Osteoporosis is a systemic skeletal disease characterised by low bone mass and microarchitectural deterioration of bone tissue(1). Osteoporotic fracture is a public health concern and a major economic burden and is associated with reduced quality of life and increased mortality(Reference Lips and vanSchoor2–Reference Bliuc and Center4). Medical and care costs for hip fracture in Japan were estimated to be 500 billion yen (approximately 4·5 billion US dollars) annually(Reference Moriwaki and Kamae5).
Dietary nutrients are important factors associated with osteoporosis. Ca is a basic nutrient important for maintaining normal bone metabolism, and its insufficiency is a risk factor for osteoporosis(Reference Heaney, Weaver and Heaney6), especially in low Ca populations, such as East Asians(Reference Balk, Adam and Langberg7). However, the impact of low Ca intake on osteoporotic fractures in East Asian populations has yet to be fully elucidated, although a global systematic review evaluated many cohort studies and demonstrated no association between Ca intake and fracture risk(Reference Bolland, Leung and Tai8). Only a few cohort studies have assessed the relationship between Ca intake and fracture risk in East Asians(Reference Nakamura, Kurahashi and Ishihara9–Reference Kong, Kim and Hong12), and their results are conflicting, which may be due in part to differences in study design.
Vitamin D is another nutrient involved in normal bone metabolism(Reference Whiting, Calvo, Feldman, Pike and Adams13). Thus, in addition to Ca, vitamin D is an important nutrient when considering osteoporotic fractures. Vitamin K is gaining interest as a relevant nutrient, since in addition to its role in blood coagulation, it is also important for bone metabolism. Specifically, vitamin K plays a role in osteocalcin carboxylation(Reference Palermo, Tuccinardi and Onofrio14). Moreover, epidemiological studies have shown that vitamin K levels are associated with bone fracture(Reference Palermo, Tuccinardi and Onofrio14). One meta-analysis that evaluated five cohort studies found that vitamin K intake is inversely associated with fracture risk, although the design of these studies had some limitations(Reference Hao, Zhang and Gu15). East Asians have a unique intake pattern of these nutrients. For example, East Asians generally have low Ca intake(Reference Balk, Adam and Langberg7). Moreover, intakes of vitamins D and K are particularly high in Japanese due to the habitual intake of fish and fermented soyabeans, respectively(Reference Nakamura, Nashimoto and Okuda16,Reference Fujita, Iki and Tamaki17) .
The population-based Murakami Cohort Study in Japan targeted individuals aged 40–74 years (baseline n 14 364) to investigate risk factors for common musculoskeletal diseases(Reference Nakamura, Takachi and Kitamura18). We were able to leverage this framework to identify lifestyle and dietary factors associated with osteoporotic fractures. Accordingly, this study aimed to determine if intakes of basic nutrients related to bone health, including Ca, vitamin D and vitamin K, are associated with incident osteoporotic fractures in middle-aged and elderly Japanese.
Methods
Subjects
The Murakami Cohort Study investigated age-related musculoskeletal diseases of middle-aged and elderly individuals living in the Murakami region (Niigata Prefecture, Japan). Of the 34 802 residents aged between 40 and 74 years, 14 364 participated in the baseline study in 2011–2013. Among them, the following were excluded: 480 individuals who reported a history of fractures of the arm, proximal femur or low back, 155 women who reported female hormone use and 254 individuals who were undergoing osteoporosis therapy. In addition, 681 subjects whose reported dietary energy intakes fell within the upper or lower 2·5 % of the study population were excluded. The Murakami Cohort Study had a policy to exclude extreme values for dietary intake because these values may be unreliable(Reference Nakamura, Kurahashi and Ishihara9). Energy cut-off points were 3364 and 18 346 kJ (801 and 4368 kcal)/d. Ultimately, 12 794 subjects (6301 men and 6493 women) were statistically analysed. Informed consent was obtained from all subjects. The protocol of this study was approved by the Ethics Committee of Niigata University (nos. 1324 and 2018-0417). A detailed protocol of the Murakami Cohort Study has been published elsewhere(Reference Nakamura, Takachi and Kitamura18).
