Vitamin D is essential to maintain bone health, playing a key role in bone mineralisation(Reference Suda, Ueno and Fujii1), with severe vitamin D deficiency in children resulting in rickets(Reference Hutchison and Bell2). As stores of vitamin D in newborns are dependent on maternal vitamin D status(Reference Hollis and Pittard3), vitamin D deficiency during pregnancy leads to infant vitamin D deficiency and thus to increased risk of rickets(Reference Dawodu, Agarwal and Sankarankutty4). More recent evidence suggests that in addition to causing poor mineralisation of the skeleton, vitamin D insufficiency is linked to other non-skeletal health outcomes(Reference Holick5, Reference Pasco, Wark and Carlin6).
25-Hydroxyvitamin D (25(OH)D) is the storage form of vitamin D and circulating plasma concentrations of 25(OH)D are an indicator of vitamin D status. There is a lack of consensus on definitions regarding adequate vitamin D status and various cut-off levels have been used to define levels of deficiency: severe deficiency < 12·5 nmol/l(Reference Lips7); deficiency < 25 nmol/l(8); and insufficiency < 50 nmol/l(Reference Lips7, Reference Holick9), or < 80 nmol/l(Reference Hollis, Wagner and Drezner10). However, even using the most conservative estimates, evidence shows that vitamin D deficiency and insufficiency are common worldwide, particularly in northern latitudes(Reference Holick9). Given the additional demands on maternal stores during pregnancy(Reference Bowyer, Catling-Paull and Diamond11), it is not surprising that low vitamin D status has been reported among pregnant women in both America and Australia(Reference Bowyer, Catling-Paull and Diamond11, Reference Bodnar, Simhan and Powers12).
The major source of vitamin D is cutaneous synthesis following sunlight exposure (UV B irradiation). Several factors can affect the synthesis of vitamin D, including use of sunscreen, age, skin pigmentation, clothing, melanin concentration, latitude, climate type and season(Reference Webb13). In high northern latitude countries, there is a marked seasonal variation in vitamin D status throughout the year owing to a seasonal variation in UV B intensity. Indeed, during winter, the population relies on body stores and dietary vitamin D to maintain status. Vitamin D is found in small quantities in a limited number of foods such as oily fish, eggs and liver, and in fortified foods such as margarine, breakfast cereals and powdered milk(Reference Holden, Lemar and Exler14). However, pregnant women are advised to avoid liver and liver products, raw or under-cooked eggs and to limit their intake of certain fish such as tuna, thus restricting natural food sources of vitamin D(15).
There is a lack of consensus regarding the need for vitamin D supplementation during pregnancy. In the UK, the Food Standards Agency recommends that pregnant women should take supplements containing 10 μg vitamin D/d(15), while the National Institute for Health and Clinical Excellence (NICE) recommends that pregnant women should be advised about the importance of adequate vitamin D stores but falls short of recommending supplementation(16). In the USA and Canada, the adequate intake for the general population aged 0–50 years, including pregnant women, is set at 5 μg vitamin D/d(17), whilst in the Netherlands, the adequate intake for pregnant women is set at either 7·5 or 10 μg vitamin D/d, dependent on sunlight exposure(18). However, in recent years, it has been suggested that the recommended intakes for adults should be much higher than current recommendations(Reference Vieth, Bischoff-Ferrari and Boucher19).
Given the importance of adequate vitamin D status, the aim of the current study was to investigate vitamin D status in healthy Caucasian pregnant women living at 54–55°N, and to compare vitamin D status with a group of age-matched non-pregnant controls, sampled concurrently to control for seasonal variation.
Subjects and methods
Patients and procedures
The Research Ethics Committee of the University of Ulster (UUREC/99/46) approved the study, and informed consent was obtained from each participant upon recruitment. Participating in this longitudinal study were 120 healthy pregnant women and forty-one female control subjects, matched for age and BMI at recruitment, at 54–55°N as previously described(Reference Holmes, Wallace and Gilmore20). Briefly, ‘low risk’ (otherwise healthy) women attending antenatal clinics at the Royal Jubilee Maternity Hospital, Belfast were recruited on to the study during their first antenatal visit at approximately 12 weeks of gestation. Age-matched, non-pregnant women were recruited on to the study at the same time from healthcare workers and university employees to act as control subjects.
