findings, a trace-back investigation for the implicated food items was performed. With the owner's permission, one investigator reviewed the in-store surveillance camera recordings to inspect the processing of the implicated food items during the period of 16–22 April 2018. On 23 May we interviewed food handlers about symptoms before the outbreak, assignment of work and food processing.
Microbiological investigation
Stool specimens, rectal swabs, or blood samples of case-patients who sought medical care were collected and tested for Salmonella species in the hospital laboratory. The local health authorities collected further stool specimens or rectal swabs from food handlers and some case-patients who were identified via the active case finding and these were tested for foodborne pathogens. The Institute of Forensic Medicine conducted an autopsy to obtain clinical specimens from the decedent, including throat swabs and swab samples of gastric contents, large intestine, small intestine, liver, pericardial fluid, heart, lung, brain and cerebrospinal fluid for bacterial culture. The pericardial fluid and cerebrospinal fluid were tested by both aerobic and anaerobic culture in blood culture bottles. Specimens from food handlers, some case-patients and the decedent were examined for enteric bacteria at TCDC. Norovirus and rotavirus examinations were conducted only on the specimens from food handlers and some case-patients. The testing for enteric bacteria sought species including Salmonella, Vibrio cholerae, Shigella, Staphylococcus aureus, Vibrio parahaemolyticus, enterohemorrhagic Escherichia coli and Bacillus cereus.
Pulsed-field gel electrophoresis and whole-genome sequencing
Once Salmonella isolates were identified in samples, they were submitted for pulsed-field gel electrophoresis (PFGE) and whole-genome sequencing at the TCDC laboratory. Salmonella isolates were characterised with the use of the standardised PulseNet PFGE protocol [Reference Ribot22] and whole-genome sequencing (WGS) method (Illumina MiSeq platform). We analysed PFGE patterns and constructed single linkage trees with PFGE patterns and whole-genome single nucleotide polymorphism (wgSNP) profiles to assess genetic relatedness among bacterial isolates, using the tools provided in BioNumerics version 7.6.3 (Applied Maths).
Results
Epidemiological investigations
Of 202 persons identified from the notification system and active case finding, 30 could not be contacted and 74 ill persons did not eat at the restaurant before disease onset. Complete data were available from 92 of 98 persons, of which 47 case-patients (including 16 who tested positive for Salmonella species) and 44 controls were included in our case-control study (Fig. 1). The remaining one person reported only respiratory symptoms and was excluded from the analysis. The onset date for case-patients ranged from 18 to 26 April 2018 and peaked on 21 April (Fig. 2).
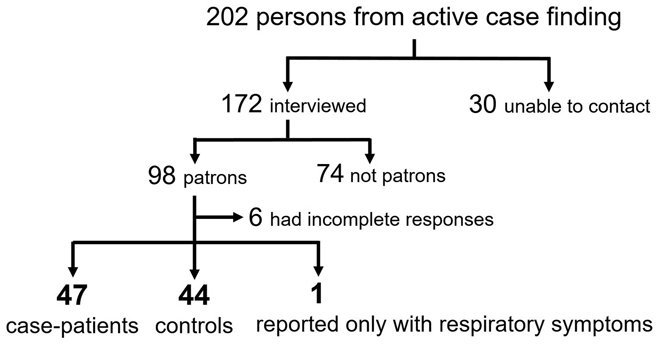
Fig. 1. A flow chart showing identification of case-patients and controls in a salmonellosis outbreak associated with a restaurant during 16–27 April 2018 in Taiwan.

Fig. 2. Case-patients of salmonellosis outbreak in a restaurant by date of onset during 16–27 April 2018 in Taiwan (n = 47).
The median age was 30 years (range, 1–68) for case-patients, compared with 30.5 years (range, 6 to 70) for controls (P = 0.60). Males accounted for 32 (68%) of case-patients, comparing with 25 (57%) of controls (P = 0.27). The main symptoms of case-patients were diarrhoea (n = 47; 100%), fever (n = 32; 68%) and abdominal pain (n = 31; 66%). The median incubation period was 10.5 h (range, 0.5 to 52.5 h). Of 47 case-patients, 14 (32%) were hospitalised and one died. The median time from onset of case-patients to symptom relief was 135.2 h (range, 12.5–288.5 h).
