Insulin secretion from pancreatic β-cells is regulated by several factors including fuels, hormones and neurotransmitters. These agents modify the intracellular concentrations of several β-cell regulators such as Ca2+, phospholipids and cyclic nucleotides that influence the amplitude and shape of the insulin secretory response(Reference Jones and Persaud1, Reference Tengholm and Gylfe2).
Ca2+ influx contributes to the insulin secretory process, regulating the docking and fusion of the secretory granules with the plasma membrane(Reference Rorsman and Renström3), whereas Ca2+ mobilised from intracellular stores is essential for the replenishment of the readily releasable pool of secretory granules(Reference Gromada, Høy and Renström4). In addition, Ca2+ contributes to the amplification of secretion by activating several enzymes such as phospholipase C (PLC) and adenylyl cyclase, which generate the intracellular messengers inositol-1,4,5-trisphosphate (IP3) plus diacylglycerol, and cAMP, respectively(Reference Leech, Castonguay and Habener5–Reference Thore, Wuttke and Tengholm9).
Various hormones and neurotransmitters acting through specific receptors, located at the plasma membrane, potentiate insulin secretion via stimulation of PLC, generating IP3 and diacylglycerol(Reference Jones and Persaud1). IP3 mediates rapid mobilisation of Ca2+ from the endoplasmic reticulum, whereas diacylglycerol stimulates protein kinase (PK) C(Reference Liu and Gylfe10, Reference Berridge, Bootman and Roderick11). PLC contributes to the insulin secretion not only when membrane receptors are activated, but also when the intracellular Ca2+ concentration increases in response to glucose(Reference Thore, Wuttke and Tengholm9).
Evidence points to an inter-relationship between cAMP and intracellular Ca2+ concentration. It is known that cAMP–PKA mobilises Ca2+ from intracellular stores and regulates the activity of the L-type Ca2+ channels(Reference Ammala, Eliasson and Bokvist12–Reference Dyachok and Gylfe15). cAMP also alters the intracellular Ca2+ concentration, modifying Ca2+ oscillations from slow to fast in the presence of stimulatory glucose concentrations(Reference Grapengiesser, Gylfe and Hellman16). In addition, PKA contributes to the first phase of insulin secretion, phosphorylating some proteins involved in the exocytotic process(Reference Seino and Shibasaki17, Reference Hatakeyama, Kishimoto and Nemoto18).
Taurine (TAU), a naturally occurring sulfur-containing amino acid, regulates several biological processes, including osmolarity(Reference Schaffer, Takahashi and Azuma19), Ca2+ binding and transport(Reference Satoh20–Reference Palmi, Youmbi and Fusi22), ion channel activity(Reference Lee, Lee and Kim23, Reference Park, Bae and Kim24), insulin secretion, and glucose homeostasis(Reference Cherif, Reusens and Dahri25–Reference Loizzo, Carta and Bennardini28). Previous data from our laboratory have shown that TAU supplementation improves glucose tolerance and insulin sensitivity in mice and increases nutrient-induced insulin secretion in isolated islets. Islets from TAU-supplemented mice show increased Ca2+ uptake and higher expression of the L-type β2 subunit Ca2+ channel(Reference Ribeiro, Bonfleur and Amaral27). It has also been demonstrated that TAU affects the kinetics of Ca2+ movement in different tissues(Reference Satoh20–Reference Lee, Lee and Kim23). Despite reports of a regulatory role of TAU in insulin secretion and Ca2+ handling(Reference Carneiro, Latorraca and Araujo26, Reference Ribeiro, Bonfleur and Amaral27), little is known about its effects on cholinergic/PLC and PKA pathways in β-cells. These mechanisms regulate and are regulated by intracellular Ca2+ concentration in β-cells(Reference Thore, Wuttke and Tengholm9, Reference Ammala, Eliasson and Bokvist12–Reference Dyachok and Gylfe15, Reference Dyachok, Idevall-Hagren and Sagetorp29).