Pregnant women had blood samples taken at 12, 20 and 35 weeks of gestation and non-pregnant control subjects were sampled concurrently in order to control for any seasonal variation. Project resources allowed sample collection and analysis in a randomly selected subgroup at 3 d post-partum (pregnant, n 21; non-pregnant, n 24). All samples were collected between January and October. At each visit, samples were obtained by venepuncture from the antecubital vein, into a K3 EDTA anticoagulant tube. Within 3 h of collection, blood samples were centrifuged at 1400 g for 15 min at room temperature. Separated plasma was stored at − 70°C until analysis.
Samples were batch analysed after the study was completed. Vitamin D status was assessed by quantitatively measuring plasma 25(OH)D using OCTEIA 25(OH)D ELISA (Immunodiagnostic Systems Ltd, Boldon, Tyne & Wear, UK), following the manufacturer's instructions. The sensitivity of the ELISA is 5 nmol/l and the intra- and inter-assay CV was 3·37 and 3·89 % respectively.
For the analysis, we classified women into groups that defined vitamin D status: severe vitamin D deficiency: 25(OH)D < 12·5 nmol/l(Reference Lips7); vitamin D deficiency: 25(OH)D < 25 nmol/l(8); and two levels of vitamin D insufficiency: 25(OH)D < 50 nmol/l(Reference Lips7, Reference Holick9) and 25(OH)D < 80 nmol/l(Reference Hollis, Wagner and Drezner10).
Season of sample collection was defined as winter (December, January, February), spring (March, April, May), summer (June, July, August) and autumn (September, October, November). Sampling at the 12-week time-point occurred mostly in winter with the remainder occurring in spring. The majority of 20-week sampling therefore occurred in spring with only 1 % occurring in winter. Sampling at the 35-week time-point occurred solely in summer.
Patient records were checked in the post-natal period to confirm that all pregnancies remained uncomplicated. Anthropometric data on cases and control subjects were obtained upon recruitment. BMI (weight (kg)/height (m)2) was based on weight and height as measured at 12 weeks gestation. All study participants were asked about their use of vitamin and mineral supplements at each time-point, and any multivitamin supplementation was noted. The amount of vitamin D contained in multivitamin supplements consumed ranged from 5 to 12·5 μg.
Statistical analysis
Statistical analyses were carried out using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Results were considered as statistically significant when P < 0·05. Analyses of the data on plasma 25(OH)D concentrations revealed a skewed distribution and, consequently, values were transformed logarithmically prior to statistical analyses to approximate normal distribution, and data are presented as medians with 5th and 95th percentiles. Data which were normally distributed are presented as means and standard deviations. Differences between the study groups were assessed using independent t tests or the χ2 test as appropriate. Data were analysed for an effect of pregnancy and time by repeated-measures ANOVA using the general linear model controlling for age, BMI and reported use of multivitamin supplements. Post hoc comparisons with Bonferroni's correction were used to test for specific comparisons between time-points and between the two groups (pregnant and non-pregnant) at each time-point.
Results
Of the 120 pregnant women recruited, vitamin D measurements were available for ninety-nine pregnant women at each time-point. All women delivered healthy babies. Of the forty-one control subjects recruited, vitamin D measurements were available for thirty-eight non-pregnant women at each time-point. In parallel with twenty-one mothers sampled at 3 d post-partum, twenty-four randomly selected non-pregnant control subjects were sampled. There was no significant difference in age, BMI or proportion of smokers between pregnant women and non-pregnant control subjects at time of recruitment (Table 1).
Table 1 Characteristics of pregnant and non-pregnant women
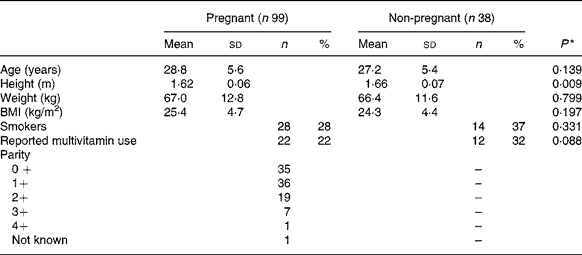
* Differences between the two groups were assessed using independent t tests or χ2 tests as appropriate.
