Over recent decades, delays in motor abilities using measures that combine both gross and fine motor scores have commonly been reported in toddlers after early complex cardiac surgery. Reference Snookes, Gunn and Eldridge1–Reference Sprong, Broeders and van der Net3 Impaired gross motor abilities for pre-school and school-age children with CHDs have been reported, with concern for low tone and poor balance skills. Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4–Reference Ricci, Fung and Moddemann6 Mild to moderate improvement in motor function has been suggested to occur between age 1 and 6 years. Reference Naef, Wehrie, Rousson and Latal7 A few studies have reported lower gross motor than fine motor scores. Reference Sprong, Broeders and van der Net3,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4,Reference Sprong, van Brussel and de Vries8–Reference Ehrler, von Rhein and Schlosser12 Reporting motor standard scores or composite scores without the individual subtest scaled scores masks differences between fine and gross motor scores and thus may decrease the recognition of individual deficits and reduce recommended clinical neurodevelopmental interventions. Reference Sprong, van Brussel and de Vries8,Reference Stieber, Gilmour and Morra11–Reference Ricci and Alton15 Recognising that long-term motor delays may be improved by early interventions, there is a need to study gross motor and fine motor scores separately to aid clinicians in the care of this population. Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4–Reference Ricci, Fung and Moddemann6,Reference Salls, Silverman and Gatty14
Reported acute care predictors of adverse motor outcomes have included a variety of different cardiac defects, especially for those after palliative surgery, imaging determined brain injury, need for anticoagulant medication, longer duration of mechanical ventilation, and longer hospital and intensive care stay. Reference Sprong, Broeders and van der Net3,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4,Reference Sprong, van Brussel and de Vries8,Reference Stegeman, Sprong and Breur9,Reference Stieber, Gilmour and Morra11–Reference Uzark, Smith, Donohue, Yu and Romano13 These previous studies have not considered the predictive role of chronic health conditions for adverse motor development of the children, including chronic health procedures resulting in decreased time in the prone position which is known to be associated with motor delay. Reference Salls, Silverman and Gatty14,Reference Ricci and Alton15 The failure to report differences in fine and gross motor scores not only can reduce appropriate developmental intervention but also has the clinical effect of preventing the identification of risk factors separately associated with each.
The clinical assessment of the gross motor abilities of very young children may be completed using The Bayley Scales Reference Bayley16–Reference Bayley and Aylward18 or other measures. Reference Uzark, Smith, Donohue, Yu and Romano13,Reference Peyton and Einspieler19–Reference Folio and Fewell24 The most common measure used as a research tool to evaluate predictors of outcome is The Bayley Scales. Reference Bayley16–Reference Bayley and Aylward18 Currently, most published research of early childhood outcomes and predictors of outcomes after early complex cardiac surgery report the cognitive, language, and motor composite scores of The Bayley Scales of Infant and Toddler Development – Third Edition (Bayley-III) Reference Bayley17 that has been shown to overestimate development Reference Acton, Biggs and Creigton10,Reference Long, Galea, Eldridge and Harris25 compared with the Bayley Scales of Infant Development – Second Edition. Reference Bayley16 Most developmental follow-up centres now use The Bayley Scales of Infant and Toddler Development – Fourth Edition Reference Bayley and Aylward18 with similar calculation of composite scores to the Bayley-III. Reference Bayley17
We hypothesised that a greater proportion of survivors after complex cardiac surgery have gross motor rather than fine motor delay such that the common reporting of the motor composite score does not adequately represent the subtest scaled scores for this population, in particular diminishing the impact of gross motor deficits. We further hypothesised that the determination of predictors (using both acute care and chronic health variables) of gross motor outcome would explain a greater proportion of the variance than of fine motor outcome, leading to a better understanding of motor delay and its associations.
We aimed to determine whether (1) the gross motor developmental scores of young children after early complex cardiac surgery were different from fine motor scores and adequately represented by the motor composite score of the Bayley-III Reference Bayley17 and, (2) the acute care and chronic health predictors of gross motor delay differ from predictors of fine motor delay. Reference Bayley17
Materials and methods
Study design
This prospective inception-cohort outcomes study is part of a developmental longitudinal follow-up project of infants after complex cardiac surgery. Reference Robertson, Sauve and Joffe26 Information about the registration and assessment process of the children has been previously published. Reference Acton, Biggs and Creigton10,Reference Robertson, Sauve and Joffe26 Our cohort was identified at the time of their first complex cardiac surgery within the first 6 months of life. Predetermined demographic, preoperative, intra-operative, and post-operative variables, and early childhood chronic health markers were prospectively collected. Ethics board approval from the University of Alberta was obtained. Parents or guardians gave signed informed consent.