In the present study, we confirm that TAU supplementation increases insulin secretion in response to glucose in isolated islets. We also show that the secretory capacity and Ca2+ handling in these islets were higher in conditions in which IP3 and cAMP production was increased. Data indicate that TAU modulation of these processes seems to be linked to the activation of cholinergic/PLC and PKA pathways.
Methods and materials
Materials
45CaCl2 and [125I]human insulin were purchased from Amersham International (Little Chalfont, Bucks, UK). Routine reagents, phorbol 12-myristate 13-acetate (PMA), carbachol (Cch), forskolin and 3-isobutyl-1-methyl-xanthine (IBMX), N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline sulfonamide (H89) were purchased from Sigma Chemical (St Louis, MO, USA). PK inhibitor-(6–22)-amide (PKI) was purchased from Calbiochem (San Diego, CA, USA).
Animals
All experiments were approved by the ethics committee at the Universidade Estadual de Campinas (UNICAMP). Swiss mice, aged 3 weeks, were obtained from the colony at UNICAMP. The mice were maintained on a 12 h light–dark cycle (lights on from 06.00 until 18.00 hours), controlled temperature (22 ± 1°C), and allowed free access to water and standard laboratory chow (rodent chow; Nutrilab, Colombo, Brazil) ad libitum. At 60 d, mice were distributed into two groups: mice that received 2 % of TAU in their drinking water for 30 d (TAU group; as previously reported by Ribeiro et al. (Reference Ribeiro, Bonfleur and Amaral27)) and those that received only water (control (CTL) group). Drinking water was changed three times per week. Daily TAU intake has been previous reported(Reference Ribeiro, Bonfleur and Amaral27).
Islet isolation and static insulin secretion
Islets were isolated by collagenase digestion of the pancreas. For static incubations, groups of four islets were first incubated for 30 min at 37°C in Krebs–Ringer bicarbonate (KRB) buffer containing 115 mm-NaCl, 5 mm-KCl, 24 mm-NaHCO3, 2·56 mm-CaCl2, 1 mm-MgCl2 and 25 mm-HEPES with 2·8 mm-glucose and 3 g bovine serum albumin per litre, and equilibrated with a mixture of 95 % O2–5 % CO2 to give pH 7·4. This medium was then replaced with fresh buffer and the islets incubated for 1 h under the following conditions: glucose (8·3 mmol/l) alone or with Cch (100 μmol/l), PMA (100 nmol/l), forskolin (10 μmol/l) and IBMX (1 mmol/l). At the end of the incubation period, the insulin content of the medium was measured by RIA using human insulin radiolabelled with 125I as tracer, rat insulin as standard (Crystal Chem Inc., Downers Grove, IL, USA) and rat insulin antibody (donated by Dr Leclerq-Meyer, Free University of Brussels, Brussels, Belgium). The charcoal–dextran method was used to separate free insulin from antibody-bound [125I]insulin(Reference Scott, Atwater and Rojas30).
Dynamic insulin secretion
Groups of freshly isolated islets were placed on Millipore SW 1300 filters (8·0 μm pore) and perifused at a flow rate of 1 ml/min with KRB buffer gassed with 95 % O2–5 % CO2 (pH 7·4) and maintained at 37°C. To study the dynamic insulin secretion in response to Cch, groups of seventy islets were perifused with Ca2+-free KRB buffer containing 8·3 mm-glucose plus 250 μm-diazoxide and 10 mm-ethylene glycol tetraacetic acid (EGTA), with or without 100 μm-Cch, as indicated in the figure legends and Results section. In another series of experiments, groups of fifty islets were perifused in a KRB containing 8·3 mm-glucose (basal condition) for 30 min and, after this period, IBMX (1 mmol/l) or forskolin (10 μmol/l) was added to the perifusion solution (for more information, see figure legends). Insulin release was measured by RIA.