The vitamin D status of the pregnant and non-pregnant women at each time-point, split by supplement users and non-users, is displayed in Fig. 1. Repeated-measures ANOVA showed an overall difference in plasma 25(OH)D concentrations between pregnant women and non-pregnant control subjects (P < 0·0001), and a significant effect of time (P < 0·0001). Post hoc analyses showed that plasma 25(OH)D concentrations were significantly lower in pregnant women when compared to non-pregnant women at 20 (P < 0·0001) and 35 weeks of gestation (P < 0·0001) and at 3 d post-partum (P < 0·0001). Statistical analyses showed that pregnant women who reported taking a multivitamin supplement had significantly higher 25(OH)D concentrations at 12 (P < 0·0001), 20 (P = 0·001) and 35 weeks (P = 0·001) when compared with those who did not report supplement use. There was no statistically significant effect of parity on vitamin D status (data not shown).

Fig. 1 25-Hydroxyvitamin D (25(OH)D) concentration of pregnant (□) and non-pregnant (![]() ) women at 12, 20 and 35 weeks gestation and 3 d post-partum, split by supplement users (A) and non-users (B). Boxes represent the 5th and 95th percentiles, with the median represented by the line; whiskers at the top and bottom of the box represent the highest and lowest values excluding outliers. Data were analysed for an effect of pregnancy and time by repeated ANOVA using the general linear model controlling for age and BMI. Values were significantly different from those of the non-pregnant group (post hoc comparisons with Bonferroni's correction): *P < 0·05. Values were significantly different from those of the pregnant non-users: †P < 0·001. Values were significantly different from those of the non-pregnant non-users: ‡P < 0·05. Pregnant, n 99 (supplement users, n 22; non-users, n 77) and non-pregnant, n 38 (supplement users, n 12; non-users, n 26) at each time-point except for 3 d post-partum (pregnant, n 21 (supplement users, n 5; non-users, n 16); non-pregnant, n 24 (supplement users, n 6; non-users, n 18)).
) women at 12, 20 and 35 weeks gestation and 3 d post-partum, split by supplement users (A) and non-users (B). Boxes represent the 5th and 95th percentiles, with the median represented by the line; whiskers at the top and bottom of the box represent the highest and lowest values excluding outliers. Data were analysed for an effect of pregnancy and time by repeated ANOVA using the general linear model controlling for age and BMI. Values were significantly different from those of the non-pregnant group (post hoc comparisons with Bonferroni's correction): *P < 0·05. Values were significantly different from those of the pregnant non-users: †P < 0·001. Values were significantly different from those of the non-pregnant non-users: ‡P < 0·05. Pregnant, n 99 (supplement users, n 22; non-users, n 77) and non-pregnant, n 38 (supplement users, n 12; non-users, n 26) at each time-point except for 3 d post-partum (pregnant, n 21 (supplement users, n 5; non-users, n 16); non-pregnant, n 24 (supplement users, n 6; non-users, n 18)).
The percentage of women below previously reported cut-off values for vitamin D deficiency and insufficiency are presented in Table 2(Reference Lips7–Reference Hollis, Wagner and Drezner10). Severe vitamin D deficiency (25(OH)D < 12·5 nmol/l) was not apparent in the non-pregnant controls at any time-point, however, 1–2 % of pregnant women were severely vitamin D deficient during pregnancy. When split by supplement users and non-users, severe vitamin D deficiency was only evident in those pregnant women who were non-supplement users. During summer (which corresponded to the 35-week time-point), vitamin D deficiency (25(OH)D < 25 nmol/l) was not evident among the control volunteers, however, 16 % of pregnant women were vitamin D deficient. Again the present finding was only apparent in the non-supplement users. The prevalence of vitamin D deficiency was higher among the pregnant women compared to the non-pregnant women in spring (which corresponded to the 20-week time-point) at 44 v. 18 %, and in winter (which corresponded to the 12-week time-point) at 35 v. 29 %. Supplement users were less likely to be vitamin D deficient at both these time-points in both the pregnant and non-pregnant women. Assuming a cut-off of vitamin D insufficiency of < 80 nmol/l which has been suggested previously(Reference Kovacs21), over 95 % of pregnant women (and indeed non-pregnant controls) were classified as insufficient at each time-point, irrespective of whether they reported supplement use or not.
Table 2 Vitamin D deficiency and insufficiency of pregnant and non-pregnant women, split by supplement use, using various cut-off levels* (Cumulative n values and percentages)
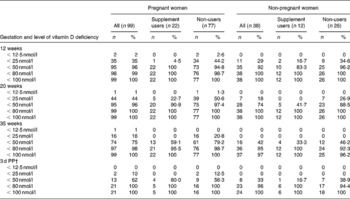
PP, post-partum.
* For details of subjects and procedures, see Subjects and methods. Data are cumulative n and cumulative %.
