- CP
cinnamon polyphenols.
Decreased insulin sensitivity or insulin resistance is associated with the signs and symptoms of the metabolic syndrome including increased visceral obesity, fasting glucose, elevated TAG, decreased HDL and hypertension. The metabolic syndrome is often a precursor of diabetes and CVD. Factors that improve insulin sensitivity usually lead to improvements in risk factors associated with the metabolic syndrome, diabetes and CVD. According to the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) criteria(1), individuals with three or more of the following have the metabolic syndrome: fasting plasma glucose level of >6·1 mmol/l (1100 mg/l), TAG ≥1·69 mmol/l (1500 mg/l), HDL-cholesterol <1·04 mmol/l (400 mg/l) for men and <1·29 mmol/l (500 g/l) for women, blood pressure ≥130/85 mm Hg and waist circumference >1·02 m for men and >0·88 m for women. The incidence of the metabolic syndrome varies from <20% among the Chinese and Korean populations to >50% among Maori and Pacific Islanders in New Zealand(Reference Qiao, Gao, Zhang, Nyamdorj and Tuomilehto2). Approximately one in four Americans has the metabolic syndrome. It is progressive and often culminates with type 2 diabetes, which increases the incidence of CVD. In patients with the metabolic syndrome the relative risk for atherosclerotic CVD ranges from 1·5 to 3·0 depending on the stage of progression. The risk for developing diabetes is fivefold higher for individuals with the metabolic syndrome compared with those without the syndrome(Reference Grundy3).
Since the metabolic syndrome is multi-factorial, strategies for reducing the incidence and consequences of metabolic syndrome must also be multi-factorial, and factors, nutrients or strategies that affect multiple factors of the metabolic syndrome are likely to yield the greatest benefits.
Chromium
The signs and symptoms of Cr deficiency are the same as those for the metabolic syndrome, i.e. elevated fasting glucose, elevated TAG, low HDL, hypertension and visceral obesity. The hallmark sign of Cr deficiency is impaired glucose tolerance and there have been numerous reports of beneficial effects on individuals with impaired glucose tolerance and type 2 diabetes(Reference Anderson4–Reference Cefalu and Hu6). In a study involving 155 subjects with type 2 diabetes a dose response to Cr has been reported for fasting glucose, postprandial glucose, fasting insulin, postprandial insulin, cholesterol and Hb A1c(Reference Anderson, Cheng, Bryden, Polansky, Chi and Feng7). Similar results have been reported by other research groups(Reference Ghosh, Bhattacharya, Mukherjee, Manna, Sinha, Chowdhury and Chowdhury8, Reference Rabinovitz, Friedensohn, Leibovitz, Gabay, Rocas and Habot9).
A key to controlling the metabolic syndrome is to prevent or alleviate visceral obesity(Reference Grundy3). A recent well-controlled study has demonstrated that weight gain in subjects with type 2 diabetes is clearly regulated by supplemental Cr(Reference Martin, Wang, Zhang, Wachtel, Volaufova, Matthews and Cefalu10). Thirty-seven subjects with type 2 diabetes were placed on sulfonylurea drugs to control blood sugar for 3 months and then randomized to receive either Cr or placebo. Subjects receiving the supplemental Cr were found to have smaller increases in body weight, percentage body fat and total abdominal fat compared with those in the placebo group. Subjects receiving Cr were also shown to have increased insulin sensitivity, corrected for fat-free mass, and decreased NEFA (Fig. 1)(Reference Martin, Wang, Zhang, Wachtel, Volaufova, Matthews and Cefalu10).

Fig. 1. Chromium decreases visceral fat, subcutaneous fat and total fat. Thirty-seven subjects with type 2 diabetes were placed on sulfonylurea medication for 3 months followed by 6 months of either 1000 μg chromium as chromium picolinate/d (![]() ) or placebo (■). After chromium supplementation body weight, glucose, insulin and NEFA were lower than for the placebo group. Mean values were significantly different from those for the placebo: *P<0·05, **P<0·01. (Adapted from Martin et al.(Reference Martin, Wang, Zhang, Wachtel, Volaufova, Matthews and Cefalu10).)
) or placebo (■). After chromium supplementation body weight, glucose, insulin and NEFA were lower than for the placebo group. Mean values were significantly different from those for the placebo: *P<0·05, **P<0·01. (Adapted from Martin et al.(Reference Martin, Wang, Zhang, Wachtel, Volaufova, Matthews and Cefalu10).)