Taurine islet and tissue content analysis
The analysis of TAU content in isolated islets, pancreas and liver was performed with the reverse-phase HPLC method. The islets and tissue fragments were disrupted using a Polytron PT 1200 C (Brinkmann Instruments, Westbury, NY, USA) or a Polytron PT 10-95 (Kinematica, Lucerne, Switzerland) homogeniser in a 5-sulfosalicylic acid (35 %, w/v) solution. The homogenates were incubated at room temperature for 1 h to extract the amino acids and were then centrifuged at 20 800 g at 20°C for 10 min. The amino acids present in the supernatant fraction were derivatised with triethylamine and phenylisothiocyanate (PTC) to form free amino acid–phenylthiocarbamyl derivatives(Reference Bidlingmeyer, Cohen and Tarvin31). The PTC–amino acid derivatives in each sample were suspended in 5 mm-sodium phosphate buffer containing 5 % acetonitrile and then were separated and quantified by UV (254 nm) in 20 μl samples using an automated Milton Roy LDC MP3000 HPLC system (Milton Roy, Ivyland, PA, USA) and Picotag C18 5 μm column (Waters Corp., Milford, MA, USA). Elution was performed at 1 ml/min at 38°C using a gradient of 0·14 m-sodium acetate, 0·05 % triethylamine (pH 5·7) as solution A, and acetonitrile–water (3:2, v/v) as solution B. Amino acid standards (Standard H; Pierce Protein Reagent Products, Pittsburgh, PA, USA) were derivatised and analysed together with the samples. Tissue protein content was determined by the Bradford method(Reference Bradford32) using bovine serum albumin as the standard curve and Bradford reagent (Bio-Agency Lab., São Paulo, SP, Brazil). Amino acid content for islet samples was expressed in pmol/μg protein as previously reported(Reference Bustamante, Lobo and Alonso33) and for liver and pancreas as nmol/mg protein.
Uptake of 45Ca
Groups of 150 to 200 islets, derived from the same batch of islets, were pre-incubated for 30 min at 37°C in a KRB buffer containing 2·8 mm-glucose (pH 7·4). The islets were then incubated for 30 min in 200 μl of the same medium containing 45CaCl2 (2·22 MBq; 60 μCi/ml) and 8·3 mm-glucose alone or with IBMX (1 mmol/l), or H89 (10 μmol/l) or PKI (5 μmol/l). At the end of the incubation period, 800 μl of ice-cold KRB containing 2 mm-LaCl3 (pH 7·4) was added to stop the reaction. The medium was then removed and a sample was saved to determine the amount of 45Ca in the solution. The islets were subsequently washed three times with ice-cold KRB containing La3+ and islets were then placed in a Petri dish. Groups of ten islets were transferred to counting vials containing 1 ml EGTA (50 mmol/l). The uptake of 45Ca was expressed as pmol Ca2+ per islet per 30 min of incubation.
Cytoplasmic Ca2+ oscillations
Fresh pancreatic islets were incubated with fura-2-acetoxymethyl ester (FURA 2 AM) (5 μmol/l) for 1 h at room temperature in KRB buffer containing 5·6 mm-glucose (pH 7·4) and supplemented with bovine serum albumin. Islets were washed with the same medium and placed in a chamber that was thermostatically regulated at 37°C on the stage of an inverted microscope (Nikon UK, Kingston upon Thames, Surrey, UK). Islets were then perifused with Ca2+-free KRB continuously gassed with 95 % O2–5 % CO2 (pH 7·4) at 37°C containing 8·3 mm-glucose plus 250 μm-diazoxide and 10 mm-EGTA with or without 100 μm-Cch. A ratio image was acquired approximately at every 5 s with an ORCA-100 CCD camera (Hammamatsu Photonics Iberica, Barcelona, Spain), in conjunction with a Lambda-10-CS dual filter wheel (Sutter Instrument Company, Novato, CA, USA), equipped with 340 and 380 nm, 10 nm bandpass filters, and a range of neutral density filters (Omega Opticals, Stanmore, Middlesex, UK). Data were obtained using the ImageMaster3 software (Photon Technology International, Birmingham, NJ, USA).