Participants
During 2009–2019, 272 infants born in Northern Alberta with complex cardiac surgery at <6 months of age at the Stollery Children’s Hospital were referred to the Complex Pediatric Therapies Developmental Assessment Clinic at the Glenrose Rehabilitation Hospital, Edmonton, Alberta. This clinic is part of the Complex Pediatric Therapies Follow-up Program. Reference Robertson, Sauve and Joffe26 Our focus for this study were those toddlers assessed with reliable motor outcome scores in order to study the relationships of the gross motor and fine motor scores. Excluded were those who died before assessment age, were lost to follow-up, had parental refusal, were tired or too ill to participate in the assessment, were assessed at an age older than the regular assessment age, or who had known chromosomal abnormalities (Fig. 1). All infants were tested for chromosomal abnormalities with the available tests for the era of the complex cardiac surgery, with further testing done as needed. In addition, we excluded 8 (3.2%) of 244 toddlers seen in clinic who had a clinical developmental level of below 2 months of age and therefore could not be reliably tested on the Bayley-III (Fig. 1). Those requiring extracorporal membrane oxygenation and/or heart transplantation in addition to the complex cardiac surgery were not excluded. Follow-up assessments occurred for 227 (93.3%) of the 244 survivors. After exclusions, there were 171 (78.8% of all survivors without known chromosomal abnormality) study subjects.
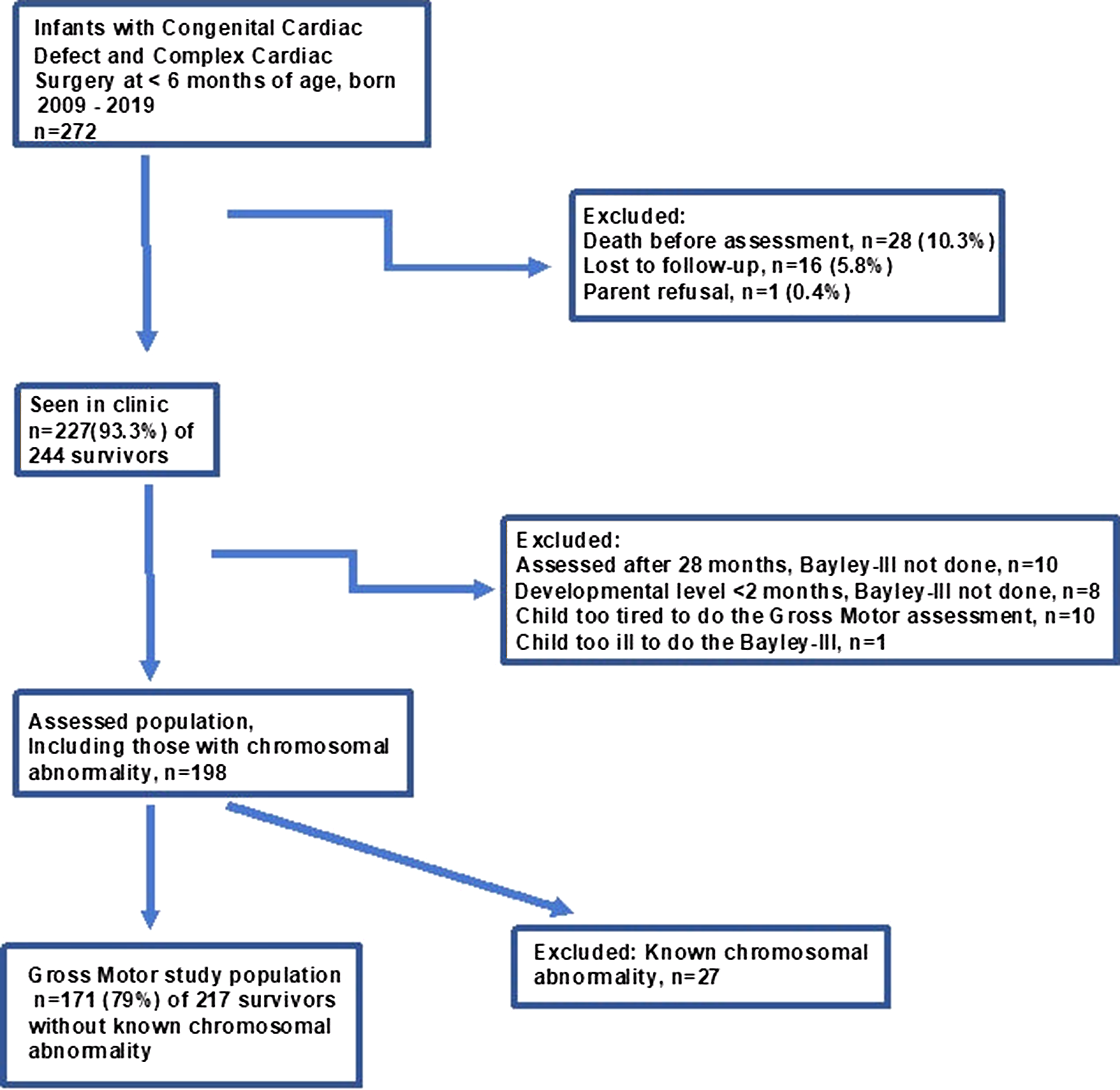
Figure 1. Flow chart for gross motor study for children after early complex cardiac surgery.