Baseline survey
A self-administered questionnaire survey was conducted between 2011 and 2013. The questionnaire elicited information on age, sex, marital status, education, occupation, height, weight, lifestyle and history of osteoporotic fracture. BMI was calculated by weight (kg) divided by height squared (m2). Physical activity levels were estimated by calculating the metabolic equivalents (MET) score (MET-h/d), which was obtained by multiplying the time spent on activities per d by its MET intensity(Reference Fujii, Yamamoto and Takeda-Imai19). Participants were also asked about their smoking habits ((1) non-smoker; (2) past smoker; (3) 1–20 cigarettes/d; (4) ≥20 cigarettes/d), alcohol consumption ((1) non- or rare-drinkers; (2) 1–149 g ethanol/week; (3) ≥150 g ethanol/week)(Reference Inoue and Tsugane20) and consumption of green tea and coffee ((1) <1 cup/week; (2) 1–6 cups/week; (3) 1–3 cups/d; (4) ≥4 cups/d). Information on use of Ca, vitamin D and vitamin K supplements was also obtained. Dietary intakes of Ca, vitamin D, vitamin K and total energy were assessed with a validated FFQ(Reference Yokoyama, Takachi and Ishihara21). Each participant was asked how often she/he had consumed specific foods and beverages on average during the previous month. The amount of each food consumed was calculated by multiplying the frequency of consumption with the portion size. Intakes of nutrients, including Ca, vitamin D and vitamin K, were calculated using the Standard Tables of Food Composition in Japan 2010(22). The validity of the FFQ with regard to assessing intake was evaluated against 12-d dietary records collected as a reference during each of the four seasons. Spearman’s correlation coefficients between the FFQ and the reference 12-d records in men and women were 0·58 and 0·42 for Ca, 0·32 and 0·49 for vitamin D and 0·56 and 0·52 for vitamin K, respectively(Reference Yokoyama, Takachi and Ishihara21). Detailed categories and characteristics of demographic, lifestyle and nutritional factors are shown in online Supplementary Table S1.
Incident cases of osteoporotic fracture during follow-up
We collected all incident cases of major osteoporotic limb fractures, including those of the distal forearm (radius or ulna), neck of humerus, neck or trochanter of femur and lumbar or thoracic spine(Reference Kanis23), at medical facilities affiliated with the Murakami Cohort Study, including Murakami General Hospital, Sakamachi Hospital, Sanpoku Tokushukai Hospital, Sasaki Orthopaedic Clinic, Takahashi Orthopaedic Clinic, Arakawa Chuo Clinic in Murakami City and Nakajo Chuo Hospital in Tainai City(Reference Nakamura, Takachi and Kitamura18). Briefly, we visited the orthopaedic departments of these hospitals and clinics every year and retrieved information related to the limb fractures mentioned above and vertebral compression fractures of newly diagnosed patients between baseline and 31 December 2017, using each facility’s database. We then carefully checked the clinical diagnosis, date of fracture occurrence and the orthopaedist’s review of each diagnostic X-ray film from each patient’s medical record. Symptomatic vertebral fractures in the lumbar or thoracic region were diagnosed using criteria of the Japanese Society of Bone and Mineral Metabolism(Reference Soen, Fukunaga and Sugimoto24). Only low-energy trauma fractures, that is, fractures due to falls from a standing height or lower, were included(Reference Center, Marcus, Feldman, Dempster and Luckey25). Fractures due to high-energy trauma, such as traffic accidents, were not included.
Statistical methods
Means and standard deviations were calculated for continuous variables. Extreme values exceeding 3 sd of BMI (n 23) and MET score (n 113) were excluded as outliers. Pearson’s correlation coefficients were calculated between energy-adjusted Ca, vitamin D and vitamin K to check multicollinearity. The variance inflation factor was calculated to assess multicollinearity. A variance inflation factor value >10 was considered to reflect multicollinearity. The incidence of fracture was calculated by the number of cases divided by person-years. The Cox proportional hazards model was used to calculate unadjusted and adjusted hazard ratios (HR) of fracture. Among the 12 794 study subjects, multivariate analysis was performed in 12 306 (96·2 %) after excluding those with incomplete questionnaire data (including food items). Sample size calculation for this cohort study has been described elsewhere(Reference Nakamura, Takachi and Kitamura18).
We first evaluated the HR for total fractures (limb and vertebral fractures) according to levels of energy-unadjusted nutritional intake by sex. HR were presented in the unadjusted model, age-adjusted model and multivariable-adjusted model. In multivariable models, the HR was adjusted for all other variables, including age (continuous), marital status (dummy variable), education level, occupation (dummy variable), BMI (continuous), MET score (continuous), smoking, alcohol consumption, Ca intake (continuous), vitamin D intake (continuous) and vitamin K intake (continuous), as covariates. Next, HR for total, limb and vertebral compression fractures, according to levels of energy-adjusted Ca, vitamin D and vitamin K intakes in women, were calculated. HR for energy-adjusted Ca, vitamin D and vitamin K were calculated by the residual method(Reference Willett26) by sex. Because users of Ca, vitamin D and vitamin K supplements were rare (2·1, 0·6 and 0·2 %, respectively), we did not include supplement information in the statistical analyses.
