1. Introduction
The primary plant cell wall (CW) is a multi-layered structure in which each layer (lamella) consists of load bearing cellulose microfibrils laterally interconnected possibly with xyloglucan and embedded into a pectin matrix (Zhang et al., Reference Zhang, Tang, Vavylonis and Cosgrove2019a; Reference Zhang, Yu, Wang, Durachko, Zhang and Cosgrove2021a). The properties of CW are being constantly modified to allow for morphological changes that are necessary for plant growth and development both in the shoot (Gruel et al., Reference Gruel, Landrein, Tarr, Schuster, Refahi, Sampathkumar, Hamant, Meyerowitz and Jonsson2016; Hamant et al., Reference Hamant, Heisler, Jonsson, Krupinski, Uyttewaal, Bokov, Corson, Sahlin, Boudaoud, Meyerowitz, Couder and Traas2008; Hervieux et al., Reference Hervieux, Tsugawa, Fruleux, Dumond, Routier-Kierzkowska, Komatsuzaki, Boudaoud, Larkin, Smith, Li and Hamant2017; Landrein et al., Reference Landrein, Kiss, Sassi, Chauvet, Das, Cortizo, Laufs, Takeda, Aida, Traas, Vernoux, Boudaoud and Hamant2015; Majda et al., Reference Majda, Grones, Sintorn, Vain, Milani, Krupinski, Zagorska-Marek, Viotti, Jonsson, Mellerowicz, Hamant and Robert2017; Pien et al., Reference Pien, Wyrzykowska, McQueen-Mason, Smart and Fleming2001; Reinhardt et al., Reference Reinhardt, Wittwer, Mandel and Kuhlemeier1998; Sampathkumar et al., Reference Sampathkumar, Yan, Krupinski and Meyerowitz2014; Takatani et al., Reference Takatani, Verger, Okamoto, Takahashi, Hamant and Motose2020) and root (Barbez et al., Reference Barbez, Dunser, Gaidora, Lendl and Busch2017; Hurny et al., Reference Hurny, Cuesta, Cavallari, Otvos, Duclercq, Dokladal, Montesinos, Gallemi, Semeradova, Rauter, Stenzel, Persiau, Benade, Bhalearo, Sykorova, Gorzsas, Sechet, Mouille, Heilmann and Benkova2020; Mielke et al., Reference Mielke, Zimmer, Meena, Dreos, Stellmach, Hause, Voiniciuc and Gasperini2021; Pacifici et al., Reference Pacifici, Di Mambro, Dello Ioio, Costantino and Sabatini2018; Ramakrishna et al., Reference Ramakrishna, Ruiz Duarte, Rance, Schubert, Vordermaier, Vu, Murphy, Vilches Barro, Swarup, Moirangthem, Jorgensen, van de Cotte, Goh, Lin, Vobeta, Beeckman, Bennett, Gevaert, Maizel and De Smet2019; Vermeer et al., Reference Vermeer, von Wangenheim, Barberon, Lee, Stelzer, Maizel and Geldner2014). Mechanical properties of the CW are regulated by a variety of agents including expansins (Cosgrove, Reference Cosgrove2000; McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992), glucanases (Yoshida & Komae, Reference Yoshida and Komae2006; Yuan et al., Reference Yuan, Wu and Cosgrove2001; Zhang et al., Reference Zhang, Tang, Vavylonis and Cosgrove2019a), pectin methylesterases (Goldberg et al., Reference Goldberg, Morvan, Jauneau and Jarvis1996; Peaucelle et al., Reference Peaucelle, Louvet, Johansen, Hofte, Laufs, Pelloux and Mouille2008; Wang et al., Reference Wang, Wilson and Cosgrove2020), calcium ions (Bou Dahner et al., Reference Bou Dahner, Chen, Bozorg, Clough, Jonsson and Braybrook2018; Wang et al., Reference Wang, Wilson and Cosgrove2020) and others. While endoglucanases and other enzymes typically decrease the number of linkages between cellulose and other CW molecules (i.e., mediate CW remodelling, see the Glossary) leading to a weaker (i.e., more easily breakable) wall, α-expansins induce creep—an irreversible time-dependent CW enlargement (Cosgrove, Reference Cosgrove2016a; Park & Cosgrove, Reference Park and Cosgrove2012a; Wang et al., Reference Wang, Park, Caporini, Rosay, Zhong, Cosgrove and Hong2013; Yuan et al., Reference Yuan, Wu and Cosgrove2001). These types of biomechanical modifications should be distinguished. Thus, the timing and location of growth are controlled by spatial- and time-specific modification of the mechanical properties of the CW. Here we review recent contributions on the role of α-expansins in the control of biomechanical CW properties, focusing primarily on their role in plant development and abiotic stress response.
2. Expansin discovery and evolution
Expansins were discovered in plants as proteins that play a crucial role in CW loosening (McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992), as they induce stress relaxation and extension in plant CWs during pH-dependent ‘acid growth’ (Rayle & Cleland, Reference Rayle and Cleland1992). Since then, expansins have been shown to be involved in many aspects of plant growth and development. Expansins are present to the best of our knowledge in all plant species, although some gene loss is observable in highly adapted aquatic species (Hepler et al., Reference Hepler, Bowman, Carey and Cosgrove2020). Expansins can also be found in fungi and bacteria, probably as a result of horizontal gene transfer (Georgelis et al., Reference Georgelis, Nikolaidis and Cosgrove2015). However, the presence of these genes in all eukaryotic microorganisms that use cellulose as a structural component of their CW suggests that expansins evolved in ancient marine microorganisms long before the evolution of land plants (Chase et al., Reference Chase, Zhaxybayeva, Rocha, Cosgrove and Shapiro2020). Expansins from diverse bacteria and fungi assisting plant–microbe interactions in nature have often been utilised in industrial applications to facilitate lignocellulose degradation that is used further in the conversion of biomass into alternative fuels (Georgelis et al., Reference Georgelis, Nikolaidis and Cosgrove2015; Liu et al., Reference Liu, Ma and Zhang2015).
3. The expansin (super)family
Based on phylogenetic sequence homology, four distinct genetic subfamilies of expansins are currently recognised in vascular plants: α-expansin (EXPA), β-expansin (EXPB), expansin-like A (EXLA) and expansin-like B (EXLB) (Sampedro & Cosgrove, Reference Sampedro and Cosgrove2005). Two of these subfamilies, the α and β expansins have been demonstrated experimentally to induce CW loosening (Cosgrove et al., Reference Cosgrove, Bedinger and Durachko1997; McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992). EXPA is the most numerous subfamily, for example in Arabidopsis thaliana there are 26 EXPA genes, 6 EXPB, 3 EXLA and 1 EXLB. Apart from Arabidopsis, rice and poplar (Sampedro & Cosgrove, Reference Sampedro and Cosgrove2005), genome-wide identification and expression profile analysis of expansin gene families have recently been performed in sugarcane (Santiago et al., Reference Santiago, Pereira, de Souza, Steindorff, Cunha, Gaspar, Favaro, Formighieri, Kobayashi and Molinari2018), wheat (Han et al., Reference Han, Liu, Deng, Liu, Liu, Hu and Yan2019; Zhang et al., Reference Zhang, Xu, Dong, Peng, Feng, Wang, Li, Miao, Yao, Zhao, Feng, Hu and Li2018a), potato (Chen et al., Reference Chen, Zhang, Li, Lei, Kong, Yang and Gong2019), Chinese jujube (Hou et al., Reference Hou, Zhang, Dou, Zhang, Pang and Li2019), cotton (Lv et al., Reference Lv, Zuo, Wang, Cheng, Zhang, Wang, Song and Ma2020) and Brassica species (Li et al., Reference Li, Ma, Shen, Zhao, Ma, Wang, Fan, Tang and Wei2021a).