The decedent is a 24-year-old male without underlying diseases. He ate in this restaurant on the evening of 21 April 2018. He had abdominal pain and diarrhoea the next morning and was found dead 2 days later. The results of the autopsy and microbiological examination demonstrated Salmonella bacteremia and the causes of death were metabolic failure and septic shock.
Among 70 food items consumed by case-patients and controls, only the French toast sandwich was significantly associated with illness (OR: 102.4; 95% CI: 18.7–952.3) (Table 1). All other associations between 69 food items and illness were not statistically significant.
Table 1. Univariate analysis results for food items consumed by case-patients (n = 47) and controls (n = 44) at a restaurant associated with a salmonellosis outbreak during 16–27 April 2018 in Taiwan

Note: This table lists only the top two or three food items of each category consumed by case-patients and controls.
Environmental and food investigation
The restaurant operations were suspended by the local health authorities on 27 April 2018. Because the restaurant was cleaned after suspension and no historical food safety inspection record was available, we were unable to assess actual sanitation and food processing conditions in the restaurant when conducting a walk-through inspection on 1 May 2018.
According to the food handlers, the ingredients of the French toast sandwich included an egg mixture, mayonnaise, cucumber slices, ham, pork floss, bread and pan-fried egg. Food handlers prepared the egg mixture daily by breaking and mixing eggs in a bowl. They assembled the ingredients in the bread, dipped the sandwich into the egg mixture, fried it on a heated griddle and put a pan-fried egg in it. After the bread turned golden, a piece of ham was put on the outside of the sandwich. The French toast sandwich could be made-to-order or were ready to serve.
We identified several lapses in the food preparation when we reviewed the surveillance camera recordings. They revealed that eggshells were dropped into the mixing bowl and then retrieved by the food handlers during the egg mixture preparation. The recordings also showed that the mixing bowl was not washed during operation hours. The egg mixture was stored at room temperature for 18 h after preparation. Moreover, food handlers reserved the leftover egg mixture and continuously used it for 3 days. In addition, the recordings also showed that raw eggs, raw ingredients and cooked food items had been placed on the same cooking counter. Food handlers cooked foods right after handling raw ingredients, without washing their hands. These findings violated the GHP guidelines of TFDA due to the possibility of cross-contamination.
From 17 to 21 April 2018 we randomly selected 179 servings of the French toast sandwich and calculated the cooking time on the heated griddle. The median cooking time was 80 s (range, 42–255 s). We found that the food handlers did not use a probe food thermometer to ensure internal temperature for each servings, though this is recommended by TFDA. Therefore, we were not sure whether the temperature was high enough to inactivate Salmonella. This could leave the contents of the French toast sandwich undercooked and raise contamination risk.
Food items and environmental samples all tested negative for Salmonella species, other enteric bacteria species, norovirus and rotavirus. The food items also tested negative for Staphylococcus aureus enterotoxin. The implicated French toast sandwiches and egg mixture were discarded before the investigation and not available for microbiological testing.
The trace-back investigation of eggs was conducted on 8 May 2018; none of the same batches of eggs was left and laying hens were all culled. We randomly sampled 20 fresh eggs, 20 washed eggs and 20 cloacal swabs of laying hens from other flocks for Salmonella testing and all were negative.
Microbiological investigation
Of 47 case-patients, samples from 17 were tested. Salmonella species were isolated from 16 case-patients (of whom 12 ate a French toast sandwich), including the decedent. Among the 16 confirmed case-patients, one was also positive for norovirus and another one was also positive for Bacillus cereus. Thirteen specimens from the decedent, including liver, heart, small intestine and cerebrospinal fluid, were positive for Salmonella species. All of the nine food handlers had no symptoms and their specimens were negative for enteric bacteria, norovirus and rotavirus.