To evaluate the relative contribution of each food item (cereals, potatoes and starches, beans, vegetables, fruits, fish, meats, milk and dairy products) on nutrition intake, we calculated coefficients of determination (R 2) using multiple linear regression analysis with a forward selection method. Statistical analyses were performed with SAS statistical software (release 9.13, SAS Institute Inc.). P < 0·05 was considered statistically significant.
Results
In the total study population, mean age was 58·8 (sd 9·3) years and the mean follow-up period was 5·0 (sd 0·7) years. Subject characteristics at baseline according to quartile intakes of Ca, vitamin D and vitamin K by sex are shown in Table 1. Older age and lower proportions of current smokers and drinkers were associated with higher quartile energy-adjusted intakes of Ca, vitamin D and vitamin K in both sexes. Higher MET score was associated with lower quartiles of Ca intake in men and higher quartiles of vitamin K intake in women. BMI was not associated with any of the quartiles of nutrient intake. Intakes of Ca, vitamin D and vitamin K were associated with quartile energy-adjusted intakes of each of the three nutrients. Pearson’s correlation coefficients for energy-adjusted nutrient intakes in men and women were 0·23 and 0·06 for Ca v. vitamin D, 0·51 and 0·33 for Ca v. vitamin K and 0·26 and 0·13 for vitamin D v. vitamin K, respectively (all P < 0·001). Variance inflation factor values for energy-adjusted intakes of Ca, vitamin D and vitamin K were all <1·5.
Table 1. Subject characteristics at baseline according to quartile (Q) energy-adjusted intakes of calcium, vitamin D and vitamin K by sex
(Mean values and standard deviations)
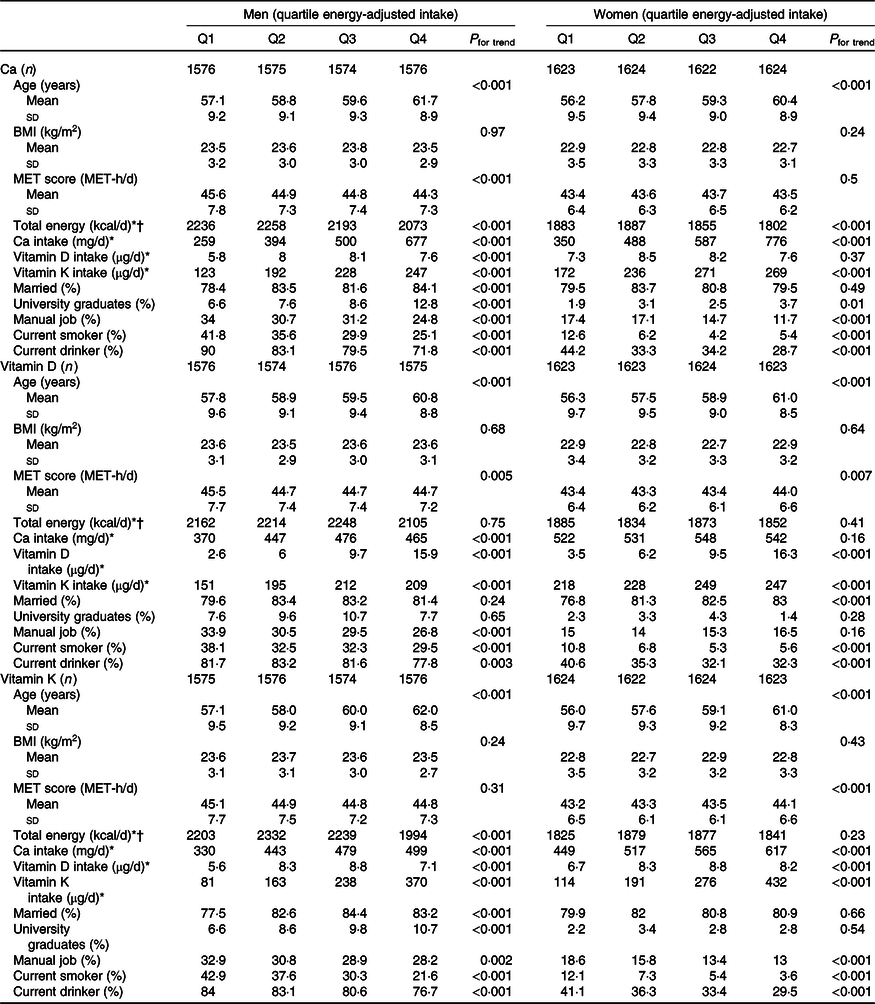
* Median.