Although the main focus of this review is on EXPA, it is worth mentioning that the group of β-expansins expanded significantly in grasses (Sampedro et al., Reference Sampedro, Guttman, Li and Cosgrove2015). As an example, EXPB1 (also called Zea m 1) is a member of group-1 grass pollen allergens and its crystal structure has been resolved suggesting the role of EXPB1 in the local movement and stress relaxation of (arabino)xylan-cellulose networks within the wall (Yennawar et al., Reference Yennawar, Li, Dudzinski, Tabuchi and Cosgrove2006). Detailed characterisation of EXPB1 function in extracted maize CWs revealed that the protein primarily binds glucuronoarabinoxylan, the major polysaccharide in grass CWs (Wang et al., Reference Wang, Chen, Tabuchi, Cosgrove and Hong2016a) that is largely absent in primary CWs of dicots (Carpita, Reference Carpita1996; Vogel, Reference Vogel2008). In maize, the group is needed for pollen separation and stigma penetration (Valdivia et al., Reference Valdivia, Stephenson, Durachko and Cosgrove2009).
4. Expansin structure and mode of action
4.1. Expansin structure
Expansins are modular, torpedo-shaped proteins that consist of two tightly packed, structured domains of 200–250 amino acids, connected by a short linker and preceded by a signal peptide. The N-terminal domain (D1) is a six-stranded double-psi (ω) β-barrel related to family 45 glycoside hydrolases (GH45), but lacks the critical catalytic Asp required for hydrolytic activity (Cosgrove, Reference Cosgrove2015; Georgelis et al., Reference Georgelis, Nikolaidis and Cosgrove2015; Kerff et al., Reference Kerff, Amoroso, Herman, Sauvage, Petrella, Filée, Charlier, Joris, Tabuchi, Nikolaidis and Cosgrove2008; Yennawar et al., Reference Yennawar, Li, Dudzinski, Tabuchi and Cosgrove2006). The C-terminal domain (D2) with a β-sandwich fold is related to group-2 grass pollen allergens and resembles the carbohydrate binding module (CBM) family 63 (Chase et al., Reference Chase, Zhaxybayeva, Rocha, Cosgrove and Shapiro2020; Georgelis et al., Reference Georgelis, Yennawar and Cosgrove2012). Both domains are required for full CW loosening activity (Georgelis et al., Reference Georgelis, Tabuchi, Nikolaidis and Cosgrove2011; Sampedro & Cosgrove, Reference Sampedro and Cosgrove2005). The Expansin Engineering Database (ExED; https://exed.biocatnet.de) is a useful navigation and classification tool for expansins and their homologues and is based on newly created profile hidden Markov models of the two expansin domains (Lohoff et al., Reference Lohoff, Buchholz, Le Roes-Hill and Pleiss2020).
Despite the rather long history of expansin research, many of the details of the functional and structural properties underlying the molecular mechanism of expansin action in enabling CW expansion still remain undiscovered. One of the reasons for this knowledge gap is that, unlike bacterial or fungal expansins, plant α-expansins have proven difficult to produce in the active form using heterologous expression systems (Gaete-Eastman et al., Reference Gaete-Eastman, Morales-Quintana, Herrera and Moya-Leon2015). Nonetheless, computational 3D models built through comparative modelling and molecular dynamics simulations have yielded the first structural approximation of several α-expansins (Gaete-Eastman et al., Reference Gaete-Eastman, Morales-Quintana, Herrera and Moya-Leon2015; Mateluna et al., Reference Mateluna, Valenzuela-Riffo, Morales-Quintana, Herrera and Ramos2017; Pastor et al., Reference Pastor, Davila, Perez-Rueda and Segovia2015; Valenzuela-Riffo et al., Reference Valenzuela-Riffo, Ramos and Morales-Quintana2018; Reference Valenzuela-Riffo, Gaete-Eastman, Stappung, Lizana, Herrera, Moya-Leon and Morales-Quintana2020) and confirmed that expansins can form a stable complex with cellulose via the flat aromatic surface of the C-terminal domain (Valenzuela-Riffo et al., Reference Valenzuela-Riffo, Ramos and Morales-Quintana2018). Based on the model, the expansins also interacted with the xyloglucan XXFG ligand, but were less likely to bind the XXXG ligand; they did not interact with pectin (Valenzuela-Riffo et al., Reference Valenzuela-Riffo, Gaete-Eastman, Stappung, Lizana, Herrera, Moya-Leon and Morales-Quintana2020), the latter being in contrast to experimental data (Nardi et al., Reference Nardi, Escudero, Villarreal, Martinez and Civello2013). Recently, the protein structure of several expansins was determined by the AlphaFold protein prediction algorithm (Figure 1a) proven to be highly reliable in terms of the predicted protein structure (Jumper et al., Reference Jumper, Evans, Pritzel, Green, Figurnov, Ronneberger, Tunyasuvunakool, Bates, Zidek, Potapenko, Bridgland, Meyer, Kohl, Ballard, Cowie, Romera-Paredes, Nikolov, Jain, Adler and Hassabis2021; Varadi et al., Reference Varadi, Anyango, Deshpande, Nair, Natassia, Yordanova, Yuan, Stroe, Wood, Laydon, Zidek, Green, Tunyasuvunakool, Petersen, Jumper, Clancy, Green, Vora, Lutfi and Velankar2021).

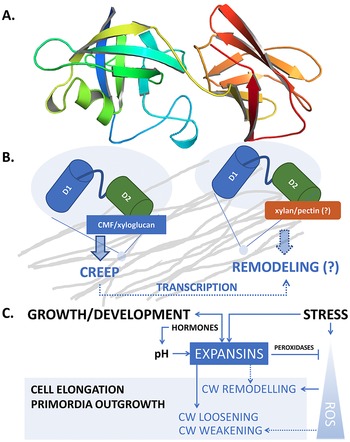
Fig. 1. (a) Structure of AtEXPA1 determined by the AlphaFold algorithm. N-terminal six-stranded double-psi (ω) β-barrel D1 domain related to family 45 glycoside hydrolases (GH45) (green/blue, left) and C-terminal β-sandwich fold D2 domain related to group-2 grass pollen allergens resembling the carbohydrate binding module (CBM) family 63 (red/orange, right); the unstructured signal peptide is not shown. (b) Upon binding the load-bearing cellulose microfibril (CMF) network laterally interconnected with possible xyloglucan contribution (grey), expansins induce CW expansion via CW creep. By interfering with CW remodelling enzymes via binding to xylan and/or pectin or through transcriptional feedback regulations in a response to changed CW biomechanics, expansins might contribute to CW remodelling, too. (c) Expansin expression and localization is regulated during plant development, ensuring expansin action in a manner that is specific to their dose and the particular developmental context. Conversely, expansin action on CW biomechanics affects plant development and growth responses by regulating cell elongation and/or primordia specification/outgrowth. Expansins are activated in response to various stresses associated with ROS production. Expansin expression might be mediated by developmental- and stress-regulated hormone production, controlling expansin activity also via spatial-specific CW acidification. Expansins could mitigate ROS effects by upregulating CW peroxidases. In turn, ROS also contribute to the regulation of CW biomechanical properties. While short-term or low-level ROS production leads to growth inhibition by inducing crosslinking of CW components, high ROS levels/long-term ROS production leads to OH°-radical formation that was hypothesised to allow restoration of cell expansion via polymer cleavage, leading to CW weakening. See the main text for a more detailed description.