Western blotting
Isolated islets from TAU and CTL groups were solubilised in 100 μl homogenisation buffer containing: 100 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) (pH 7·5), 10 mm-sodium pyrophosphate, 100 mm-sodium fluoride, 10 mm-EDTA, 10 mm-sodium vanadate, 2 mm-phenylmethylsulfonyl fluoride and 1 % Triton-X 100. The islets were disrupted using a Polytron PT 1200 C homogeniser (Brinkmann Instruments, Westbury, NY, USA), employing 10 s pulses for three times. The extracts were then centrifuged at 12 600 g at 4°C for 5 min to remove insoluble material. The protein concentration in the supernatant fractions was assayed using the Bradford method(Reference Bradford32). For SDS gel electrophoresis and Western blot analysis, the samples were treated with a Laemmli sample buffer containing dithiothreitol. After heating to 95°C for 5 min, the proteins were separated by electrophoresis (55 μg protein/lane, 10 % gels). Following electrophoresis, proteins were transferred to nitrocellulose membranes. The nitrocellulose filters were overnight treated with a blocking buffer (5 % non-fat dried milk, 10 mm-Tris, 150 mm-NaCl and 0·02 % Tween 20) and were subsequently incubated with rabbit polyclonal antibody to PLCβ2 (1:500; H-255; catalogue no. sc-9018), or PKAα (1:500; C-20; catalogue no. sc-903), or mouse monoclonal antibody to PKCα (1:1000; H-7; catalogue no. sc-8393) at 4°C. All primary antibodies used were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Visualisation of specific protein bands was made by incubating the membranes for 2 h with a peroxidase-conjugated secondary antibody (1:10 000; Zymed Laboratories, Inc., San Francisco, CA, USA), followed by detection with enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL, USA) and exposure to X-ray film (Kodak, Manaus, AM, Brazil). The band intensities were quantified by optical densitometry (Scion, Image, Frederick, MD, USA). After assaying the target proteins, Western blotting was repeated using rabbit monoclonal antibody to β-actin (1:10 000; catalogue no. ab8227; Abcam, Cambridge, MA, USA) as an internal control.
Statistical analysis
Results are presented as mean values with their standard errors for the number of determinations (n) indicated. The statistical analyses were carried out using one-way ANOVA followed by the Tukey post test for multiple comparisons or Student's t test for two-group comparisons (P ≤ 0·05) and performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Taurine supplementation
Table 1 shows that the supplementation protocol applied in the present study efficiently increased tissue TAU concentrations, since TAU islet content in isolated islets from supplemented mice was 27 % higher when compared with islets from CTL mice (P < 0·04). In addition, TAU-supplemented mice showed an increase of 60 and 44 % in liver and pancreas TAU content, respectively, when compared with CTL mice (P < 0·04).
Table 1 Taurine content in isolated islet (pmol/μg protein), pancreas and liver (nmol/mg protein) extracts from taurine-supplemented (TAU) and control (CTL) mice
(Mean values with their standard errors)
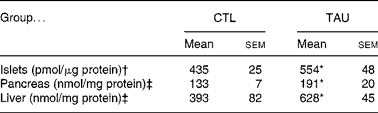
* Mean value was significantly different from that for the CTL mice (P < 0·05).
† The islet samples (n 9) correspond to 100 isolated islets from different mice.
‡ Pancreas and liver extracts were from three or four mice for each group.
Insulin secretion in taurine-supplemented mice islets
Fig. 1 shows the insulin secretion induced by sub- and supra-threshold glucose concentrations. Under basal glucose conditions, insulin secretion was similar between groups. Islets from TAU-supplemented mice released significantly more insulin in the presence of 8·3 and 22·2 mm-glucose, compared with the CTL group (P < 0·03 and P < 0·01, respectively). Since, at a physiological concentration of glucose (8·3 mmol/l), insulin released in TAU islets was significantly different from CTL islets this glucose concentration was used in all subsequent insulin release experiments.