In a study involving twenty male and twenty female swimmers receiving 400 μg Cr as chromium picolinate/d, Cr was found to significantly increase lean body mass (3·3%), decrease fat mass (−4·6%) and decrease percentage body fat (−6·4%) compared with the placebo group(Reference Bulbulian, Pringle and Liddy11). Females were found to have a greater change for percentage body fat compared with males (−8·2 and −4·7% respectively). Effects were not significant after 12 weeks but became significant only after 24 weeks. This study supports the concept that studies involving Cr supplementation and lean body mass should be longer than 12 weeks and should involve ≥400 μg supplemental Cr/d(Reference Anderson12).
Cr also decreases cortisol concentration in human subjects(Reference Anderson, Bryden, Polansky and Thorp13), which is important in relation to weight control because cortisol increases circulating insulin and increases fat accumulation(Reference Freedman, Horwitz and Stern14). Adrenalectomy of obese rats leads to a normalizing of insulin and decreased fat accumulation, and after glucocorticoid administration there is a return to elevated insulin levels and accumulation of fat(Reference Strack, Sebastian, Schwartz and Dallman15).
Studies involving improved lean body mass as a result of supplemental Cr in human subjects are supported by animal studies conducted mainly using pigs; Cr increases longissimus muscle area and decreases percentage fat in pigs(Reference Page, Southern, Ward and Thompson16–Reference Wang and Xu18). Following the original studies showing beneficial effects of Cr on lean body mass, pig producers started adding Cr to the feed of sows, which would also affect the Cr status of the young pigs(Reference Mooney and Cromwell19). Goats fed a high-refined-carbohydrate low-Cr diet also have increased feed consumption and corresponding weight gain compared with animals consuming the same diet with added Cr(Reference Frank, Anke and Danielsson20). The increases in weight gain are attributed to the antilipolytic effects of increased insulin leading to accumulation of TAG in the adipose tissue. Elevated insulin levels in the animals receiving the low-Cr diet would also lead to decreased glucagon. As glucagon stimulates lipolysis, decreased glucagon may lead to decreased lipolysis and subsequent accumulation of body fat and weight gain. There were no effects until after 28 weeks on the low-Cr diet of low nutritional quality(Reference Frank, Anke and Danielsson20). If it takes >28 weeks to detect significant changes in body weight in rapidly-growing goats, it is not surprising that most of the human studies, which are usually ⩽12 weeks in duration, are also unable to detect significant changes in individuals consuming conventional diets.
A meta-analysis of several human studies has reported that there is a significant reduction in body weight caused by Cr, but it states that ‘a body weight reduction of 1·1 to 1·2 kg during an intervention period of 10 to 13 weeks (i.e. 0·08 to 0·1 kg/week) seems too small to be clinically meaningful’(Reference Pittler, Stevinson and Ernst21). Improvements in this range, if sustained, could lead to a loss, or prevention of gain, of approximately 4 kg/year, which certainly could lead to large changes over time. Even if Cr only prevents the increase in body weight of 0·5–1 kg/year, it becomes consequential with time. Improvements in insulin-related variables that affect body weight and lean body mass are a result of changes in metabolism and should not be confused with those associated with changes in dietary intake and energy expenditure. Lasting changes in insulin sensitivity and changes in metabolism could lead to lasting changes in body weight and composition. Additional long-term studies in this area are needed.
Increased insulin resistance also leads to an increased incidence of CVD(Reference Grundy3). It has been shown that improvements in risk factors associated with the metabolic syndrome also lead to improvements in heart function (Fig. 2)(Reference Vrtovec, Vrtovec, Briski, Kocijancic, Anderson and Radovancevic22). Sixty patients with type 2 diabetes were randomly assigned to two groups and one group was given 1000 μg Cr/d and the other the placebo. After 3 months, QT interval (a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) corrected for heart rate for the patients receiving Cr was found to be decreased. The rate-corrected QT interval is a powerful predictor of total mortality, cardiac death and future stroke in patients with type 2 diabetes mellitus, and is inversely related to insulin sensitivity. BMI was found to be the only variable predictive of the shortening of the rate-corrected QT interval(Reference Vrtovec, Vrtovec, Briski, Kocijancic, Anderson and Radovancevic22).

Fig. 2. Supplemental chromium decreases the QT interval (a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) corrected for heart rate (QTc interval). Thirty patients with type 2 diabetes received 1000 μg chromium as chromium picolinate and thirty received placebo. It has been shown that: QTc is a strong predictor of total mortality and stroke; in patients with diabetes QTc is a independent of other risk factors and related to impaired glucose metabolism; QTc interval is inversely related to insulin sensitivity; Cr also improves cholesterol, DL, LDL and TAG. a,bValues with unlike superscript letters were significantly different (P<0·05). (Adapted from Vrtovec et al.(Reference Vrtovec, Vrtovec, Briski, Kocijancic, Anderson and Radovancevic22).)