† To convert kcal to kJ, multiply by 4·184.
Table 2 shows the number of fractures that occurred during the follow-up period by sex, age group and fracture site. The number of distal forearm fractures was the highest, followed by fractures of vertebra, femur and humerus. Incidence rates of total fractures in men and women were 1·5 and 7·1 per 1000 person-years, respectively, and the adjusted HR of total fractures for women to men was 3·92 (95 % CI 2·51, 6·11).
Table 2. Number of fractures* occurring during the follow-up period by sex, age group and fracture site

* Fractures caused by high-energy trauma, such as a motor vehicle accident, were excluded.
Incidence rates and adjusted HR of total fractures, limb fractures and vertebral fractures according to levels of energy-adjusted Ca, vitamin D and vitamin K intakes in men and women are shown in Tables 3 and 4, respectively. In men, there were null associations between incident fractures and each of the three nutrient intakes. In women, when total fractures were the outcome, lower energy-adjusted Ca intake was significantly associated with higher multivariable adjusted HR of total fractures (P for trend = 0·005), with the lowest quartile group having a significantly higher HR (1·66, 95 % CI 1·13, 2·44) than the highest quartile group (reference). Moreover, lower energy-adjusted vitamin K intake was marginally associated with higher adjusted HR (P for trend = 0·08). For limb fractures, lower energy-adjusted Ca intake was marginally associated with higher adjusted HR (P for trend = 0·054). For vertebral fractures, lower energy-adjusted Ca and vitamin K intakes were significantly associated with higher adjusted HR (P for trend = 0·03 and 0·006, respectively), with the lowest quartile group for both nutrients having significantly higher HR (2·03, 95 % CI 1·08, 3·82 and 2·26, 95 % CI 1·19, 4·26, respectively). Graphs of multivariable-adjusted associations of energy-adjusted Ca and vitamin K intake with vertebral fractures in women are shown in online Supplementary Fig. S1. Vitamin D intake was not associated with any of the assessed fracture types.
Table 3. Incidence rates and adjusted hazard ratios (HR) of total fractures, limb fractures and vertebral fractures according to quartiles (Q) of energy-adjusted* calcium, vitamin D and vitamin K intakes in men (Hazard ratios and 95 % confidence intervals)
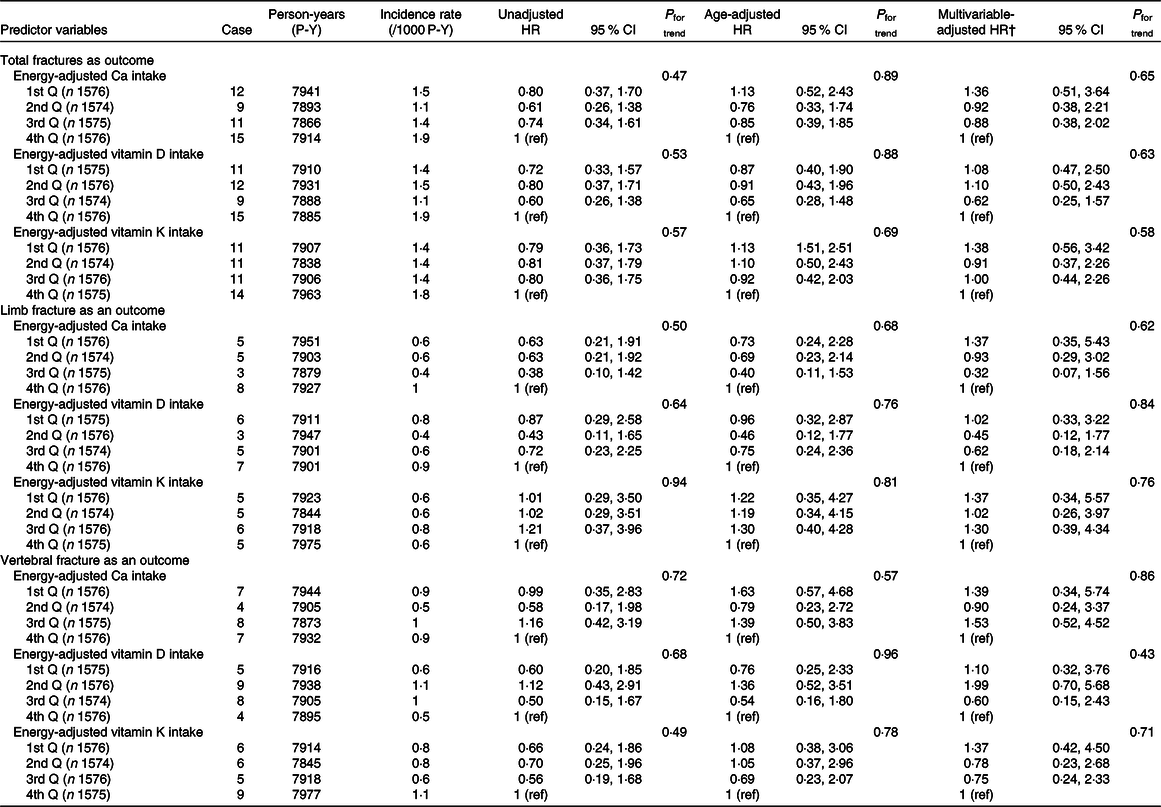
ref, Reference.