Variables and definitions
Variables considered as potential predictors of outcomes
Variables for potential prediction of developmental outcomes were chosen by a multidisciplinary committee, including cardiologists, cardiovascular surgeons, intensivists, and developmental paediatricians based on the literature and findings from our own past research. Clinical demographic, neonatal, and surgical variables related to all open-heart surgeries prior to the 21-month neurodevelopmental assessment (primarily one surgery for those with biventricular defects and two palliative procedures for those with single-ventricle defects) included birth weight (kg), gestational age (weeks), sex, antenatal diagnosis, year of first surgery, age at surgery (days), single or bi-ventricular heart anatomy, total time on cardiopulmonary bypass (min) up to age of assessment, known brain infarctions, highest modified inotrope score, Reference Wernovsky, Wypij and Jonas27 highest plasma lactate (mmol/L), lowest arterial pH and lowest PaO2 (mmHg), total ventilation and hospitalisation time (days), and “increased risk” occurrence, defined as one or more of the following (each occurring in fewer than 10% of the cohort, hence combined): convulsions, cardiopulmonary resuscitation, sepsis, dialysis, extracorporal membrane oxygenation, or heart transplantation. Variables reflecting the family background and chronic health conditions of the child, collected at the time of developmental assessment, included family socio-economic status Reference Blishen, Carroll and Moore28 based on the prestige level, required education and associated income, of the occupation of the main wage earner of the family, with a population mean (standard deviation [SD] of 43 [15]), mother’s years of schooling, gastrostomy any time since first hospitalisation, number of interval hospitalisations due to cardiac and non-cardiac reasons, number of current medical specialists, and prescribed pulmonary or cardiac medications, yes/no.
Measures of outcome
The Bayley-III motor tests were completed by registered paediatric-experienced psychologists/psychometrists. The fine motor subtest measures visual-motor integration, visual spatial skills, and motor control skills of the hands, and the gross motor subtest measures large complex body movements. From the chronological age or, if required, the corrected-for-prematurity age of the child and individualised testing of the floor and ceiling were identified for each child. From passed items, the gross and fine motor subtests, recorded as scaled scores, with a normative population mean (SD) of 10 (3), were converted into motor composite scores, with a normative population mean (SD) of 100 (15). Reference Piper and Darrah23 Differences between the fine and gross motor scaled scores were considered significant at p < 0.05 if they reach ≥ 2.93; Reference Piper and Darrah23 according to the Manual, this difference allows the determination of the proportion of children with significantly different fine and gross motor scaled scores. Reference Piper and Darrah23
Statistical analysis
Continuous variables are presented as mean (SD) or median [interquartile range (IQR)] and categorical variables as counts and percentages. Differences between the fine motor and gross motor abilities are reported as statistically significant when differences between continuous scaled scores were ≥ 2.93 (≤0.05) based on normative data Reference Piper and Darrah23 and when differences in the proportion of toddlers with scaled scores below −2 SD had p-value ≤ 0.05. Comparisons for continuous scores were completed using paired two-sample t-tests; for comparing two paired proportions, the McNemar’s chi-squared test was used. A total of 24 predictor variables were included, 16 recorded from the complex cardiac surgery and 8 collected from the time of follow-up assessment reflecting background and health. These were analysed using univariate linear and stepwise multivariate linear regression, after screening for multicollinearity. Each of two outcomes analysed, fine motor scaled score and gross motor scaled score, began with univariate analysis of each variable with results expressed as effect size with 95% confidence interval; variables with a p-value <0.1 were then selected for the multivariate regressions. To seek combinations of significant predictor variables, two stepwise multivariate regressions were done for each of the outcomes, one using the predictors from the complex cardiac surgery period only and a second using predictors from the complex cardiac surgery plus variables reflecting family background and chronic health conditions at the assessment period. The percentage of variance accounted for is reported for each model. Analyses were performed using SPSS, version 25 and R software, version 4.0.5.
Results
Cohort descriptive variables
Table 1 shows the frequency of the descriptive variables for this study. Thirty (17.5%) children were born at < 37 completed weeks of gestation. Due to collinearity with the birth weight variable, prematurity was not entered into the multivariate regressions. As the deliveries of children with complex cardiac disease were considered high risk, 144 (84.2%) of the 171 were delivered within tertiary hospitals in the same city as the complex cardiac surgery. The weight at surgery was mean 3.6 (1.2) (median 3.3 [interquartile range 2.9, 3.7]) kg and with 9 (5.9%) of children weighing < 2.5 kg. Of the 171 toddlers, 44 (25.7%) were considered at increased risk due to one or more of sepsis, seizures, cardiopulmonary resuscitation, dialysis, extracorporal membrane oxygenation, or heart transplantation.
Table 1. Descriptive variables of acute care surgery for complex cardiac defects and chronic health conditions at age 21 months for 171 toddlers, 2009–2019.