Pulsed-field gel electrophoresis and whole-genome sequencing
The Salmonella isolates were identified to be Salmonella enterica serotype Enteritidis. All the isolates shared a common PFGE pattern, SEX.010 (Fig. 3). SEX.010 was the predominant PFGE pattern for S. Enteritidis; it accounted for 70.2% (372/530) of the S. Enteritidis isolates recovered in Taiwan in 2018 (unpublished data). Therefore, we further compared the whole-genome single nucleotide polymorphism (wgSNP) profiles of six isolates from the outbreak and 21 from the previous collection from 2006 to 2018. The wgSNP tree indicated that the six isolates obtained in this outbreak had an identical wgSNP profile but were distinct from the 21 collected from 2006 to 2018 (Fig. 4).

Fig. 3. Pulse-field gel electrophoresis (PFGE) patterns for Salmonella isolates collected from a restaurant associated with a salmonellosis outbreak in Taiwan during 16–27 April 2018. Note: These isolates included 13 from the decedent, two from a case-patient and one from an ill person who was positive for Salmonella species but not included in our case-control study because of incomplete interview responses.

Fig. 4. A single linkage tree constructed with whole-genome single nucleotide polymorphism (wgSNP) profiles for Salmonella enterica serotype Enteritidis isolates with PFGE pattern SEX.010. Six S. Enteritidis isolates were collected in this outbreak and 21 were obtained from 2006 to 2018. The six isolates recovered from this outbreak are marked in yellow and asterisks indicate two isolates from the decedent.
Discussion
In this foodborne salmonellosis outbreak, S. Enteritidis isolates from case-patients shared a common PFGE pattern or an identical wgSNP fingerprint, indicating that it should be the aetiological agent. The result of the case-control study suggested that the French toast sandwich was the most likely vehicle of infection. Eggshell contamination, the long holding time of the egg mixture at room temperature and continuous use of the leftover egg mixture during the French toast sandwich preparation might have contributed to this outbreak.
In this investigation, we could not demonstrate the presence of S. Enteritidis in samples from foods, the environment and food handlers. Previous studies have suggested that S. Enteritidis is usually linked to the use of chicken meat, raw eggs, or egg products in foodborne salmonellosis outbreaks [Reference Jackson23, Reference Braden24]. S. Enteritidis could contaminate eggs during their formation in the reproductive tract or organs of laying hens; it could also penetrate eggshells via contaminated faeces or bedding materials [Reference Gantois25, Reference Whiley and Ross26]. Therefore, the egg mixture used in the French toast sandwich might have been contaminated by S. Enteritidis on the eggshell or in the egg contents. Once contamination occurred, the prolonged holding time of the egg mixture at room temperature could make S. Enteritidis proliferate [Reference Sakha and Fujikawa27]. The continuous use of leftover egg mixture could further lead S. Enteritidis to persist and raise infection risk during the preparation and serving of implicated food. These risk factors have been noted in previous studies [Reference Lee28, Reference Gormley29].
Among 16 case-patients who tested positive for S. Enteritidis, four did not eat a French toast sandwich. Foodborne salmonellosis outbreaks associated with restaurants sometimes have involved more than one vehicle in causing illness [Reference Gaulin30, Reference Liu31]. The violations identified via surveillance camera recordings strongly suggested that cross-contamination occurred in this restaurant. Surveillance camera or closed-circuit television had been used in disease outbreak investigations [Reference Diskin32, Reference Ki33]. Public health professionals checked the recordings to trace movements of case-patients, identify contacts and assess transmission risks. In our study, we used it to identify the possible causes of contamination and transmission routes during food preparation in the restaurant. This technique may provide investigators, especially for those involved in foodborne outbreaks, another chance to find more clues when lacking food safety inspection and microbiological evidences.