* Adjusted by residual method.
† Adjusted for intake of the other two nutrients, age, marital status, education level, occupation, BMI, MET score, smoking and drinking.
Table 4. Incidence rates and adjusted hazard ratios (HR) of total fractures, limb fractures and vertebral fractures according to levels of energy-adjusted* calcium, vitamin D and vitamin K intakes in women (Hazard ratios and 95 % confidence intervals)
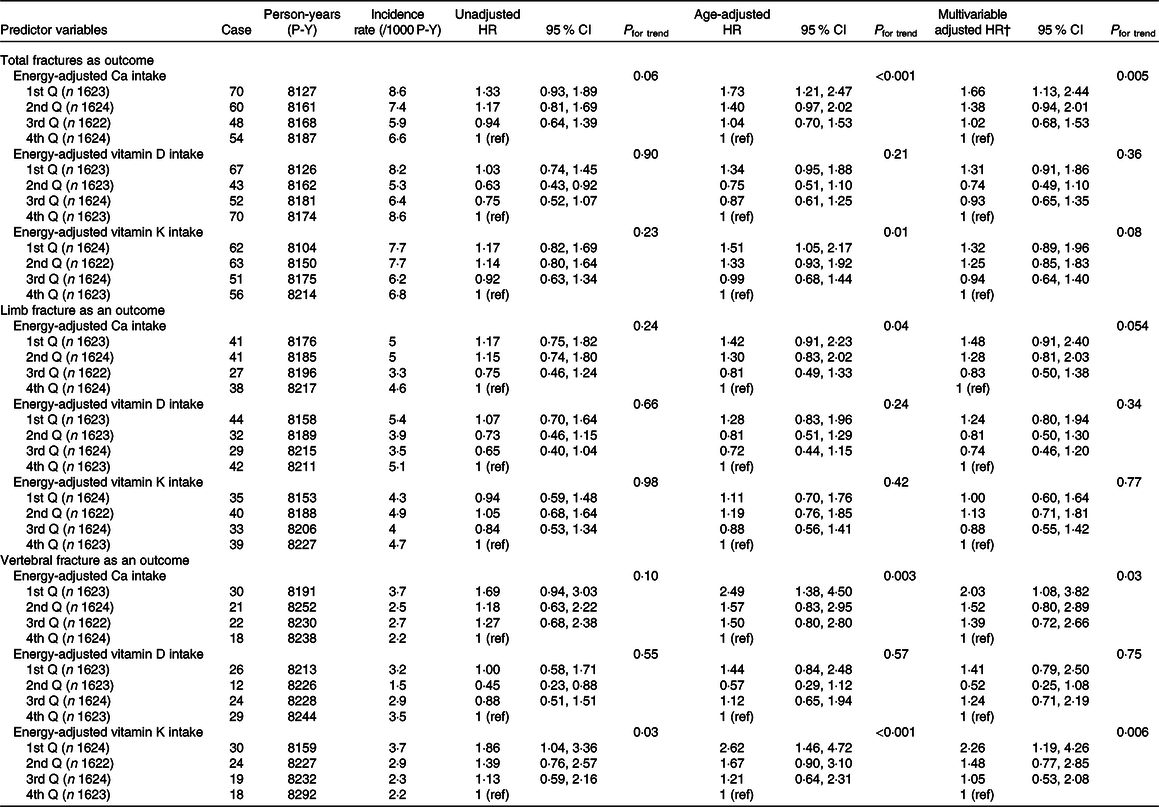
Q, quartile; ref, reference.