† Pregnant, n 21 (supplement users, n 5; non-users, n 16); non-pregnant, n 24 (supplement users, n 6; non-users, n 18).
Discussion
The present results show a high prevalence of vitamin D deficiency and insufficiency during pregnancy in apparently healthy women considered traditionally as at ‘low risk’ of vitamin D deficiency. While vitamin D status was significantly lower in the pregnant women than in the non-pregnant control population, the present results also suggest poor vitamin D status in all women of child-bearing age. Furthermore, although reported vitamin supplement use during pregnancy was associated with higher vitamin D status, it did not prevent vitamin D deficiency (25(OH)D < 25 nmol/l) during winter and spring time or insufficiency (25(OH)D < 50 nmol/l) during any season.
While there are numerous studies reporting a high prevalence of vitamin D deficiency among pregnant women, most of these studies focus on women who are highly pigmented or are veiled(Reference Bowyer, Catling-Paull and Diamond11, Reference Bassir, Laborie and Lapillonne22–Reference van der Meer, Karamali and Boeke24). Few studies have examined vitamin D status in Caucasian women living in northern latitudes. Javaid et al. (Reference Javaid, Crozier and Harvey25) reported vitamin D deficiency (25(OH)D < 27·5 nmol/l) and insufficiency (25(OH)D 27·5–50 nmol/l) in 18 and 31 %, respectively, of white mothers living in southern England, while a study from northern USA reported vitamin D deficiency (25(OH)D < 37·5 nmol/l) and insufficiency (25(OH)D 37·5–80 nmol/l) in 5 and 42·1 %, respectively, of white women(Reference Bodnar, Simhan and Powers12). The current study reports higher rates of vitamin D deficiency and insufficiency than previously reported, with 35 % (12 weeks gestation), 44 % (20 weeks gestation) and 16 % (35 weeks gestation) of pregnant women vitamin D deficient (25(OH)D < 25 nmol/l), and 96 % (12 and 20 weeks gestation) and 75 % (35 weeks gestation) of pregnant women vitamin D insufficient (25(OH)D < 50 nmol/l). It is possible that the prevalence of low vitamin D status is higher in the present study because sampling did not take place over an entire year, with few sampling time-points in autumn, when status would be higher. However, in the present cohort the prevalence of vitamin D deficiency during late pregnancy, sampled during summer compared to matched non-pregnant controls, would presumably be much higher if late pregnancy was at the end of winter. The most likely explanation for the lower vitamin D status reported in the current population is the latitude at which the study was undertaken and the low average hours of sunshine at a sufficient intensity to facilitate vitamin D synthesis. Northern Ireland is located between 54 and 55°N and as such is ‘in the dark’, in terms of UV intensity, with respect to vitamin D synthesis for 5 months of the year. Furthermore, the climate in summer is often cloudy, with a daily average of 3–4 h of sunshine in summer in the current population compared to 7–8 h of daily sunshine in the populations previously studied(Reference Bodnar, Simhan and Powers12, Reference Javaid, Crozier and Harvey25, 26). Marked geographical variations in vitamin D status in adults aged 18–65 years living in the UK have been reported recently(Reference Hirani, Mosdøl and Mishra27).
Several explanations may be proposed for the lower vitamin D status in pregnancy, including, haemodilution and decreased exposure to the sun. Such factors are, however, unlikely to explain fully the difference observed, and we speculate that the lower vitamin D status in pregnant women compared to non-pregnant controls is largely determined by fetal demand for this essential nutrient. During pregnancy, approximately 25–30 g calcium are transferred to the fetal skeleton. Increased maternal intestinal calcium absorption together with an increase in renal calcium loss owing to an increase in glomerular filtration rate results in significant changes in maternal vitamin D metabolism. Furthermore, circulating concentrations of the active metabolite, 1,25-dihydroxyvitamin D3, markedly increase from early to late pregnancy and have been shown to be higher in pregnant compared to non-pregnant women(Reference Seki, Makimura and Mitsui28). Together, these pregnancy-related changes in calcium and vitamin D metabolism may result in an increased demand for vitamin D from early to late pregnancy which may explain the significant differences in vitamin D status between pregnant and non-pregnant women in the current study at 20 and 35 weeks but not at 12 weeks gestation. Observational studies and vitamin D supplementation trials among pregnant women at high risk of vitamin D deficiency, as reviewed by Specker(Reference Specker29), showed improved neonatal handing of calcium with improved vitamin D status.