4.2. Bacterial expansins
Because of the aforementioned limitations, our knowledge of the mode of expansin action at atomic resolution is limited to bacterial expansins. Cellulose binding was demonstrated for Bacillus subtilis expansin EXLX1, a bacterial expansin that can loosen plant CWs. Through hydrophobic interactions of three linearly arranged, highly conserved aromatic residues (W125, W126 and Y157) in the D2 domain, EXLX1 binds tightly to crystalline cellulose rather than to linear oligosaccharides (Boraston et al., Reference Boraston, Creagh, Alam, Kormos, Tomme, Haynes, Warren and Kilburn2001; Georgelis et al., Reference Georgelis, Yennawar and Cosgrove2012; Kim et al., Reference Kim, Ko, Kim, Nam, Choi and Kim2013). Molecular dynamics simulations suggest that the expansin has both a cellulose-weakening and a cellulose-binding activity that depends on substrate crystallinity (Orłowski et al., Reference Orłowski, Artzi, Cazade, Gunnoo, Bayer and Thompson2018). Indeed, adsorption of EXLX1 onto a cellulose film decreased the crystallinity index, disrupted hydrogen bonding, and increased the surface area of cellulose, indicating greater accessibility of the substrate to proteins (Duan et al., Reference Duan, Ma, Zhao, Huang, Su, Qi and He2018). It is this characteristic that makes expansin and expansin-like proteins that act synergistically with cellulases during hydrolysis useful for industry, and they are often used as biological pre-treatments to disrupt and open up recalcitrant lignocellulose complexes for industrial applications (Georgelis et al., Reference Georgelis, Tabuchi, Nikolaidis and Cosgrove2011; Reference Georgelis, Nikolaidis and Cosgrove2015; Kerff et al., Reference Kerff, Amoroso, Herman, Sauvage, Petrella, Filée, Charlier, Joris, Tabuchi, Nikolaidis and Cosgrove2008; Kim et al., Reference Kim, Lee, Bang, Choi and Kim2009).
Other investigations of EXLX1 adsorption onto cellulose, using quartz crystal microbalance with dissipation (QCM-D), confirmed that cellobiose and xylose enhanced EXLX1 adsorption at low concentrations but inhibited it at high concentrations (Zhang et al., Reference Zhang, Ma, Cui, Wang, Huang, Su, Qi, He and Thielemans2020). Monitoring real-time adsorption of endo/exo-glucanases with EXLX1 and the enzymatic hydrolysis of cellulose showed synergistic effects. This increased activity can be due to easier access of the cellulase to the cellulose chains, but other effects such as electrostatic or other physical interactions between the adsorbed EXLX1 and cellulases cannot be ruled out (Zhang et al., Reference Zhang, Su, Duan, Cui, Huang, Qi, He and Thielemans2021b). However, bacterial expansins have much weaker cellulose binding and wall-loosening activity than plant α-expansins (Kerff et al., Reference Kerff, Amoroso, Herman, Sauvage, Petrella, Filée, Charlier, Joris, Tabuchi, Nikolaidis and Cosgrove2008; Kim et al., Reference Kim, Lee, Bang, Choi and Kim2009), and recent results suggest that although EXLX1 is homologous with plant expansins, it possibly has distinct effects on plant CWs (Hepler & Cosgrove, Reference Hepler and Cosgrove2019).
4.3. Expansin-mediated CW loosening
According to the loosening theory (Cosgrove, Reference Cosgrove2015), well-hydrated non-growing cells reach osmotic equilibrium with wall stresses counter-balancing the outward turgor pressure against the wall. In growing cells, however, walls are loosened (primarily via pH-dependent action of expansins), which means that the load-bearing part of the wall is relaxed, releasing the tensile stress and simultaneously reducing cell turgor. Consequently, water flows into the cell, expanding the wall and restoring turgor and wall stress, together driving cell growth (Cosgrove, Reference Cosgrove2015; Reference Cosgrove2018a). Importantly, cell expansion starts with CW loosening/relaxation, followed by a decrease and a subsequent increase of cell turgor, not vice versa (Cosgrove, Reference Cosgrove1993).
There is a significant body of evidence suggesting that expansins themselves are incapable of hydrolysing the polysaccharide substrate itself (Kerff et al., Reference Kerff, Amoroso, Herman, Sauvage, Petrella, Filée, Charlier, Joris, Tabuchi, Nikolaidis and Cosgrove2008; McQueen-Mason & Cosgrove, Reference McQueen-Mason and Cosgrove1995; McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992). Nevertheless, pH-dependent, expansin-mediated CW loosening promotes relaxation of the CW structure, thus contributing to CW remodelling by allowing different hydrolases to access their polysaccharide substrates (Cosgrove, Reference Cosgrove2000; Reference Cosgrove2005; Whitney et al., Reference Whitney, Gidley and McQueen-Mason2000).
4.4. Apoplast acidification is necessary for expansin-mediated cell expansion
According to the ‘acid growth theory’ (Hager et al., Reference Hager, Menzel and Krauss1971; Rayle & Cleland, Reference Rayle and Cleland1970), auxin triggers extrusion of protons (H+) into the apoplast, which activates expansins that subsequently loosen the CW and allow growth (McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992). The most important players in this process are plasma membrane P-type H+-ATPases which pump out protons to the wall matrix, consequently leading to apoplast acidification (Takahashi et al., Reference Takahashi, Hayashi and Kinoshita2012). Later it was discovered that the transport inhibitor response1/auxin signaling F-box—auxin/indole-3-acetic acid (TIR1/AFB-Aux/IAA) auxin signalling machinery transcriptionally upregulates the SMALL AUXIN UP-RNA 19 (SAUR19) expression levels (Fendrych et al., Reference Fendrych, Leung and Friml2016). SAUR19 inhibits the activity of TYPE 2C PROTEIN PHOSPHATASES (PP2C), thus maintaining the H+-ATPase in an active state (Spartz et al., Reference Spartz, Ren, Park, Grandt, Lee, Murphy, Sussman, Overvoorde and Gray2014). Pumping protons causes plasma membrane hyperpolarisation and also activates K+ channels that (in a short term) electrically balance the H+ efflux and (in the long term) maintain intracellular osmotic potential low, thus allowing sustained water uptake and turgor pressure forcing the CW to extend (Thiel & Weise, Reference Thiel and Weise1999; for review see Arsuffi & Braybrook, Reference Arsuffi and Braybrook2018).