The effects of Cr on lean body mass, blood lipids, glucose, insulin and related variables vary among the studies and a large number of studies do not report beneficial effects of supplemental Cr. The fact that not all studies show beneficial effects of supplemental Cr(Reference Anderson12, Reference Vincent23, Reference Kobla and Volpe24) is consistent with the expected observations that not all individuals are marginally or overtly deficient in Cr. In addition to the selection of subjects, duration of study and form of Cr, the effects of Cr may be masked by poor diets and a sedentary lifestyle. Cr should be considered as one factor that affects insulin sensitivity and related lean body mass but is certainly not, for most individuals, the dominant factor(Reference Anderson12).
Another possible reason for the variable response to Cr may be combined altered glucose and cholesterol metabolism(Reference Pattar, Tackett, Liu and Elmendorf25). Cr added to adipocytes (3T3-L1 cells) induces a loss of plasma membrane cholesterol that is linked to GLUT4 translocation. GLUT4 redistribution in cells treated with chromium picolinate occurs only in cells treated with high glucose, conditions that resemble the diabetic state, and not in cells cultured under normal conditions. There may need to be both impaired glucose and cholesterol homeostasis for supplemental Cr to be beneficial(Reference Pattar, Tackett, Liu and Elmendorf25).
Cinnamon
In 1990 it was reported that compounds found in cinnamon (Cinnamomon cassia) have insulin-potentiating properties and may be involved in the alleviation of the signs and symptoms of diabetes and CVD related to insulin resistance(Reference Khan, Bryden, Polansky and Anderson26). Aqueous extracts of cinnamon have been shown to potentiate insulin activity >20-fold, higher than any other compound tested at comparable dilutions, in an in vitro assay of the insulin-dependent utilization of glucose(Reference Broadhurst, Polansky and Anderson27). Water-soluble cinnamon compounds also stimulate the autophosphorylation of the insulin receptor(Reference Imparl-Radosevich, Deas, Polansky, Baedke, Ingebritsen, Anderson and Graves28) and inhibit phophotyrosine phosphatase, an enzyme functioning in the dephosphorylation of the insulin receptor(Reference Imparl-Radosevich, Deas, Polansky, Baedke, Ingebritsen, Anderson and Graves28). This inhibition is specific since there is no inhibition of alkaline phosphatase. The activation of the phosphorylation and the inhibition of the dephosphorylation of the insulin receptor leads to increased phosphorylation of the insulin receptor, which is associated with increased insulin sensitivity. Subjects with type 2 diabetes mellitus have reduced phosphorylation of the insulin receptor(Reference Cusi, Maezono, Osman, Pendergrass, Patti, Pratipanawatr, DeFronzo, Kahn and Mandarino29).
In rats fed a control diet, the administration of aqueous extracts of cinnamon improves glucose metabolism and potentiates the action of insulin(Reference Qin, Nagasaki, Ren, Bajotto, Oshida and Sato30). Euglycaemic clamp studies have shown that after 3 weeks of oral administration of an aqueous cinnamon extract at 30 and 300 mg kg/body weight there is greater glucose utilization. Skeletal-muscle insulin-stimulated insulin receptor-β and insulin receptor substrate-1 tyrosine phosphorylation levels and insulin receptor substrate-1: phosphoinositide 3-kinase are also increased. These results suggest that increased glucose uptake is a result of enhancing of the insulin-signalling pathway(Reference Qin, Nagasaki, Ren, Bajotto, Oshida and Sato30). Cinnamon extract fed to animals consuming a high-fructose diet also prevents the development of the metabolic syndrome(Reference Qin, Nagasaki, Ren, Bajotto, Oshida and Sato31).