* Adjusted by residual method.
† Adjusted for intake of the other two nutrients, age, marital status, education level, occupation, BMI, MET score, smoking and drinking.
Associations between levels of energy-adjusted Ca and vitamin K intakes and incident total fractures in women, stratified by age- and BMI-subgroups, are shown in Table 5. In the age-stratified analysis (<60 v. ≥60 years), similar associations between energy-adjusted Ca intake and incident total fractures were observed, with a marginal significance in the older subgroup (P for trend = 0·054). Lower energy-adjusted vitamin K intake was associated with a higher incidence of total fractures in the older group (P for trend = 0·009), but not in the younger subgroup (P for trend = 0·67). In the BMI-stratified analysis (<22·4 v. ≥22·4 (median)), lower energy-adjusted Ca intake had a higher HR of total fractures only in the lower BMI subgroup (P for trend = 0·009).
Table 5. Incidence rates and adjusted hazard ratios (HR) of total fractures according to quartiles (Q) of energy-adjusted* calcium and vitamin K intakes in women by age and BMI
(Hazard ratios and 95 % confidence intervals)
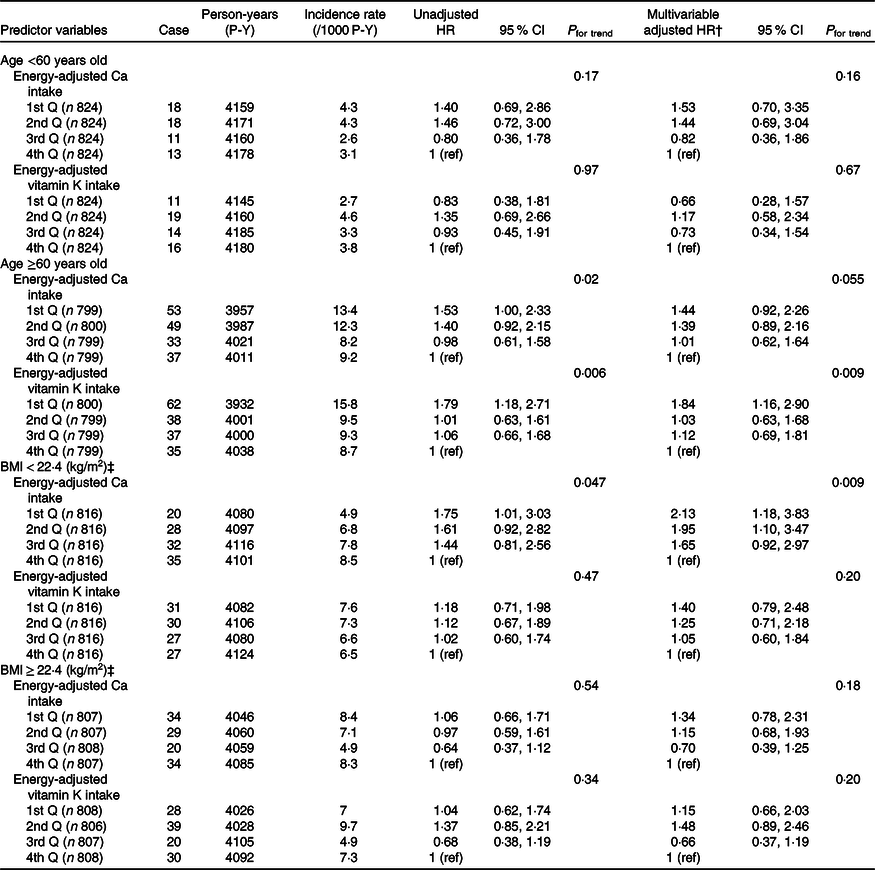
ref, Reference.
* Adjusted by residual method.
† Adjusted for intake of the other nutrient (energy-adjusted Ca or vitamin K), energy-adjusted vitamin D, age, marital status, education level, occupation, BMI, MET score, smoking and drinking.
‡ Median.