Fig. 1 Glucose-induced insulin secretion in islets from taurine-supplemented (■) and control (□) mice. Groups of four islets were incubated for 1 h with different glucose (G) concentrations: 2·8 mm (G2·8); 8·3 mm (G8·3); 22·2 mm (G22·2). Values are means (n 12–21), with standard errors represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
Carbachol and phorbol 12-myristate 13-acetate-induced insulin secretion
When the islets were incubated in the presence of 100 μm-Cch, insulin secretion was higher in TAU than CTL islets (Fig. 2; P < 0·001). However, the increment in insulin secretion, induced by 100 nm-PMA (that activates PKC), was similar between groups.

Fig. 2 Insulin secretion induced by carbachol (Cch; 100 μm) or phorbol 12-myristate 13-acetate (PMA; 100 nm) in islets from taurine-supplemented (■) and control (□) mice. Islets were incubated for 1 h at 8·3 mm-glucose (G8·3), with or without Cch and PMA. Values are means (n 15), with standard errors represented by vertical bars. * Mean value was significantly different from that of the respective control (P < 0·05).
Carbachol-induced intracellular Ca2+ mobilisation
In the next series of experiments, we analysed intracellular Ca2+ mobilisation in TAU and CTL islets. For this purpose, 100 μm-Cch was added to a perifusion system with a Ca2+-free medium, containing 8·3 mm-glucose, 250 μm-diazoxide and 10 mm-EGTA. Fig. 3(a) shows that the Cch-induced increase in intracellular Ca2+ concentration was higher in TAU than in CTL islets. The area under the curves (AUC) and the amplitude of intracellular Ca2+ concentration were significantly higher in TAU, compared with CTL islets (1·06 (sem 0·1) F340:F380 × min and 0·19 (sem 0·02) ΔF340:F380 v. 0·61 (sem 0·08) F340:F380 × min and 0·13 (sem 0·01) ΔF340:F380, respectively; P < 0·04), where F340:F380 is the fluorescence ratio at 340 and 380 nm. In accordance, the dynamic insulin release was also higher in the TAU group, compared with the CTL group, when challenged with the same concentrations of Cch (Fig. 3(b)). The AUC of the total insulin released (8–20 min) and peak of secretion (at 9 min) were 24 (sem 3) ng per seventy islets × min and 4·3 (sem 0·5) ng per seventy islets v. 17 (sem 2) ng per seventy islets × min and 2·5 (sem 0·4) ng per seventy islets, respectively (P < 0·05), for TAU and CTL islets.

Fig. 3 Carbachol (Cch; 100 μm)-induced internal Ca2+ mobilisation (a) and insulin secretion (b) from taurine-supplemented (—; ■) and control (- - -; □) islets. The experiments were performed in a perifusium system in a Ca2+-free medium containing 8·3 mm-glucose (G8·3), 250 μm-diazoxide and 10 mm-ethylene glycol tetraacetic acid (EGTA). For Ca2+, values are fluorescence ratios at 340 and 380 nm (F340:F380) registered for each group. Data are means obtained from four to six independent perifusion experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
Forskolin and 3-isobutyl-1-methylxanthine-induced insulin secretion
Forskolin (10 μmol/l) and IBMX (1 mmol/l), which increase cAMP by adenylyl cyclase stimulation or by phosphodiesterase inhibition (respectively), significantly stimulated secretion in both types of islets (Fig. 4(a)). However, while the increase in insulin secretion provoked by IBMX was higher in TAU compared with CTL islets (P < 0·05), the increment in the insulin secretion stimulated by forskolin was similar in both groups (Fig. 4(a)). Using a perifusion system, we confirmed that IBMX-potentiated insulin release was significantly higher in TAU compared with CTL islets (Fig. 4(b)). Total insulin release during 15–50 min was 842 (sem 152) and 359 (sem 139) ng per fifty islets × min, respectively (P = 0·05). Dynamic insulin release induced by forskolin was also analysed (Fig. 4(c)). The amount of insulin secreted during the presence of forskolin in the perfusate (15–50 min) was similar between TAU and CTL islets (507 (sem 60) and 380 (sem 27) ng per fifty islets × min, respectively).