Following the observations that cinnamon potentiates insulin action in vitro, a human study was conducted involving sixty subjects with type 2 diabetes (thirty males and thirty females) who were taking sulfonylurea drugs(Reference Khan, Safdar, Ali Khan, Khattak and Anderson32). The subjects were divided randomly into six groups. Groups 1, 2 and 3 received 1, 3, or 6 g cinnamon/d for 40 d. From day 40 to day 60 there was a washout period in which subjects did not receive capsules. Groups 4, 5 and 6 received the same number of placebo capsules as the corresponding cinnamon groups. After 40 d all three levels of cinnamon were found to have reduced mean fasting serum glucose (18–29%; three groups, each of ten subjects), TAG (23–30%), total cholesterol (12–26%) and LDL-cholesterol (7–27%). Values after the 20 d washout period were returning to baseline but were still lower than the values at the onset of the study. In a separate study involving twenty-two subjects with the metabolic syndrome, subjects were divided into two groups and given daily either 500 mg commercially-available aqueous extract of cinnamon (Cinnulin PF®; Integrity Nutraceuticals, Sarasota, FL, USA) or a placebo for 12 weeks. Subjects in the group receiving the capsules containing the aqueous extract of cinnamon were reported to display decreases in fasting blood glucose (–8·4%) and systolic blood pressure (–3·8%) and increases in lean mass (+1·1%) compared with the placebo group. There were also significant decreases in body fat (–0·7%) in the cinnamon-treatment group(Reference Ziegenfuss, Hofheins, Mendel, Landis and Anderson33).
Oxidative stress, which is increased in obesity, plays an important role in the development of diabetes and CVD in obese individuals(Reference Yu and Lyons34). Hyperglycaemia causes the auto-oxidation of glucose, glycation of proteins and the activation of polyol metabolism(Reference Robertson35). Cinnamon has been reported to improve the antioxidant status of subjects with the metabolic syndrome(Reference Roussel, Hininger, Ziegenfuss and Anderson36). Plasma malondialdehyde levels were found to be reduced by the aqueous extract of cinnamon, indicating decreased lipid peroxidation, while plasma SH groups were increased, indicating a protection of antioxidant SH groups against oxidation. In the group receiving cinnamon, plasma SH groups were found to be increased after 12 weeks of supplementation, suggesting that cinnamon acts in protecting both lipids and proteins against oxidation. In parallel, the ferric-reducing ability power, which is a measure of the total antioxidant capacity of plasma, was shown to be increased, thereby providing a contributory factor to the protective effects of cinnamon supplementation(Reference Roussel, Hininger, Ziegenfuss and Anderson36).
Polycystic ovary syndrome is one of the most common endocrinopathies among women of child-bearing age, affecting 5–10% of the population(Reference Wang, Anderson, Graham, Chu, Sauer, Guarnaccia and Lobo37). Insulin resistance and the compensatory hyperinsulinaemia are present in 50–70% of the women with polycystic ovary syndrome and maybe as high as 95% in overweight women. Excess insulin secretion may also be implicated in the increased metabolic and cardiovascular risks reported in this disorder. Since insulin-sensitizing agents such as Cr(Reference Lucidi, Thyer, Easton, Holden, Schenken and Brzyski38) and troglitazone(Reference Bayram, Unluhizarci and Kelestimur39) have been shown to be beneficial in the treatment of polycystic ovary syndrome, it has been postulated that the insulin-potentiating water-soluble polyphenolic compounds found in cinnamon may also be beneficial for women with polycystic ovary syndrome(Reference Wang, Anderson, Graham, Chu, Sauer, Guarnaccia and Lobo37). During an 8-week treatment period oral cinnamon extract (500 mg/d) was shown to reduce fasting glucose as well as insulin resistance, with oral glucose tolerance tests also showing a 21% reduction in mean glucose and an increase in Matsuda's insulin sensitivity index(Reference Matsuda and DeFronzo40). The cinnamon extract was found to improve insulin resistance of the women with polycystic ovary syndrome compared with the control women.
The beneficial effects of cinnamon were greater in the study of Khan et al.(Reference Khan, Safdar, Ali Khan, Khattak and Anderson32) than those observed in the studies of Ziegenfuss et al.(Reference Ziegenfuss, Hofheins, Mendel, Landis and Anderson33), Mang et al. (Reference Mang, Wolters, Schmitt, Kelb, Lichtinghagen, Stichtenoth and Hahn41) and Wang et al.(Reference Wang, Anderson, Graham, Chu, Sauer, Guarnaccia and Lobo37), but the subject populations were very different. Subjects in the Khan et al. (Reference Khan, Safdar, Ali Khan, Khattak and Anderson32) study had type 2 diabetes and were taking sulfonylurea drugs that increase insulin secretion. Since compounds found in cinnamon increase insulin sensitivity, they are likely to have larger effects in subjects taking sulfonylurea drugs. Insulin resistance would also be larger in subjects with type 2 diabetes than in subjects who are still prediabetic. The duration of the supplementation is also important to consider, since in the studies of Ziegenfuss et al.(Reference Ziegenfuss, Hofheins, Mendel, Landis and Anderson33) and Roussel et al.(Reference Roussel, Hininger, Ziegenfuss and Anderson36) no effects on blood glucose were found after a 6-week intervention of supplementation with an aqueous cinnamon extract (500 mg/d), but were found after 12 weeks. Similarly, no beneficial effects were found in post-menopausal women with type 2 diabetes after only 6 weeks(Reference Vanschoonbeek, Thomassen, Senden, Wodzig and van Loon42). Antioxidant effects were also not found to be significant after 6 weeks but were significant after 12 weeks in the study of Roussel et al.(Reference Roussel, Hininger, Ziegenfuss and Anderson36). Whether differences in hormonal milieu affect the potential interaction between cinnamon supplementation and glucose control is unknown at this time. In all the human studies involving cinnamon, or aqueous extracts of cinnamon, there have been no reported adverse events and subjects with the poorest glycaemic control appear to benefit the most.