Given the different effects of auxins on shoots compared with roots (for review see Du et al., Reference Du, Spalding and Gray2020; Dunser & Kleine-Vehn, Reference Dunser and Kleine-Vehn2015; Li et al., Reference Li, Gallei and Friml2021b), the acid growth theory seems to be more complex in roots, suggesting possible non-transcriptional regulations (Pacheco-Villalobos et al., Reference Pacheco-Villalobos, Diaz-Moreno, van der Schuren, Tamaki, Kang, Gujas, Novak, Jaspert, Li, Wolf, Oecking, Ljung, Bulone and Hardtke2016). In line with that, the non-transcriptional branch of the cytosolic TIR1/AFB pathway was demonstrated to trigger a rapid Cyclic Nucleotide-Gated Channel 14 (CNGC14)-mediated Ca2+ influx and an unknown channel or transporter-mediated H+ influx leading to apoplast alkalization inhibiting the growth (Fendrych et al., Reference Fendrych, Akhmanova, Merrin, Glanc, Hagihara, Takahashi, Uchida, Torii and Friml2018; Li et al., Reference Li, Gallei and Friml2021b). Recently, it was shown that the cell surface-based TRANSMEMBRANE KINASE1 (TMK1) directly binds and activates plasma membrane H+-ATPases thus promoting CW acidification in both shoots and roots (Li et al., Reference Li, Verstraeten, Roosjen, Takahashi, Rodriguez, Merrin, Chen, Shabala, Smet, Ren, Vanneste, Shabala, De Rybel, Weijers, Kinoshita, Gray and Friml2021c; Lin et al., Reference Lin, Zhou, Tang, Takahashi, Pan, Dai, Ren, Zhu, Pan, Zheng, Gray, Xu, Kinoshita and Yang2021), acting antagonistically to the noncanonical TIR1/AFB pathway (Li et al., Reference Li, Gallei and Friml2021b).
However, not only auxin can control apoplastic pH. Cytokinins were proposed to upregulate the expression of genes for H+-ATPases AHA2 and AHA7, facilitating thus EXPA1-mediated induction of cell elongation in the root transition zone (Pacifici et al., Reference Pacifici, Di Mambro, Dello Ioio, Costantino and Sabatini2018). Furthermore, Großeholz et al. (Reference Großeholz, Wanke, Glöckner, Rausch, Rohr, Scholl, Scacchi, Spazierer, Shabala, Shabala, Schumacher, Kummer and Harter2021) recently proposed a new model in which brassinosteroid-mediated cell elongation response depends on the amount and activity of H+-ATPases in the plasma membrane. Also here, the K+ antiport, this time mediated via CNGC10, is necessary to compensate for H+ efflux, thus keeping the plasma membrane potential constant. Using microelectrode ion flux estimation measurements, Großeholz et al. (Reference Großeholz, Wanke, Glöckner, Rausch, Rohr, Scholl, Scacchi, Spazierer, Shabala, Shabala, Schumacher, Kummer and Harter2021) demonstrated net H+ influx in the root meristematic zone while H+ efflux in the root transition zone. The resulting pH gradient is proposed to be instructive for the cell elongation in the root transition/elongation zone. Altogether, not only the spatiotemporal specificity of EXPAs expression and protein localization but also the spatial-specific control over H+ fluxes leading to the changes in the apoplastic pH are important factors controlling the EXPA-mediated cell expansion.
5. Expansins and CW biomechanics
5.1. Historical overview of the primary CW models
Previous depictions of accepted CW models (Carpita & Gibeaut, Reference Carpita and Gibeaut1993; Fry, Reference Fry1989; Hayashi, Reference Hayashi1989; Nishitani, Reference Nishitani1998) presented cellulose microfibrils as well-spaced and non-contacting rods with xyloglucan covering most cellulose surfaces and tethering them together to form the load-bearing network. Indeed, it was confirmed that enlargement of the CW required separation of cellulose microfibrils; however, high resolution (FESEM and AFM) images from slowly extended CWs in vitro and control non-extended samples, appeared indistinguishable (Marga et al., Reference Marga, Grandbois, Cosgrove and Tobias2005). CW can therefore extend slowly through creep but without passive reorientation of the innermost microfibrils, suggesting that the loosening agents act selectively on the cross-linking polymers between parallel microfibrils, rather than more generally on the wall matrix, increasing microfibril spacing but without reorienting them (Marga et al., Reference Marga, Grandbois, Cosgrove and Tobias2005).
In 2008, Cavalier et al. (Reference Cavalier, Lerouxel, Neumetzler, Yamauchi, Reinecke, Freshour, Zabotina, Hahn, Burgert, Pauly, Raikhel and Keegstra2008) showed that Arabidopsis xyloglucan-deficient (xylosyltransferase1/xylosyltransferase2; xxt1/xxt2) mutant plants were reduced in size, but otherwise seemed to develop normally. Nevertheless, stress/strain assays performed by Park and Cosgrove (Reference Park and Cosgrove2012b) showed that the xxt1/xxt2 walls were more pliant than wild-type (WT) walls but less extensible in the creep and stress-relaxation processes mediated by α-expansin, suggesting that xyloglucan plays a CW strengthening role. Similarly, loosening agents that act on xylans and pectins elicited greater extension in creep assays of the mutant xyloglucan-deficient CWs, demonstrating that these polymers take on a larger mechanical role in the absence of xyloglucan. The results also indicated that growth reduction in xxt1/xxt2 plants is likely due to the absence of the native target for CW loosening by α-expansins (Park & Cosgrove, Reference Park and Cosgrove2012b).
Although xyloglucan has the ability to bind tightly to cellulose, NMR analyses of complex CWs showed that very little of the cellulose microfibril surface is actually in contact with xyloglucan (Bootten et al., Reference Bootten, Harris, Melton and Newman2004; Dick-Perez et al., Reference Dick-Perez, Zhang, Hayes, Salazar, Zabotina and Hong2011). On the other hand, pectin content is approximately 3-fold that of xyloglucan in Arabidopsis CWs (White et al., Reference White, Wang, Park, Cosgrove and Hong2014) and makes the majority of matrix contacts with cellulose surfaces. The binding of xyloglucan is restricted to a minor component that appears to be closely intertwined with cellulose at discrete sites designated as ‘biomechanical hotspots’ (Cosgrove, Reference Cosgrove2014; Park & Cosgrove, Reference Park and Cosgrove2015). Indeed, substantial wall loosening by substrate-specific endoglucanases (CXEG) was traced to the digestion of a specific component comprising <1% of the xyloglucan in the wall, indicating that only a small number of sites may control wall extensibility (Park & Cosgrove, Reference Park and Cosgrove2012b). This picture of a few biomechanical junctions is also consistent with the low density of α-expansin binding sites in the CW (McQueen-Mason & Cosgrove, Reference McQueen-Mason and Cosgrove1995).