Fig. 4 (a) Forskolin (10 μm) and 3-isobutyl-1-methylxanthine (IBMX; 1 mm)-induced insulin secretion in islets from taurine-supplemented (■) and control (□) mice. Islets were incubated for 1 h at 8·3 mm-glucose (G8·3), with or without forskolin and IBMX. (b, c) Dynamic insulin secretion in response to 8·3 mm-glucose with or without IBMX or forskolin in taurine-supplemented and control islets. Data are means obtained from fourteen or fifteen repetitions for static incubation and from four independent perifusion experiments for dynamic measurements, with standard errors represented by vertical bars. * Mean value was significantly different from that of the respective control (P < 0·05).
3-Isobutyl-1-methylxanthine and protein kinase A inhibition effects on 45Ca uptake
As previously observed, TAU-supplemented islets showed increased Ca2+ uptake in the presence of high glucose concentrations(Reference Ribeiro, Bonfleur and Amaral27). In order to analyse the involvement of PKA in Ca2+ handling by the islets, we measured 45Ca uptake in the presence of 8·3 mm-glucose with or without agents that stimulate (IBMX) or inhibit (H89 and PKI) PKA. At 8·3 mm-glucose, the Ca2+ uptake was higher in TAU than CTL islets (Table 2; P < 0·02). IBMX significantly increased Ca2+ uptake in TAU and CTL islets by 31 and 36 %, compared with the respective controls (glucose alone) (Table 2). At the end of the incubation period, both groups of islets reached similar values of Ca2+ uptake. The administration of H89 significantly reduced Ca2+ uptake in TAU (P < 0·0001), but only marginally in CTL islets (P = 0·19). In the presence of PKI, both groups of islets showed a significant decrease in Ca2+ uptake, reaching 54 % in TAU and 24 % in CTL islets, related to the respective control (glucose alone; P < 0·01 and P < 0·001, respectively). These results indicated that the participation of the cAMP–PKA pathway in Ca2+ influx, in response to glucose, was higher in islets from TAU-supplemented mice than in the CTL group.
Table 2 Islet 45Ca uptake (pmol 45Ca/islet per 30 min) in the presence of 8·3 mm-glucose (G8·3) with or without 1 mm-3-isobutyl-1-methylxanthine (IBMX) or 10 μm-N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline sulfonamide (H89) or 5 μm-protein kinase inhibitor-(6–22)-amide (PKI)
(Mean values with their standard errors of three independent experiments with fourteen to thirty-six groups of islets)
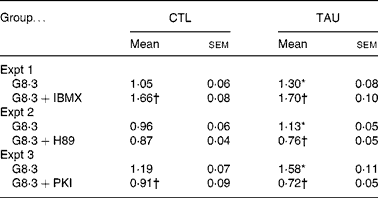
CTL, control; TAU, taurine.
* Mean value was significantly different from that for the G8·3 CTL mice (P < 0·05).
† Mean value was significantly different from that for the G8·3 control mice (P < 0·05).
Phospholipase Cβ2, protein kinase Cα and protein kinase Aα protein expression
Western blotting analysis showed that the expressions of PLCβ2 (Fig. 5(a)) and PKAα (Fig. 5(c)) were two-fold higher in TAU compared with CTL islets (P < 0·02 and P < 0·002, respectively), whereas the expression of PKCα was similar between groups (Fig. 5(b)).

Fig. 5 Phospholipase Cβ2 (PLCβ2), protein kinase (PK) Cα and PKAα protein expressions in islets from taurine (TAU)-supplemented and control (CTL) mice, determined by optical densitometry. Protein extracts were processed for Western blot detection of PLCβ2 (a), PKCα (b), PKAα (c) and β-actin (internal control). Values are means (n 3–5), with standard errors represented by vertical bars. * Mean value was significantly different from that of the CTL (P < 0·05).