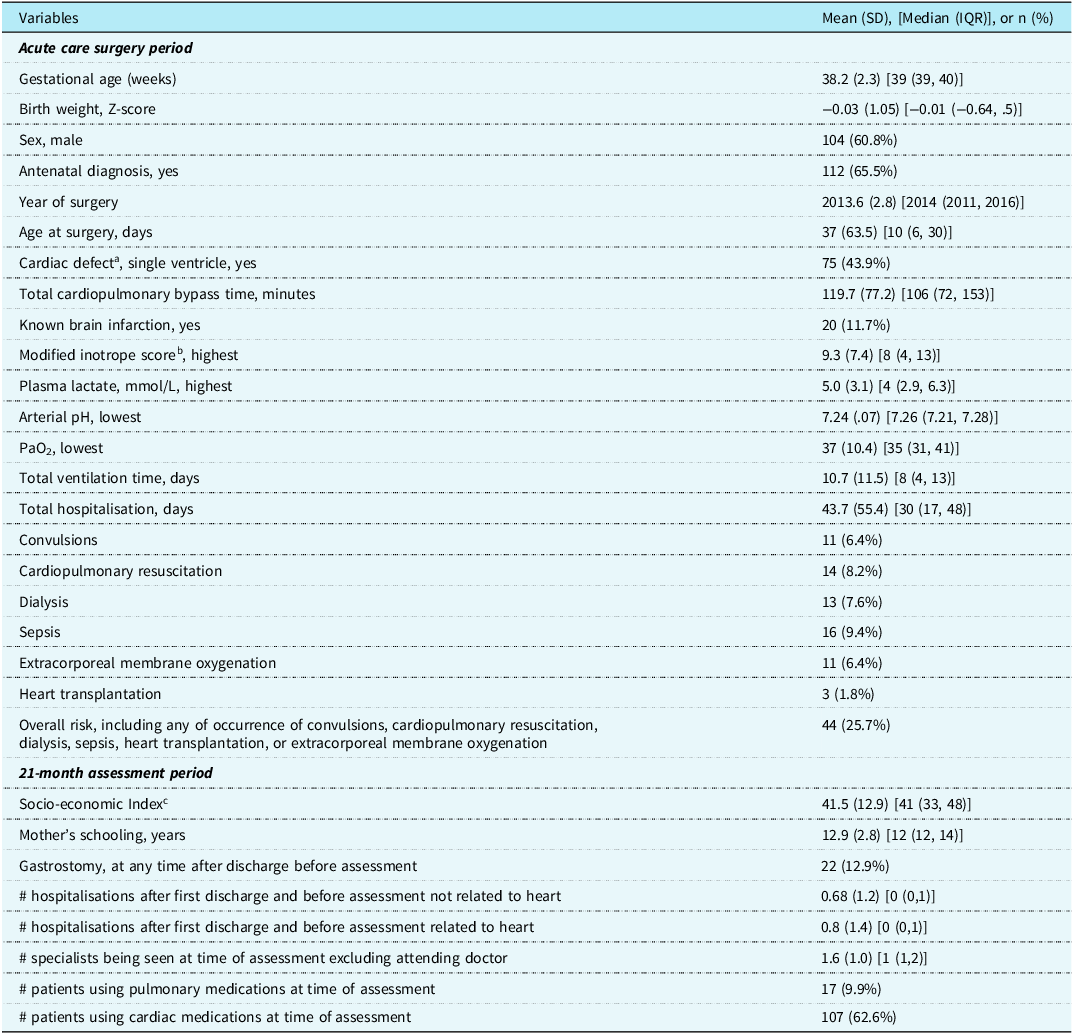
a Left single ventricle, 41; right single ventricle, 34; transposition of the great vessels, 33; truncus arteriosus, 8; total anomalous pulmonary venous drainage, 10; tetralogy of Fallot, 10; pulmonary vein atresia/stenosis, 13; hypoplastic aortic arch, 8; interrupted aortic arch, 5; isomerism, 2; double-outlet right ventricle, 5; mitral valve hypoplasia, 1; aorto-pulmonary window, 1.
b Modified inotrope score. Reference Wernovsky, Wypij and Jonas27
c Blishen Index. Reference Blishen, Carroll and Moore28
Gross and fine motor scores
The motor composite scores for the 171 study subjects was mean (SD) 89.7 (14.2) (median 94 [interquartile range 82, 100]) and 16 (9.4%) with scores below −2 SD. Table 2 shows the fine and gross motor scaled scores and their statistically significant differences. Direct comparison of scores shows 12 (7.1%) of the 171 children had the same fine and gross motor scaled score, 14 (8.2%) had fine motor scores lower than gross motor, while the remaining 145 (84.7%) had gross motor scores lower than fine motor. Relating to normative data, 4 (2.4%) children had significantly different (≥2.93, p ≤ 0.05) lower fine motor scaled scores, and 70 (40.9%) children had significantly different (≥2.93, p < 0.05) lower gross motor scaled scores. Of the six children with cerebral palsy (five with unilateral spastic arm and leg involvement and one with athetosis), two had the same fine as gross motor scaled scores and four had lower gross motor scaled scores.