The biomechanical ‘hotspot hypothesis’ proposes that wall extensibility is controlled at discrete sites where microfibrils come into close contact with one another (Zhang et al., Reference Zhang, Mahgsoudy-Louyeh, Tittmann and Cosgrove2014) via a monolayer of xyloglucan binding the hydrophobic surfaces of the two microfibrils together (Cosgrove, Reference Cosgrove2018b). These may be the selective sites of CW loosening by expansins or by CXEG-type enzymes where the microfibrils slide or separate, perhaps at a rate that is influenced by the bulk viscoelasticity of the microfibril–matrix network (Park & Cosgrove, Reference Park and Cosgrove2015). Disruption of such non-covalent bonds allows ‘slippage’ of carbohydrate polymers at load-bearing elements of the CW. Although the CW models assume non-covalent bonding between cellulose and hemicelluloses such as xyloglucan, Equisetum hetero-trans-β-glucanase (HTG) covalently attaches cellulose onto xyloglucan oligosaccharides in vitro. Interestingly, recombinant bacterial expansin EXLX1 strongly augmented the cellulose:xyloglucan endotransglucosylase activity that produces cellulose–xyloglucan covalent bonds in the CWs of structural plant tissues in vitro (Herburger et al., Reference Herburger, Frankova, Picmanova, Loh, Valenzuela-Ortega, Meulewaeter, Hudson, French and Fry2020).
The current view of the primary CW is represented by a mesoscale coarse-grained molecular dynamics model (Zhang et al., Reference Zhang, Yu, Wang, Durachko, Zhang and Cosgrove2021a). The assembled epidermal CW is based on the supramolecular structure of cellulose and matrix polysaccharides that resembles (real) physics and tensile mechanics. The multi-layered CW has a cross-lamellate organisation in which individual layers (lamellae) of stiff cellulose microfibrils form a laterally interconnected network binding noncovalently to hemicellulose that is embedded in pectin, forming a gel-like matrix. Individual lamellar microfibrils are aligned in the same direction and appear anisotropic in terms of in-plane stress resistance; however, the complete (real) CWs, consisting of many lamellae (approx. 100) are highly isotropic. Interestingly, the simple non-covalent-bonding generated cellulose network in which fibril–fibril sliding of aligned cellulose bundles bears most of the stress despite frequent xyloglucan bridging between microfibrils, and pectin abundance. Overall, in this dynamic load-bearing network, tensile forces are transmitted primarily through direct lateral contacts between cellulose microfibrils, rather than by matrix polysaccharides. Thus, although the action of expansins and other wall-modifying proteins was not part of it, the model clearly highlights the importance of the lateral cellulose microfibrils contacts and its potential modulators (particularly expansins) in the overall transmission of in-plane tensile forces.
5.2. Expansin-mediated changes in the CW biomechanics
The CW can undergo several types of deformation that can be measured either in situ (ideally in living plant tissues) or in simplified models, most frequently using onion epidermis peels clamped in a custom-made mechanical testing device (Cosgrove, Reference Cosgrove1989; Reference Cosgrove2011; Durachko & Cosgrove, Reference Durachko and Cosgrove2009; Durachko et al., Reference Durachko, Park, Zhang and Cosgrove2017; Wang et al., Reference Wang, Wilson and Cosgrove2020; Zhang & Cosgrove, Reference Zhang and Cosgrove2017; Zhang et al., Reference Zhang, Tang, Vavylonis and Cosgrove2019a). In some cases, slightly more complex systems such as de-frosted Arabidopsis petioles (Park & Cosgrove, Reference Park and Cosgrove2012a; Xin et al., Reference Xin, Lei, Zheng, Zhang, Pingali, O’Neill, Cosgrove, Li and Gu2020), cucumber and Arabidopsis hypocotyls (Boron et al., Reference Boron, Van Loock, Suslov, Markakis, Verbelen and Vissenberg2015; Cosgrove, Reference Cosgrove1989; Marga et al., Reference Marga, Grandbois, Cosgrove and Tobias2005; Park & Cosgrove, Reference Park and Cosgrove2012b) or wheat coleoptiles (Hepler & Cosgrove, Reference Hepler and Cosgrove2019) have been used. The advantage of using onion epidermal peels is that the mechanical properties of isolated CW fragments can be measured, largely neglecting the contribution of neighbouring cells, cell size or shape that might possibly influence the results when using indentation-based (AFM) measurements (Cosgrove, Reference Cosgrove2018b and references therein). However, new technologies such as non-contact, optical Brillouin spectroscopy are emerging as tools to probe biomechanical properties of CWs in developing organs at the cellular (Scarcelli et al., Reference Scarcelli, Polacheck, Nia, Patel, Grodzinsky, Kamm and Yun2015) or tissue level (Elsayad et al., Reference Elsayad, Werner, Gallemí, Kong, Sanchez Guajardo, Zhang, Jaillais, Greb and Belkhadir2016; Samalova et al., Reference Samalova, Elsayad, Melnikava, Peaucelle, Gahurova, Gumulec, Spyroglou, Zemlyanskaya, Ubogoeva and Hejatko2020).
When CWs become mechanically softer/more pliant (meaning more easily deformed by out-of-plane mechanical force, see the Glossary), they do not necessarily result in wall relaxation and cell growth. On the other hand, α-expansins cause in-plane stress relaxation and prolonged enlargement of CWs, but they do not change the CW viscoelastic properties, as measured by tensile tests (Cosgrove, Reference Cosgrove2018a; Yuan et al., Reference Yuan, Wu and Cosgrove2001). In other words, reducing the wall stiffness doesnot necessarily lead to CW loosening. One such observation was made by Wang et al. (Reference Wang, Wilson and Cosgrove2020) with pectin methylesterase (PME) that selectively softened the onion epidermal wall yet reduced expansin-mediated creep. Similarly, driselase, a potent cocktail of wall-degrading enzymes, removed cellulose microfibrils in superficial lamellae sequentially, and softened the wall (reduced its indentation-measured mechanical stiffness), yet did not induce wall loosening (Zhang et al., Reference Zhang, Tang, Vavylonis and Cosgrove2019a).
In contrast to this, expansins, despite possessing no obvious enzymatic activity, are able to induce irreversible time-dependent expansion of CWs without affecting its compliance as discussed above. Expansins cause almost immediate in vitro CW extension, allowing to extend the cell length 100 times when compared to its meristematic initials (Cosgrove, Reference Cosgrove2016b and references therein). Thus, to loosen CW, expansins probably modify non-covalent bonds in the cellulose microfibril network, laterally interconnected with a possible contribution of xyloglucans bound to the hydrophobic face of the cellulose microfibrils (Cosgrove, Reference Cosgrove2018b and references therein). The consequent fibril–fibril sliding seems to allow CW extension and in-plane stress release of the multi-lamellate CW structure (Zhang et al., Reference Zhang, Tang, Vavylonis and Cosgrove2019a; Reference Zhang, Yu, Wang, Durachko, Zhang and Cosgrove2021a).