With regard to the contribution of food items on nutrients, Ca intake was accounted for by intake of milk and dairy products (39 %), vegetables (18 %), beans (5 %), fish (3 %), cereals (1 %) and fruit (1 %) in men; and milk and dairy products (50 %), vegetables (17 %), beans (5 %) and fish (1 %) in women. Vitamin K intake was accounted for by intake of natto (29 %), vegetables (22 %), meat (4 %) and fish (1 %) in men; and vegetables (37 %), natto (26 %), meat (2 %) and fish (1 %) in women.
Discussion
In the present study, lower Ca intake was found to be associated with a higher incidence of total fractures and vertebral fractures in women, whereas lower vitamin K intake was associated with a higher incidence of vertebral fractures in women. These findings suggest that lower Ca intake in women is associated with a high incidence of osteoporotic fractures. In contrast, there were null associations between incident fractures and each of the three nutrient intakes in men.
According to a systematic review by Bolland et al. (Reference Bolland, Leung and Tai8), dietary Ca intake is not associated with the risk of fracture(Reference Bolland, Leung and Tai8). This may be explained by a relatively high Ca intake in Western countries(Reference Bolland, Leung and Tai8), as most of the forty-seven cohort studies included in the review were conducted in Europe or North America; only four cohort studies targeted East Asians (mean Ca intake, 679 mg/d(Reference Kung, Lee and Ho27), 415 mg/d(Reference Nakamura, Kurahashi and Ishihara9), 586 mg/d(Reference Nakamura, Saito and Oyama11), and not described(Reference Koh, Wu and Wang10)). In one of the cohort studies targeting East Asians, Kung et al. (Reference Kung, Lee and Ho27) followed 1435 Chinese postmenopausal women (mean age, 63·4 years) for 5 years and found that those with dietary Ca intake <400 mg/d had a significantly higher risk of osteoporotic fractures (relative risk = 3·1) than those with Ca intake ≥400 mg/d. In another cohort study, Nakamura et al. (Reference Nakamura, Kurahashi and Ishihara9) followed 41 835 Japanese men and women aged between 40 and 69 years for 10 years and found that lower energy-adjusted Ca intake was significantly associated with a higher incidence of self-reported vertebral fractures, with the lowest quartile of energy-adjusted Ca intake having a significantly higher relative risk (1·9) than that of the highest quartile. The remaining two cohort studies(Reference Koh, Wu and Wang10,Reference Nakamura, Saito and Oyama11) failed to detect an association between Ca intake and osteoporotic fractures, partly because Ca intake was not a primary focus of those studies. Thus, the association between dietary Ca intake and osteoporotic fractures in East Asian populations has not been fully clarified; in this respect, the present study provides important findings on this topic.
Ca intake among women in this study was inversely associated with the risk of vertebral fractures, but not with limb fractures. This finding is consistent with that of a randomised controlled trial of Ca supplement use which targeted Japanese peri- and post-menopausal women. That trial found that Ca supplements had an effect on vertebral bone mineral density (BMD), but not on hip BMD(Reference Nakamura, Saito and Kobayashi28). Although we cannot fully explain the mechanism underlying the present findings, one potential explanation involves differences in trabecular and cortical BMD. Specifically, while cortical bone BMD begins to decrease at menopause, trabecular BMD begins to decrease before middle age and continues throughout life(Reference Riggs, Melton and Robb29). This suggests that insufficient Ca intake among women early in life may affect trabecular bone-rich vertebra more adversely than cortical bone-rich limb bones.
We found that higher vitamin K intake in women was significantly associated with a lower risk of vertebral fractures and marginally associated with total fractures. Hao et al. (Reference Hao, Zhang and Gu15) recently conducted a meta-analysis of five cohort and nested case–control studies on the relationship between dietary vitamin K intake and risk of fractures and found the pooled relative ratio of highest v. lowest dietary vitamin K intake for all fractures to be 0·78 (95 % CI 0·56, 0·99). Our findings are consistent with this. Two Japanese cohort studies(Reference Fujita, Iki and Tamaki17,Reference Ikeda, Iki and Morita30) reported that a higher intake of natto (fermented soyabeans), high in vitamin K2 (phylloquinone), is associated with higher BMD. These two studies support our results. Natto is a popular food also in the Murakami area, and intake of vitamin K in the present population (264 μg/d) was higher than that of cohorts of North American and European studies (mean or median intakes, 67–169 μg/d)(Reference Feskanich, Weber and Willett31–Reference Apalset, Gjesdal and Eide34).