The incubation period of salmonellosis is usually 6–72 h [Reference Chai34]. We found several case-patients had their incubation period less than 6 h and the shortest one was 0.5 h. The incubation period could be determined by the infective dose and the host's condition. However, there is the possibility of co-infection of other enteric pathogens which have a short incubation period. For example, there were two case-patients in this outbreak who also tested positive for Bacillus cereus or norovirus in addition to S. Enteritidis. The short incubation could also be due to recall bias of eating and onset time for case-patients.
WGS is increasingly applied in foodborne outbreak investigations for tracing the source of infection [Reference Inns8, Reference Mohammed35, Reference Thompson36]. Studies showed that WGS could provide higher discriminatory power (to distinguish closely related strains) than traditional typing methods such as PFGE and multiple-locus variable-number tandem repeat analysis (MLVA) [Reference Gaulin30, Reference Hopkins37, Reference Simon38]. The PFGE pattern in this outbreak has been common in Taiwan in recent years. To confirm the outbreak isolates were of a common source, we performed WGS to compare the outbreak isolates with some epidemiologically unrelated isolates of the same PFGE pattern. Our study indicated that the WGS-based genotyping method can strengthen the findings of the investigation by providing an excellent discriminatory power in discerning the outbreak isolates from the epidemiologically unrelated isolates.
Limitations were noted in our investigation. First, we could not prove our suspicion about the source of infection due to the lack of microbiological evidence. However, the epidemiological investigation provided evidence of the association between the French toast sandwich and disease occurrence. Second, the delay between outbreak notification and investigation hindered sampling of foods, the egg mixture and the environment. This may explain the negative results of testing. Because of the delay, we also could not observe the actual food preparation to find the cause of this outbreak. Nevertheless, with the assistance of surveillance camera recordings, the lapses during food preparation and poor sanitation in the restaurant were identified. This complementary evidence facilitated our investigation. Third, we mostly recruited customers who left contact information in the restaurant for a telephone interview. Comparing with customers without contact information, customers with contact information in the restaurant were more likely to order takeaway for breakfast. These might have led to selection bias and underestimation of the magnitude of the outbreak. To reduce the possible effects of selection bias, we also recruited customers from other sources by active case finding, including interviewees' referrals, self-reporting customers and patients notified in hospitals. Fourth, because the investigation was conducted 2 weeks after the outbreak, we could not rule out information bias due to the retrospective nature of the study. Despite these limitations, our investigation presented substantial evidence of traditional epidemiological findings, supported by environmental and analytical results.
In conclusion, we investigated a foodborne salmonellosis outbreak associated with the consumption of a French toast sandwich. The outbreak might be attributed to an undercooked egg mixture or contaminated eggs. To prevent similar outbreaks in the future, we recommend that restaurants use pasteurised egg mixture for raw or lightly cooked egg dishes. Otherwise, restaurants should use a food thermometer to ensure adequate cooking temperature with appropriate time (e.g. 74 °C for 15 s) and an internal temperature over 71 °C for egg dishes, to inactivate Salmonella. Also, restaurants should avoid holding egg mixtures at room temperature for over 2 h before cooking and should avoid continous use of the leftover egg mixture. Food handlers should be strictly adherent to GHP guidelines, including separating raw ingredients and cooked foods in different areas, ensuring appropriate storage temperatures according to recommendations for each food and maintaining good personal hygiene during food processing.
Acknowledgements
The authors gratefully acknowledge the contributions of the Chiayi County Health Bureau and Chiayi City Health Bureau for epidemiological investigation. The authors thank the Council of Agriculture, Public Health Bureau of Yunlin County, Animal and Plant Disease Control Center of Yunlin County and Animal Technology Laboratories, Agricultural Technology Research Institute, for their efforts in the egg trace-back investigation. The authors also thank the Institute of Forensic Medicine of the Ministry of Justice for the autopsy of the decedent and Taiwan Food and Drug Administration for the microbiological investigation of food items.
Conflict of interest
The authors declare no conflicts of interest.
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the Taiwan Centers for Disease Control.