Discussion
Recently, we demonstrated that pancreatic islets from TAU-supplemented mice secreted more insulin in response to glucose and that this effect seems to be linked to a higher Ca2+ mobilisation. We also showed increased protein expression of the β2 subunit of the L-type Ca2+ channel in these islets(Reference Ribeiro, Bonfleur and Amaral27). Here, we investigated the mechanisms involved in the higher insulin release in islets from TAU-supplemented mice, and the main findings of the present study suggest that TAU supplementation increases the β-cells' sensitivity to cholinergic/PLC and cAMP–PKA pathways with a higher Ca2+ recruitment from intra- and extra-cellular compartments.
In β-cells, Cch provokes an increase in intracellular Ca2+ concentration in a biphasic manner. The first phase occurs by a rapid mobilisation of intracellular Ca2+, induced by IP3, and the second one depends on Ca2+ influx through the Ca2+ store-operated channels located at the cell plasma membrane(Reference Liu and Gylfe10). The intracellular Ca2+ mobilisation, stimulated by the activation of the PLC and IP3 production by acetylcholine, has been reported to increase insulin granule movement in a PKC-independent manner(Reference Niwa, Matsukawa and Senda34). Here, we observed that insulin secretion in response to PMA, and PKCα protein expression, did not differ between TAU and CTL islets. However, increased PLCβ2 protein levels in TAU islets suggest that augmented activation of PLC followed by IP3 production may account for increased intracellular Ca2+ mobilisation in Cch-stimulated islets from TAU-supplemented mice. These data give support to a possible effect of TAU upon IP3 production, in turn amplifying β-cell response to fuel secretagogues.
It is known that PLC may be activated when the β-cell is depolarised. Since all PLC isoforms require Ca2+(Reference Rhee7)), it is possible that, in β-cells, the increase in intracellular Ca2+ concentration activates this enzyme, leading to an increase in IP3 and diacylglycerol production that may account for an enhanced insulin secretion in response to glucose. Supporting this view, PLCδ1 activity has been found to increase in the presence of stimulatory glucose concentrations, resulting in a cycle of synthesis and degradation of plasma membrane phosphatidylinositol-4,5-bisphosphate(Reference Thore, Dyachok and Gylfe8, Reference Thore, Wuttke and Tengholm9).
We show, in the present study, that islets from TAU-supplemented mice increase insulin secretion in response to IBMX and express more PKAα protein. Thus, in addition to PLC signals, cAMP and PKA may contribute to increasing insulin secretion and islet functionality in the presence of glucose. In support of this assumption, Ca2+ uptake, in response to glucose in TAU islets, was significantly reduced in the presence of different PKA inhibitors (Table 2). As such, TAU supplementation seems to alter islet Ca2+ handling either by increasing the expression of the β2 subunit Ca2+ channels (see Ribeiro et al. (Reference Ribeiro, Bonfleur and Amaral27)) or by increasing the expression and/or activity of PKA, since the inhibition of the enzyme provoked only a minor effect on Ca2+ uptake in CTL islets.
Recently, a direct coupling between Ca2+ and cAMP has been reported. Dyachok et al. (Reference Dyachok, Idevall-Hagren and Sagetorp29) who monitored the alterations in cAMP levels in β-cells by observing the dissociation of PKA catalytic from the regulatory subunits. These authors showed that glucose induces cAMP level oscillations and that each oscillation was preceded and enhanced by the increase in intracellular Ca2+ concentration. In addition, cells from an insulinoma cell line (INS-1), exposed to glucagon-like peptide-1 or IBMX, exhibited cAMP–PKA oscillations synchronised with increases in intracellular Ca2+ concentration(Reference Dyachok, Isakov and Sagetorp35).