Table 2. Bayley-III fine and gross motor subtest scaled scores, and proportion of delay for 171 toddlers after complex cardiac surgery.
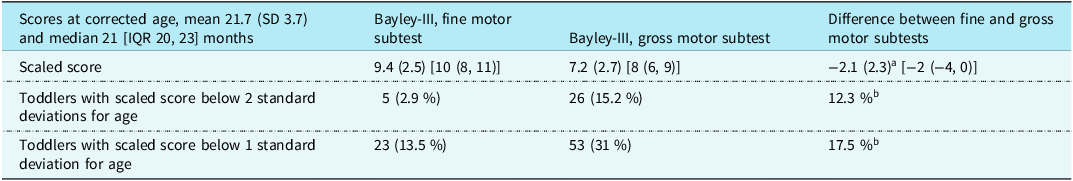
Values given as mean (standard deviation), [median (interquartile range)], and n (%). Population mean (standard deviation) for scaled scores are 10 (3).
a Paired two-sample t-test = <0.001.
b McNemar’s chi-squared test to compare two paired proportions = <0.001.
The proportion of toddlers with scores below −2 SD were determined for the 44 with increased risk versus the remaining 127: fine motor scaled score, 1 (2.3%) of 44 versus 4 (3.1%) of 127, p = 0.766; gross motor scaled score, 13 (29.5%) of 44 versus 13 (10.2%) of 127, p = 0.006. Gross motor delay occurred 5.2 times more often than fine motor delay for the cohort of 171, 12.7 times more often for those in the increased risk group, and 3.3 times more often in those without a defined risk.
Prediction of motor scores
Table 3 shows variables from (1) the complex cardiac surgery period and (2) the combined complex cardiac surgery and 21-month assessment periods associated with the fine and gross motor scaled scores which have a p-value < 0.1 on univariate linear regression analyses. Using these variables, stepwise multivariate linear regression analyses for fine and gross motor scaled scores were completed (Table 4). The variables combined to explain 21.2% of the variance for fine motor scaled scores, with the health variables at 21 months adding 5.9% to the variance provided by the acute care variables. Of