The impact of maternal vitamin D status on neonatal outcomes is significant, owing to the strong relationship between maternal and fetal circulating 25(OH)D concentrations(Reference Hollis and Pittard3). Children born to vitamin D-deficient mothers show an increased incidence of rickets(Reference Dawodu, Agarwal and Sankarankutty4), while maternal vitamin D insufficiency is associated with a deficit in bone-mineral accrual in children that persists to age 9 years(Reference Javaid, Crozier and Harvey25). Furthermore, there is emerging evidence that in utero or early life vitamin D deficiency is associated with non-skeletal health outcomes including increased risk of schizophrenia(Reference McGrath, Saari and Hakko30), type 1 diabetes(Reference Zipitis and Akobeng31) and asthma(Reference Litonjua and Weiss32). In terms of maternal health, 25(OH)D concentration < 37·5 nmol/l has been reported as an independent risk factor for pre-eclampsia(Reference Bodnar, Catov and Simhan33). The women in the study reported here were considered as ‘low risk’ in terms of vitamin D deficiency, yet vitamin D deficiency and insufficiency was prevalent and of considerable concern given the potential health implications for both mother and neonate.
In the USA and Canada, the adequate intake for vitamin D in pregnancy is 5 μg/d(17), while in the UK the reference nutrient intake is set at 10 μg/d(15), however, current guidelines fall short of recommending vitamin D supplementation for all pregnant women(16). The present results suggest a benefit of supplement use, in improving vitamin D status, even in women considered at low risk of vitamin D deficiency. However, even in the face of supplementation, median 25(OH)D concentration remained below 50 nmol/l, with 99 % of women having levels < 80 nmol/l, a cut-off widely used by researchers to indicate vitamin D sufficiency(Reference Hollis, Wagner and Drezner10). Although supplement usage was low at only 22 % and we did not have any information regarding compliance of supplement use, these supplements were most commonly pregnancy-specific multivitamins, containing relatively low doses of vitamin D (between 5 and 12·5 μg/d). We would suggest that there is an urgent need for studies to ascertain the dietary intake and/or supplementation of vitamin D needed to maintain vitamin D status during pregnancy, in individuals at risk of vitamin D deficiency, but also in the general population. Such evidence could then be used to underpin guidelines for dietary vitamin D intake and indeed for supplement use during pregnancy.
To our knowledge, the present study is the first to measure vitamin D status in free-living Caucasian women with uncomplicated pregnancies, which collected samples longitudinally throughout pregnancy whilst concurrently sampling non-pregnant age-matched controls. This design allowed us to examine definitively the effect of pregnancy on vitamin D status while controlling for the marked effects of season on vitamin D status. Clearly, the present study would have benefited from detailed data on individual sunlight exposure and dietary intake of vitamin D which would have provided an opportunity to assess the impact of these key factors on vitamin D status. Importantly, however, we did, have information on supplement usage, which allowed us to show that while ad hoc supplement use improves status in women at low risk of vitamin D deficiency, it does not prevent vitamin D deficiency or insufficiency, particularly during the winter period.
In summary, we report a high prevalence of both vitamin D deficiency and insufficiency in pregnant Caucasian women considered at low risk of vitamin D deficiency living at 54–55°N. Women reporting multivitamin supplement usage during pregnancy did have higher vitamin D status, but many remained vitamin D insufficient. Suboptimal vitamin D status has significant consequences for maternal and neonatal health and, therefore, further research is needed to determine the dietary vitamin D intake required to maintain vitamin D sufficiency during pregnancy, and to underpin guidelines for supplement use during pregnancy.
Acknowledgements
The work was supported by funding from the Health and Personal Social Services Research & Development Office, Belfast, UK. Queen's University Belfast, UK provided funding for the vitamin D analysis. The authors wish to thank all the women who participated in the study and the Midwives from the Royal Jubilee Maternity Hospital, Belfast for their assistance in obtaining samples. J. M. W. W., V. A. H. and H. D. A. were involved in designing the study. V. A. H. recruited all participants and secured funding for the vitamin D analysis. P. M. was involved in subject recruitment. M. S. B. did the vitamin D assays. M. S. B. and J. M. W. W. analysed the data. All authors were involved in data interpretation and manuscript preparation. None of the authors have a conflict of interest. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Research Ethics Committee of the University of Ulster (UUREC/99/46). Written informed consent was obtained from all subjects.