6. Involvement of expansins in various aspects of plant growth and development
6.1. Cell elongation: The more (expansin) the better?
Expansins were identified as factors that primarily enhance cell elongation. The CW fraction from the actively growing (apical) portion of cucumber hypocotyls was able to induce creep of heat-inactivated cucumber hypocotyls when measured by a constant load extensometer. The observed CW extension required acidic pH and was also seen upon application of cucumber extracts to CW isolated from actively growing tissues (hypocotyls, leaves, petioles and coleoptiles) from other plant species. The CW extracts from the basal (non-growing) hypocotyls were unable to induce cell extension of apical hypocotyl fragments. Nonetheless, even the (active) CW extracts from the apical regions were unable to induce CW extension of the basal hypocotyl fragments, suggesting maturation-associated changes in CW structure limiting susceptibility to these extension-inducing factors (McQueen-Mason et al., Reference McQueen-Mason, Durachko and Cosgrove1992).
Cell expansion is a developmental response that is most frequently associated with upregulation of endogenous expansins in various tissues from a number of species. These include petiole elongation associated with RpEXPA1 upregulation and CW acidification in response to ethylene entrapment following flooding in Rumex palustris (Vreeburg et al., Reference Vreeburg, Benschop, Peeters, Colmer, Ammerlaan, Staal, Elzenga, Staals, Darley, McQueen-Mason and Voesenek2005), enlargement of floral organs and internodes due to overexpression of PhEXPA1 in petunia (Zenoni et al., Reference Zenoni, Fasoli, Tornielli, Dal Santo, Sanson, de Groot, Sordo, Citterio, Monti and Pezzotti2011), changes in petiole and leaf-blade size associated with up- and down-regulation of AtEXPA10 in Arabidopsis, root hair-specific expression of AtEXP7 and AtEXP18 (Cho & Cosgrove, Reference Cho and Cosgrove2002), and AtEXPA1-mediated cell elongation in the Arabidopsis root transition zone (Pacifici et al., Reference Pacifici, Di Mambro, Dello Ioio, Costantino and Sabatini2018).
However, the correlation between cell extension and expansin activity is not absolute. Only a partial correlation between the activities of LeEXP2 and LeEXP18 and cell elongation has been observed in tomato. This implies the existence of another factor, acting in concert with expansins, that may control growth under certain physiological conditions (Caderas et al., Reference Caderas, Muster, Vogler, Mandel, Rose, McQueen-Mason and Kuhlemeier2000). In line with that, chemically regulated expression of CsEXP1 in tobacco suggested the existence of a specific developmental phase, when the leaf is sensitive to upregulated expansin (Sloan et al., Reference Sloan, Backhaus, Malinowski, McQueen-Mason and Fleming2009). Consistent with this, downregulating several expansins being transcriptionally active during the phase of maximal leaf-cell expansion (AtEXPA1,3, 5 and 10) using inducible amiRNA resulted in leaf growth repression in the latter stages of leaf development. Surprisingly, the smaller leaves had larger cells, suggesting organ and cell context-specific outputs of expansin gene expression (Goh et al., Reference Goh, Sloan, Dorca-Fornell and Fleming2012). In rice seedlings with inducible OsEXP4 expression, OsEXP4 protein levels were correlated with growth, but constitutive expression of the same gene resulted in growth retardation (Choi et al., Reference Choi, Lee, Cho and Kende2003). Dose-dependent effects were observed in Arabidopsis (over)expressing cucumber CsEXPA1 using a DEX-inducible system (Craft et al., Reference Craft, Samalova, Baroux, Townley, Martinez, Jepson, Tsiantis and Moore2005). While low levels of CsEXPA1 were able to broaden leaf lamina, high levels had strong negative effects, particularly on the enlargement of fast-growing (expanding) tissues like hypocotyls or petioles (Goh et al., Reference Goh, Sloan, Malinowski and Fleming2014). Finally, both overexpression of CsEXPA1 and amiRNA-based downregulation of endogenous expansins (AtEXPA1,3, 5 and 10) impaired hypocotyl elongation in etiolated Arabidopsis seedlings (Ilias et al., Reference Ilias, Negishi, Yasue, Jomura, Morohashi, Baharum and Goh2019). Overall, the action of expansins on CW enlargement seems to be specific, with regard to both dose (expression level) and the particular developmental context.
6.2. Do expansins control CW enlargement by modulating CW remodelling?
As with the examples in the previous sections, transgenic tomato lines with high levels of CsEXPA1 showed overall growth inhibition. Notably, hypocotyls from CsEXPA1 OE tomatoes were less sensitive to exogenously applied expansin in the constant-load extensometer assay (Rochange et al., Reference Rochange, Wenzel and McQueen-Mason2001). The authors proposed that the observed CW tension resistance can be partly due to CW adaptation to the excessive amount of CW-loosening expansins through ‘a decrease in the abundance or activity of secondary loosening agents, or stiffening of the CWs via other components (such as the de-esterification of pectins or extensin crosslinking)’ (quote taken from Rochange et al., Reference Rochange, Wenzel and McQueen-Mason2001).
There are several other pieces of evidence supporting a possible role for expansins as modulators of CW remodelling. Downregulation of PhEXPA1 in petunia led to CW thickening and reduction in crystalline cellulose content, suggesting involvement of PhEXPA1 in the cellulose synthesis or deposition (Zenoni et al., Reference Zenoni, Reale, Tornielli, Lanfaloni, Porceddu, Ferrarini, Moretti, Zamboni, Speghini, Ferranti and Pezzotti2004). Further in PhEXPA1 OE CWs, the relative abundance of CW polymers was altered (in this case less pectin and hemicellulose, but unchanged cellulose content). Another example is overexpression of root-specific OsEXPA8 in rice, leading to changed root architecture (longer main root, more lateral roots and root hairs), taller plants and larger leaves. The OsEXPA8 overexpression was associated with lower (AFM-measured) CW stiffness and an increase in the polysaccharide/lignin ratio as measured using FTIR (Ma et al., Reference Ma, Wang, Qiu, Kang, Che, Wang and Huang2013). The observed changes in the CW composition could be achieved by changes in substrate availability due to the binding of expansins also to other CW polymers besides cellulose (Zenoni et al., Reference Zenoni, Reale, Tornielli, Lanfaloni, Porceddu, Ferrarini, Moretti, Zamboni, Speghini, Ferranti and Pezzotti2004). In support of this mechanism, the CBM of strawberry expansin 2 (CBM-FaExp2) was shown to bind not only cellulose/xyloglucans but also other CW polymers including xylan and pectin. The presence of CBM-FaExp2 decreased the activity of CW degrading enzymes such as polygalacturonase, endoglucanase, pectinase and xylanase in an in vitro assay, probably due to CBM-FaExp2 binding to the enzyme substrates (Nardi et al., Reference Nardi, Escudero, Villarreal, Martinez and Civello2013). Notably, the CBM of FaEXP2 shows a high level of similarity to CBMs of AtEXPA1, AtEXPA2 and potato CBM-Pot-BG097738. Furthermore, the aromatic residues of CBM-FaExp2 are conserved in CBM-Pot-BG097738, and they were proposed to be involved in binding CW polysaccharides (Nardi et al., Reference Nardi, Escudero, Villarreal, Martinez and Civello2013). Thus, the CW stiffening recently observed in Arabidopsis lines with high levels of AtEXPA1 (Samalova et al., Reference Samalova, Elsayad, Melnikava, Peaucelle, Gahurova, Gumulec, Spyroglou, Zemlyanskaya, Ubogoeva and Hejatko2020) could be explained by a similar mechanism, that is, interference of AtEXPA1 binding to CW components with enzyme activity mediating CW softening. Furthermore, expansin-mediated changes in the accessibility of CW-modifying enzymes were also proposed to be how EXP1-controlled fruit softening in tomato (Brummell et al., Reference Brummell, Harpster, Civello, Palys, Bennett and Dunsmuir1999). However, the role of feedback regulations leading to changes in the expression of genes for several CW remodelling proteins could also contribute to the EXPA overexpression-induced changes in CW composition (Ilias et al., Reference Ilias, Negishi, Yasue, Jomura, Morohashi, Baharum and Goh2019).