the variables, two were not modifiable, sex and birth weight; two reflected acute care illness that may be potentially modifiable, lowest arterial pH and total days of ventilation; and one variable, the number of medical specialists caring for the child at 21 months reflected the degree of poor health of the toddler. Considering the same variables in the regressions for the gross motor scaled scores as for the fine motor scaled scores, the total variance explained was 36.9%, > 15% more than for the fine motor scaled scores. By using clinical health variables from the toddler’s 21-month assessment to combine with the acute care variables, the variance was increased by 11.4%. The combined variance included two non-modifiable variables, birth weight, and single-ventricle anatomy; one potentially modifiable acute care variable, total hospitalisation days; and three variables reflecting the degree of toddler ill health, need for cardiac medication, need for gastrostomy, and the number of medical specialists seen at 21 months.
Table 3. Univariate linear regressions variables with p-value <0.1 from acute care surgery period and from the 21-month assessment in relation to the Bayley-III fine motor and gross motor scaled scores for 171 toddlers after complex cardiac surgery.
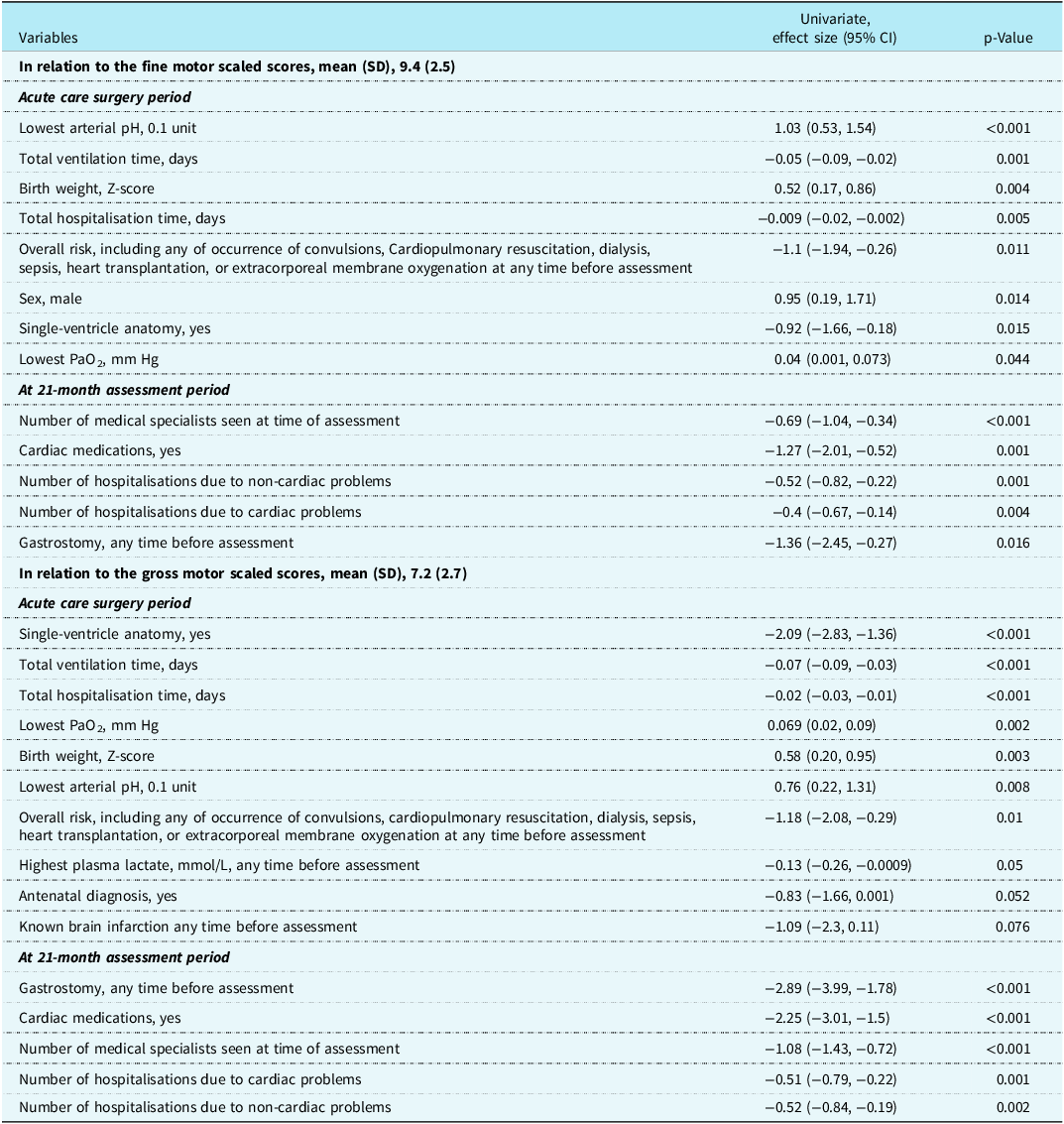
Table 4. Stepwise multivariate regression for prediction of the Bayley-III fine motor and gross motor scaled scores for 171 toddlers after complex cardiac surgery.
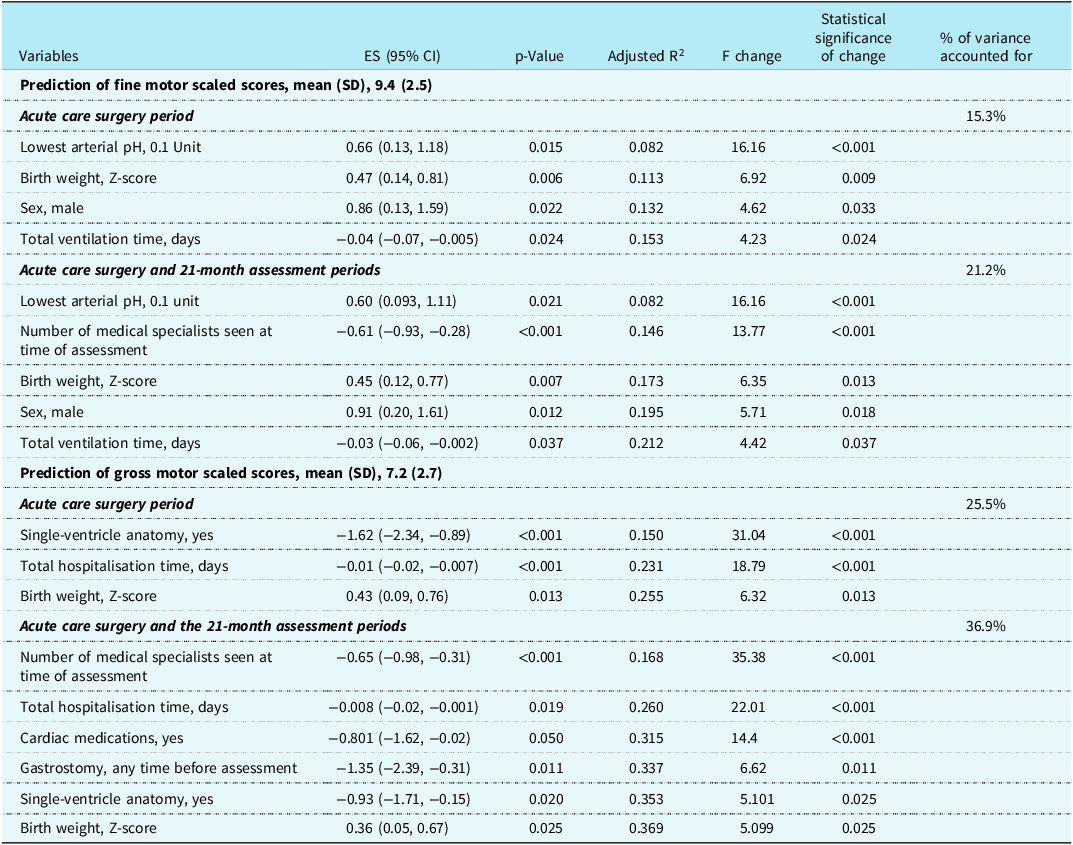
ES = effect size.