Model of cinnamon effects
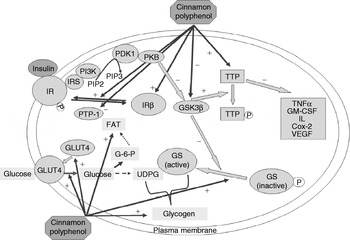
A model of the potential diverse effects of cinnamon is depicted in Fig. 3(Reference Cao, Polansky and Anderson43). Cinnamon polyphenols (CP) affect multiple steps related to glucose and insulin function. CP activate insulin receptors by increasing their tyrosine phosphorylation activity and by decreasing phosphatase activity that inactivates the insulin receptor(Reference Imparl-Radosevich, Deas, Polansky, Baedke, Ingebritsen, Anderson and Graves28). CP also increase the amount of insulin receptor β and GLUT4 protein(Reference Cao, Polansky and Anderson43). CP increase glycogen synthase activity and glycogen accumulation(Reference Jarvill-Taylor, Anderson and Graves44) with decreased glycogen synthetase kinase-3 β activity(Reference Jarvill-Taylor, Anderson and Graves44). CP also increase the amount of the early-response anti-inflammatory protein, tristetraprolin(Reference Cao, Polansky and Anderson43). All these activities and other potential activities may eventually lead to more efficient glucose transport and utilization. In addition, CP-induced tristetraprolin accumulation may provide one of the molecular bases for the beneficial effects of cinnamon in improving the conditions of individuals with metabolic syndrome and insulin resistance by down regulating the synthesis of pro-inflammatory cytokines. It has also been reported that improvements in postprandial blood glucose response are related to gastric emptying rate but that the effects on postprandial glucose are greater than those on gastric emptying rate(Reference Hlebowicz, Darwiche, Bjorgell and Almer45).

Fig. 3. A model of actions of cinnamon polyphenols (CP) in the insulin signal transduction pathway leading to beneficial effects in subjects with glucose intolerance or type 2 diabetes: (1) CP activate insulin receptors (IR) by increasing their tyrosine phosphorylation activity and by decreasing phosphatase activity that inactivates the receptor; (2) CP increase the amount of insulin receptor-β and GLUT4 proteins; (3) CP increase glycogen synthase activity and glycogen accumulation; (4) CP decrease glycogen synthetase (GS) kinase-3 β (GSK3β) activity; (5) CP increase the amount of tristetraprolin (TTP) protein; (6) CP may increase the activity of TTP by decreasing its phosphorylation through inhibition of GSK3β activity. IRS, insulin receptor substrate; PI3K, 1-phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PTP-1, protein tyrosine phosphatase-1; PDK1, phosphatidylinositol-dependent protein kinase 1; FAT, fat; G-6-P, glucose 6-phosphate; PKB, protein kinase B; UDPG, uridine diphosphoglucose; GM-CSF, granulocyte–macrophage colony-stimulating factor; Cox2, cyclooxygenase-2; VEGF, vascular endothelial growth factor; –, negative effect; +, positive effect. (From Cao et al.(Reference Cao, Polansky and Anderson43).)
Conclusions
In summary, naturally-occurring insulin-potentiating compounds such as Cr and CP lead to increased insulin sensitivity characterized by improvements in characteristics of the metabolic syndrome and decreases in risk factors associated with diabetes and CVD. Individuals with metabolic syndrome, and the subsequent diabetes and CVD, have both decreased insulin sensitivity and decreased antioxidant status. Animal and human studies involving subjects with the metabolic syndrome, type 2 diabetes and polycystic ovary syndrome show beneficial effects of Cr, whole cinnamon and aqueous extracts of cinnamon on glucose, insulin, lipids and antioxidant status. There also may be effects on lean body mass and body composition, and inflammatory response. All these effects would lead to decreased risk factors associated with diabetes and CVD and improvements in the metabolic syndrome leading to decreased incidences of these diseases.