In the β-cell, nutrient stimulation leads to membrane depolarisation and Ca2+ influx. The increase in intracellular Ca2+ concentration may stimulate or inhibit cAMP formation because β-cells express different adenylyl cyclases(Reference Leech, Castonguay and Habener5). The adenylyl cyclase type VIII, present in β-cells, is a Ca2+-sensitive isoform and, in the presence of glucose, may account for the increase in cAMP levels(Reference Delmeire, Flamez and Hinke6). This increase represents an important signal for intracellular Ca2+ concentration regulation and insulin exocytosis in β-cells. Moreover, IBMX and forskolin markedly increased the intracellular cAMP levels and, consequently, intracellular Ca2+ concentration in β-cells(Reference Rajan, Hill and Boyd36), probably via phosphorylation of the α1 subunit of the L-type Ca2+ channel by PKA(Reference Leiser and Fleischer13). This effect of PKA was also observed in other cell types. PKA phosphorylation of the L-type Ca2+ channel (skeletal muscle, cardiac cells and neurons) increases the open probability of the channel, shifts the voltage dependence of activation and slows the rate of inactivation, increasing voltage-dependent facilitation of the channel(Reference Gao, Yatani and Dell'Acqua14, Reference Mundina-Weilenmann, Chang and Gutierrez37, Reference Bourinet, Charnet and Tomlinson38). PKA also phosphorylates the β2 subunit of the cardiac Ca2+ channel(Reference Gao, Yatani and Dell'Acqua14) and this effect contributes to increase Ca2+ channel activity(Reference Kamp and Hell39). In β-cells, PKA acts upon intracellular Ca2+ dynamic via IP3 receptors, increasing intracellular Ca2+ mobilisation from the endoplasmic reticulum(Reference Ammala, Eliasson and Bokvist12, Reference Dyachok and Gylfe15).
Therefore, the present results suggest that the inter-relationship between PKA and Ca2+ is enhanced in islets from TAU-supplemented mice and accounts for increased Ca2+ handling in the presence of glucose. We do not have a definitive explanation as to why TAU islets, when challenged by IBMX, but not forskolin, secreted more insulin than CTL islets, since PKAα expression was increased in the former group of islets. We speculate that the maintenance of cAMP levels, rather than the amplification of its production, is more important for the phenomenon. The other possibility, not addressed in the present study, is that phosphodiesterase activity may be higher in the TAU group, resulting in a faster degradation of that second messenger.
In summary, the present study confirms and extends previous results showing that TAU supplementation increased insulin secretion and Ca2+ handling in the presence of stimulatory concentrations of glucose(Reference Carneiro, Latorraca and Araujo26, Reference Ribeiro, Bonfleur and Amaral27). We also provide new evidence that TAU supplementation increased insulin secretion in response to the cholinergic/PLC and cAMP–PKA pathways and that these effects, at least in part, are probably due to increased protein PLCβ2 and PKAα expressions associated to a higher Ca2+ mobilisation from both external and internal pools.
Acknowledgements
The present study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP no. 2008/53811-8; FAPESP no. 2007/50365-4; FAPESP no. 2005/59707-0), Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq), Instituto Nacional de Obesidade e Diabetes (CNPq/FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are grateful to Léscio D. Teixeira for animal care and technical assistance and to Nicola Conran for editing the English. We also thank Dr Clarice Izumi, Dr Helen J. Laure and Professor Dr Lewis J. Greene from Chemistry Protein Centre (Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo) for help and technical assistance in the TAU content analysis in islet and tissue.
R. A. R. was involved in the conception of the experiment and experimental design, execution of all experiments, analyses, data interpretation and manuscript writing; E. C. V. and M. L. B. were involved in insulin secretion experiments; C. A. M. O. analysed cytoplasmic Ca2+; A. C. B. provided intellectual contribution along with work development and manuscript writing; E. M. C. was involved in conception of the experiment and experimental design, data interpretation and manuscript writing.
The authors have no conflicts of interest.