The subgroup analysis by age revealed that the importance of Ca intake did not differ between the younger and older subgroups in women. On the other hand, insufficient vitamin K intake was found to be harmful only in the older (≥60 years) subgroup, suggesting that vitamin K is especially important for maintaining bone health in older women. These findings may be useful when considering fracture prevention approaches in high-risk populations. However, further investigation will be needed to elucidate underlying mechanisms. In addition, a future study should use a longer follow-up period to clarify the effect of insufficient nutrient intakes in middle-aged people. Subgroup analysis by BMI revealed that insufficient Ca intake was harmful only in the lean (BMI < 22·4 kg/m2) subgroup, suggesting that sufficient Ca intake should be recommended, especially for lean women with lower food intake.
The estimated Ca intakes according to a validated FFQ in the present population were 442 mg/d in men and 537 mg/d in women (online Supplementary Table S1), which were lower compared with the recommended dietary intake for Ca of 650–700 mg/d in the 2015 Dietary Reference Intakes for Japanese (35). In the stratified analysis of Ca intake in women (<537 v. ≥537 mg/day (median)), intake of milk and dairy products accounted for 24 % of Ca intake in the <537 mg/d group, as compared with 68 % in the ≥537 mg/d group (data not shown). These findings can be interpreted as an indication that women with low Ca intake do not consume much milk/dairy products. In this regard, higher intakes of milk and dairy products as well as adequate Ca supplements may be helpful, especially in women with low Ca intake.
We did not find a significant association between vitamin D intake and fractures, although a number of studies have shown that a low level of vitamin D, as reflected by blood 25-hydroxyvitamin D concentration, is a risk factor for osteoporotic fracture(Reference Bischoff-Ferrari, Willett and Wong36). Blood 25-hydroxyvitamin D is provided not only by dietary vitamin D but also vitamin D that is biosynthesised in the skin by sunlight. Thus, dietary vitamin D intake may contribute less to vitamin D status in the body than vitamin D biosynthesis in the skin.
The present study has several strengths. First, the study had a large sample size, which enabled us to set osteoporotic fractures as the outcome and identify risk factors in an early-stage female elderly population (mean age, 58 years). Second, we determined all cases of osteoporotic fractures from relevant medical facilities with accurate diagnoses. Third, nutritional intake was assessed using a validated FFQ. Finally, this is the first large-scale cohort study targeting an East Asian population, which had a diet of low Ca and high vitamin K.
The present study also has some limitations worth noting. First, fracture incidence in men was much lower than that in women. Thus, we were unable to identify risk factors for men due to the smaller sample size. Second, lifestyle information, including dietary intake, was based on self-report, and thus misclassification bias may have occurred. If the misclassification was non-differential, the strength of the association between predictors and outcomes would be underestimated. Third, there were missing questionnaire data (3·8 %), which were not included in the multivariable analyses. This may have led to selection bias and potentially affected the adjusted HR. Fourth, vertebral fractures assessed in the present study were symptomatic, and thus our findings cannot be generalised to asymptomatic vertebral fractures. Finally, as this was an observational study, unknown confounders may have affected our results. A future study to address these limitations is warranted.
In conclusion, lower intakes of dietary Ca and vitamin K are independent lifestyle-related risk factors associated with osteoporotic fractures in middle-aged and elderly Japanese women. This association was robust for vertebral compression fractures, but not for osteoporotic limb fractures. These associations were not observed in men and thus should be re-examined with a longer follow-up period.
Acknowledgements
The authors acknowledge the Murakami Public Health Centre, Murakami City, Sekikawa Village, and Awashimaura Village for their valuable support in data collection. We also thank Murakami General Hospital, Sakamachi Hospital, Sanpoku Tokushukai Hospital, Sasaki Orthopaedic Clinic, Takahashi Orthopaedic Clinic and Arakawa Chuo Clinic in Murakami City, Nakajo Chuo Hospital in Tainai City, and Tsuruoka Municipal Shonai Hospital in Tsuruoka City for follow-up data collection.
This work was supported by JSPS KAKENHI grant numbers JP23249035 and JP23790708 and the National Cancer Center Research and Development Fund (23-A31(toku)) (since 2010).
K. P. and K. N. formulated the research question. K. P., Y. W., K. Ki., K. Ka., K. W., A. S. and K. N. designed the study. K. Ki., R. T., T. S., A. T., R. K., R. O., S. T., M. I., A. S., O. Y. and K. N. contributed to data collection. K. P. and K. N. analysed the data and wrote the first draft of the paper. All authors participated in interpretation of the data and approved the final version.
The authors have no conflicts of interest to report.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114520001567