The role of the C-terminal CBM and its possible functional importance in recognising cellulose and/or other CW sugar polymers was highlighted by the work of Boron et al. (Reference Boron, Van Loock, Suslov, Markakis, Verbelen and Vissenberg2015). The overexpression of AtEXLA2, a member of the expansin-like A family in Arabidopsis led only to a weak enlargement of etiolated hypocotyls. That was accompanied by CW thickening and decreased CW strength manifesting as higher rupture frequency (twice that of WT) under load during the creep test with a constant-load extensiometer. As AtEXLA2 is lacking the three conserved residues necessary for the CW loosening activity of the N-terminal D1 domain, the authors hypothesise a possible role for the C-terminal CBM in cellulose crystallisation and/or its affecting xyloglucan/cellulose interaction, leading to the observed defects in CW biomechanical properties. However, expansins may control CW remodelling independently of competition with CW modulating enzymes by binding to a wide spectrum of CW polymers as demonstrated for GbEXPATR in cotton. GbEXPATR represents a truncated version of its homologue GbEXPA2, lacking the C-terminal CBM. Interestingly, while the OE of GbEXPA2 had no significant effects on the length of mature fibres, overproduction of GbEXPATR led to longer, finer and stronger cotton fibres, probably via a GbEXPATR-mediated delay in the onset of secondary CW formation (Li et al., Reference Li, Tu, Pettolino, Ji, Hao, Yuan, Deng, Tan, Hu, Wang, Llewellyn and Zhang2016).
The CW acts as a sensing platform and plants use a dedicated system to control and maintain CW homeostasis that allows them to adapt to developmental changes as well as to environmental stresses. The wall composition and mechanical integrity are monitored by cell wall integrity (CWI) sensors and mechanosensitive ion channels (Hamann, Reference Hamann2015; Novakovic et al., Reference Novakovic, Guo, Bacic, Sampathkumar and Johnson2018). CWI signalling involves the perception of mechanical and physical changes of the plant cell environment and the generation of signals that are amplified through feedback processes. Disruption of CWI results in activation of stress responses and CW modifications that might prevent the cells from further damage, including oxidative crosslinking, productions of ROS, jasmonic acid (JA), salicylic acid (SA), ethylene, lignin or callose depositions, alterations in pectin methylesterification and finally swollen root cells and root growth arrest caused by the inhibition of cellulose synthesis (Gigli-Bisceglia et al., Reference Gigli-Bisceglia, Engelsdorf and Hamann2020; Van der Does et al., Reference Van der Does, Boutrot, Engelsdorf, Rhodes, McKenna, Vernhettes, Koevoets, Tintor, Veerabagu, Miedes, Segonzac, Roux, Breda, Hardtke, Molina, Rep, Testerink, Mouille, Höfte, Hamann and Zipfel2017). Interestingly, one of the proposed CWI sensors (reviewed in Rui & Dinneny, Reference Rui and Dinneny2020), the GPI-anchored COBRA (COB) localises predominantly to longitudinal CWs and controls the orientation of Arabidopsis root cell expansion (Schindelman et al., Reference Schindelman, Morikami, Jung, Baskin, Carpita, Derbyshire, McCann and Benfey2001). COB was shown to be involved in the regulation of cellulose crystallinity and microfibril orientation (Roudier et al., Reference Roudier, Fernandez, Fujita, Himmelspach, Borner, Schindelman, Song, Baskin, Dupree, Wasteneys and Benfey2005; Schindelman et al., Reference Schindelman, Morikami, Jung, Baskin, Carpita, Derbyshire, McCann and Benfey2001). Considering cellulose/CW matrix interaction as the primary target of EXPAs and the aforementioned role of PhEXPA in the control of cellulose crystallinity, the role of CWI and downstream feedback regulations in mediating the CW remodelling in a response to EXPA-induced changes in CW biomechanical properties cannot be excluded. However, the molecular mechanisms perceiving mechanical forces at the CW–plasma membrane interphase and controlling CWI-initiated adaptive responses remain largely unknown as it is difficult to separate them from integrated hormonal and stress signalling (Vaahtera et al., Reference Vaahtera, Schulz and Hamann2019).
Taken together, apart from their role in CW loosening, expansins seem to be involved in controlling CW properties and composition by interfering with the action of CW remodelling enzymes, possibly via mechanisms that are both dependent and independent of expansin interaction with CW carbohydrates (Figure 1b).
6.3. Organ primordia specification/outgrowth
Besides their role in organ growth, expansins were shown to be involved in the initiation of new organs both in the shoot and in the root. Sephacryl beads coated with expansin purified from cucumber hypocotyls disturbed phyllotaxis by inducing new leaf primordia on the shoot apical meristem (SAM) in tomato (Fleming et al., Reference Fleming, McQueen-Mason, Mandel and Kuhlemeier1997). Endogenous LeREXP18 was shown to be expressed in new leaf primordia in tomato (Reinhardt et al., Reference Reinhardt, Wittwer, Mandel and Kuhlemeier1998). Accordingly, local microinduction of cucumber expansin CsEXP1 in the tobacco SAM was able to induce new leaf formation and reverse the direction of new primordia appearance. Furthermore, the induction of CsEXP1 at the leaf margin changed the leaf shape by inducing ectopic leaf lamina formation (Pien et al., Reference Pien, Wyrzykowska, McQueen-Mason, Smart and Fleming2001). More recently, a possible molecular mechanism underlying the expansin-mediated primordia induction has been elucidated by placing expansin-controlled CW loosening into a previously described framework comprising a feedback loop between CW tension and microtubule orientation in the SAM (Armezzani et al., Reference Armezzani, Abad, Ali, Andres Robin, Vachez, Larrieu, Mellerowicz, Taconnat, Battu, Stanislas, Liu, Vernoux, Traas and Sassi2018; Hamant et al., Reference Hamant, Heisler, Jonsson, Krupinski, Uyttewaal, Bokov, Corson, Sahlin, Boudaoud, Meyerowitz, Couder and Traas2008; Sassi et al., Reference Sassi, Ali, Boudon, Cloarec, Abad, Cellier, Chen, Gilles, Milani, Friml, Vernoux, Godin, Hamant and Traas2014). Briefly, mechanical stress in the complex tissue of growing SAM affects the microtubule cytoskeleton, and that in turn controls morphogenesis (Hamant et al., Reference Hamant, Heisler, Jonsson, Krupinski, Uyttewaal, Bokov, Corson, Sahlin, Boudaoud, Meyerowitz, Couder and Traas2008). In parallel, auxin affects the cortical microtubule dynamics thus enhancing microtubule isotropy; together with auxin-induced softening of the CW, this seems to be sufficient to induce new organ outgrowth (Sassi et al., Reference Sassi, Ali, Boudon, Cloarec, Abad, Cellier, Chen, Gilles, Milani, Friml, Vernoux, Godin, Hamant and Traas2014). However, the changes in microtubule organisation were shown to activate the transcription of genes which potentially can induce CW loosening (PME3, XTH9 and EXPA15) independently of auxin accumulation and transport. Conversely, interfering with wall loosening promotes changes in microtubule organisation (Armezzani et al., Reference Armezzani, Abad, Ali, Andres Robin, Vachez, Larrieu, Mellerowicz, Taconnat, Battu, Stanislas, Liu, Vernoux, Traas and Sassi2018).