Discussion
A delay of childhood motor skills, usually reported as total or composite score, is commonly reported after early complex cardiac surgery. Reference Snookes, Gunn and Eldridge1–Reference Holm, Fredriksen, Fosdahl, Olstad and Vollestad5 A few studies that reported fine and gross motor scores separately raised concerns that gross motor development after complex cardiac surgery lags behind. Reference Sprong, Broeders and van der Net3,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4,Reference Sprong, van Brussel and de Vries8–Reference Stieber, Gilmour and Morra11 In this study, we confirmed gross motor skills were frequently lower than fine motor skills with 15.2 % of toddlers after complex cardiac surgery having gross motor scaled scores below −2 SD of normative data and only 2.9% of toddlers having fine motor scaled scores below −2 SD. This difference is not found in population normative data where gross and fine motor scaled scores are similar. Reference Bayley17 The proportions of scores below −2 SD found in this study were similar to those reported by Sprong Reference Sprong, van Brussel and de Vries8 for 18-month-old children with critical CHDs: composite score, 2.3%, fine motor scaled score, 0%, and gross motor scaled score, 12%. Similarly, using the Peabody Developmental Scale, Reference Folio and Fewell24 Stieber reported 20 children with CHDs at 12–26 months of age, with a mean total motor score of 90 ± 14, fine motor, 94 ± 11, and gross motor, 87 ± 12. Reference Stieber, Gilmour and Morra11 Reporting of only the motor composite score in toddlers after complex cardiac surgery masks some gross motor delay due to the relative strength of their fine motor skills.
The full assessment of the motor skills of a child during developmental follow-up allows for appropriate early developmental intervention that is so important for improving the long-term abilities of the child. Reference Sprong, Broeders and van der Net3,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4,Reference Naef, Wehrie, Rousson and Latal7,Reference Ehrler, von Rhein and Schlosser12,Reference Ricci, Moddemann, Garcia Guerra and Robertson29 The skills assessed during the age of early locomotion depend heavily on balance. Delay in the development of balance for pre-school and school-age children is not uncommon after complex cardiac surgery and early intervention is recommended. Reference Holm, Fredriksen, Fosdahl, Olstad and Vollestad5,Reference Ricci, Fung and Moddemann6,Reference Ehrler, von Rhein and Schlosser12,Reference Marino, Lipkin and Newburger30
Building on the importance of “Early Developmental Intervention,” Neonatal Developmental Care is now a large part of early intervention for the preterm infant and increasingly for the infant with complex cardiac disease. Reference Lisanti, Uzark and Harrison31,Reference Lisanti, Vittner and Peterson32 The recognition that infants with CHD are at risk for delay or disabilities in all developmental areas has resulted in the current recommendations for Individualized Family-Centered Developmental Care beginning with fetal interventions and continuing throughout the hospital period. Reference Lisanti, Uzark and Harrison31 Critical to Individualized Developmental Care is the training required by the caregivers to enable them to read and understand cues given by the infant in order to better guide the development of the infant. Reference Als33 Specific for motor development, supportive positioning and awake prone positioning improves motor skills of infants after cardiac surgery. Reference Uzark, Smith, Donohue, Yu and Romano13,Reference Salls, Silverman and Gatty14,Reference Uzark, Smith and Yu34 Developmental follow-up programmes give opportunities for discharged infants after cardiac surgery to have early access to physical and occupational therapists offering training and guidance in continuing early intervention as needed. Reference Ricci and Alton15,Reference Ricci, Moddemann, Garcia Guerra and Robertson29 This is particularly important for infants with gastrostomy or after prolonged sternal precautions who are at risk for core muscle weakness and gross motor delay. Reference Ricci and Alton15
Important for the developmental assessment of children is the choice of the measure. In this study, we used the Bayley-III as this measure adjusts for weeks of prematurity, includes children with a wide variety of developmental diagnoses and genetic conditions within the norming procedure, has strong reliability and validity, and specifically set out to have five distinct developmental scales, cognitive, receptive and expressive language, and fine and gross motor scores. Reference Bayley17 Most importantly, this measure is widely used in developmental follow-up for many at risk infants, is used in many follow-up studies after complex cardiac surgery, and was a suggested standardised measure from the American Heart Association for the developmental evaluation of children with CHD. Reference Marino, Lipkin and Newburger30 The new Bayley Scales, fourth edition Reference Bayley and Aylward18 also has five distinct developmental scales and calculates scores in a similar way; hence, comparisons among motor scores are likely to be similar to the Bayley-III. Reference Bayley17 A weakness of the Bayley III is that it is based on pass/fail of items for age and does not give the opportunity for recording muscle tone, tremor, or quality of movement. Depending on the age of the child, there are a variety of measures of motor skills that may be useful for determining the need for developmental intervention for these more specific issues. Reference Uzark, Smith, Donohue, Yu and Romano13,Reference Peyton and Einspieler19,Reference Piper and Darrah22,Reference Folio and Fewell24
To address the second hypothesis of this study, we sought associations between motor scores and clinical risk factors. Various predictors of motor outcomes have been published, primarily from acute care pre-, peri-, and post-surgical periods including a variety of different cardiac defects, especially those after palliative surgery, imaging determined brain injury, longer duration of mechanical ventilation, and longer hospital and intensive care stay. Reference Sprong, Broeders and van der Net3,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov4,Reference Sprong, van Brussel and de Vries8,Reference Stegeman, Sprong and Breur9,Reference Stieber, Gilmour and Morra11,Reference Ehrler, von Rhein and Schlosser12 A unique aspects of this study was combining variables reflecting the health of the child at 21 months with the acute care surgical variables and thus expanding the predictive risk factors studied. The overall percentage of variance determined by the variables was greater for the gross motor scaled scores than for the fine motor scaled scores. The toddler chronic health-associated variables for the gross motor scaled scores added more to the prediction explained by the complex cardiac surgery variables than for the fine motor scaled scores, suggesting toddlers with lower gross motor scores have been sicker and still required more medical interventions over time than the other children.
The same predictive variables were considered for each of the multivariate regressions for fine and gross motor outcomes. The chronic health of the child at assessment as measured by the number of medical specialists was important for each outcome and was highly associated with gross motor outcomes in the multivariate regression. This speaks to the importance of establishing the best possible post-surgery health for each child. Possibly modifiable acute care predictors for fine motor outcome included lowest arterial pH and days ventilated where improvements may be made. For gross motor outcomes, total hospital days, a well-known predictor of adverse outcomes, reflects increased illness and complications. Reference Pagowska-Klimek, Pychynska-Pokorska, Krajewski and Moll35 Reducing length of stay is a major goal for most centres. The reasons why gastrostomy was associated with reduced gross motor abilities may include hypoxic events and prolonged illness affecting muscle tone. Failure of prone positioning and reduced “tummy time” may also have contributed to the gross motor delay.
One limitation of the prediction of gross motor skills in this study was the absence of information about brain injury, especially stroke, which is vital to understanding and improving the motor outcomes of children after complex cardiac surgery. Reference Stegeman, Sprong and Breur9,Reference Ehrler, von Rhein and Schlosser12,Reference Reitz and Yerebakan36–Reference Dowling, Hynan and Lo38 While this centre does not do routine brain imaging, CT or MRI is done either peri-operatively or peri-catheterisation when there is a clinical indication. We know that 11.7 % of the children in this study had documented brain infarction, giving a p-value of < 0.10 in the univariate analysis for gross motor scaled scores, but this was not an independent predictor. Present literature on the importance of brain injury from fetal life to post-surgery is dramatically increasing and in time will help to find ways to improve outcomes. Reference Reitz and Yerebakan36–Reference Peyvandi, Lim and Marini39 This study did not attempt to find the best predictors of motor skills, rather used common predictors to make comparisons between the motor outcomes. The combined “increased risk” variable may reflect events of differing importance, hence lack significance in the regressions.
A strength of this study is that it included children at a uniform age from the same surgical site and assessed in the same developmental clinic with specific standardised training for each assessor. Other strengths include the relatively large cohort of children, prospective enrolment after early complex cardiac surgery, prospectively recorded pre-specified potential predictor variables (including chronic childhood health markers), and the high rate of follow-up achieved.
Conclusions
For toddlers after early complex cardiac surgery, we confirmed that gross motor scores often lagged behind fine motor skills and that motor composite scores did not adequately represent the gross motor scaled scores. Where possible, we recommend reporting separate fine and gross motor scores to allow for the best developmental interventions and the best determination of clinical risk factors. For this post-complex cardiac surgery population, future studies could consider de-emphasising reporting of the composite scores and instead focus on the subset motor scores. The gross motor scores could then be used as outcomes for specific developmental interventions for gross motor development, for trends in improvement of outcomes, and for determining predictors and improving both acute and chronic care.
Acknowledgements
We deeply appreciate the children and their parents for their willingness to attend developmental follow-up and their ongoing cooperation with research to improve the care of future children. We thank Helen Knorren-McGrath, registered paediatric psychologist (retired), for her supervision and assessment of these children over the years of this study.
Financial support
This study was primarily unfunded, with support from the Glenrose Rehabilitation Hospital. This hospital had no role in the design and conduct of the study; analysis or interpretation of data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Competing interests
None.
Author contribution
CMTR conceptualised and designed the study, drafted the manuscript, assisted with the planning of the developmental outcomes data collection, and had full access to all study data. JAS gave specialty knowledge for the study, as well as the interpretation of the outcome data. ARJ supervised acute care data collection and critically reviewed the manuscript. IAD and SK preformed statistical analysis. All authors read and approved the final manuscript.
Social media synopsis
Gross motor scores are often lower than fine motor scores after complex cardiac surgery; separate reporting may give improved identification of predictors of delay.