In the root, cytokinin-induced AtEXPA1 and CW acidification were suggested to induce the elongation and differentiation of cells leaving the root apical meristem (RAM) in the root transition zone (Pacifici et al., Reference Pacifici, Di Mambro, Dello Ioio, Costantino and Sabatini2018), and this is somewhat analogous to new organ primordia in the SAM. However, more recent studies seem to confirm neither cytokinin-inducible AtEXPA1 in the root transition zone nor the role of AtEXPA1 in controlling root growth (Ramakrishna et al., Reference Ramakrishna, Ruiz Duarte, Rance, Schubert, Vordermaier, Vu, Murphy, Vilches Barro, Swarup, Moirangthem, Jorgensen, van de Cotte, Goh, Lin, Vobeta, Beeckman, Bennett, Gevaert, Maizel and De Smet2019; Samalova et al., Reference Samalova, Elsayad, Melnikava, Peaucelle, Gahurova, Gumulec, Spyroglou, Zemlyanskaya, Ubogoeva and Hejatko2020). Instead, AtEXPA1 seems to be involved in radial swelling of the lateral root founder cell as an important determinant of asymmetric cell division, initiating the process of lateral root (primordia) formation (Ramakrishna et al., Reference Ramakrishna, Ruiz Duarte, Rance, Schubert, Vordermaier, Vu, Murphy, Vilches Barro, Swarup, Moirangthem, Jorgensen, van de Cotte, Goh, Lin, Vobeta, Beeckman, Bennett, Gevaert, Maizel and De Smet2019). Interestingly, also here the asymmetric swelling of the lateral root founder cell is dependent on auxin signalling and position-specific reorientation of cortical microtubules (isotropic in the position of asymmetric swelling; Vilches Barro et al., Reference Vilches Barro, Stöckle, Thellmann, Ruiz-Duarte, Bald, Louveaux, von Born, Denninger, Goh, Fukaki, Vermeer and Maizel2019). This result is another puzzle in the emerging role of mechanical interactions between pericycle and endodermis cells in lateral root formation (Vermeer et al., Reference Vermeer, von Wangenheim, Barberon, Lee, Stelzer, Maizel and Geldner2014) and more generally the role of cytoskeleton dynamics in the determination of primary CW biomechanics and cell division (reviewed in Chebli et al., Reference Chebli, Bidhendi, Kapoor and Geitmann2021; Robinson, Reference Robinson2021).
7. Expansins under abiotic stress
The transcripts of many α-expansins are up-regulated under abiotic stress (Marowa et al., Reference Marowa, Ding and Kong2016; Tenhaken, Reference Tenhaken2015). Accordingly, genetic approaches have shown that enhanced expansin expression might contribute to stress tolerance to drought (Chen et al., Reference Chen, Han, Meng, Zhou, Xiangzhu and Wei2016; Hao et al., Reference Hao, Qian, Xiao, Huabo, Junkai and Jichen2017; Liu et al., Reference Liu, Zhang, Hao, Zhang, Liu and Chen2019; Narayan et al., Reference Narayan, Dharshini, Manoj, Padmanabhan, Kadirvelu, Suresha, Subramonian, Ram, Premachandran and Appunu2019; Yang et al., Reference Yang, Zhang, An, Li, Chen, Zhao, Wu, Wang, Hao, Wang and Wang2020), high salinity (Chen et al., Reference Chen, Han, Kong, Kang, Ren and Wang2017; Reference Chebli and Geitmann2018a; Hao et al., Reference Hao, Qian, Xiao, Huabo, Junkai and Jichen2017; Lu et al., Reference Lu, Kang, Jiang, Dai, Gao and Zhang2013; Yan et al., Reference Yan, Wu, Yan, Hu, Ali and Gan2014; Zhang et al., Reference Zhang, Liu, Yang, Xu, Liu and Xu2019b), heat (Xu et al., Reference Xu, Tian, Belanger and Huang2007; Reference Xu, Xu, Shi, Xu and Huang2014), cold (Peng et al., Reference Peng, Xu, Wang, Feng, Zhao, Feng, Zhao, Hu and Li2019; Zhang et al., Reference Zhang, Xu, Dong, Peng, Feng, Wang, Li, Miao, Yao, Zhao, Feng, Hu and Li2018a), oxidative (Chen et al., Reference Chen, Ren, Zhang, An, Yang, Wang and Wang2018b) and heavy metal (cadmium) stress (Ren et al., Reference Ren, Chen, An, Zhao, Zhang, Wang and Wang2018; Zhang et al., Reference Zhang, Ding, Zhi, Li, Liu and Xu2018b). Moreover, Han et al. (Reference Han, Li, Li, Zhao and Wang2012; Reference Han, Chen, Yin, Zhang and Wang2015) described that overexpression of
![]() $\unicode{x3b2}$
-expansin TaEXPB23 also enhanced tolerance to oxidative and salt stress, similar to the
$\unicode{x3b2}$
-expansin TaEXPB23 also enhanced tolerance to oxidative and salt stress, similar to the
![]() $\unicode{x3b2}$
-expansins ZmEXPB6 and ZmEXPB8 studied by Geilfus et al. (Reference Geilfus, Ober, Eichacker, Mühling and Zörb2015) and Wu et al. (Reference Wu, Thorne, Sharp and Cosgrove2001) respectively. The changes in
$\unicode{x3b2}$
-expansins ZmEXPB6 and ZmEXPB8 studied by Geilfus et al. (Reference Geilfus, Ober, Eichacker, Mühling and Zörb2015) and Wu et al. (Reference Wu, Thorne, Sharp and Cosgrove2001) respectively. The changes in
![]() $\unicode{x3b1}$
-expansin gene activity under various abiotic stresses in different plants are summarised in Table 1.
$\unicode{x3b1}$
-expansin gene activity under various abiotic stresses in different plants are summarised in Table 1.
Table 1 Overview of published evidence on expansin role in abiotic stress response.
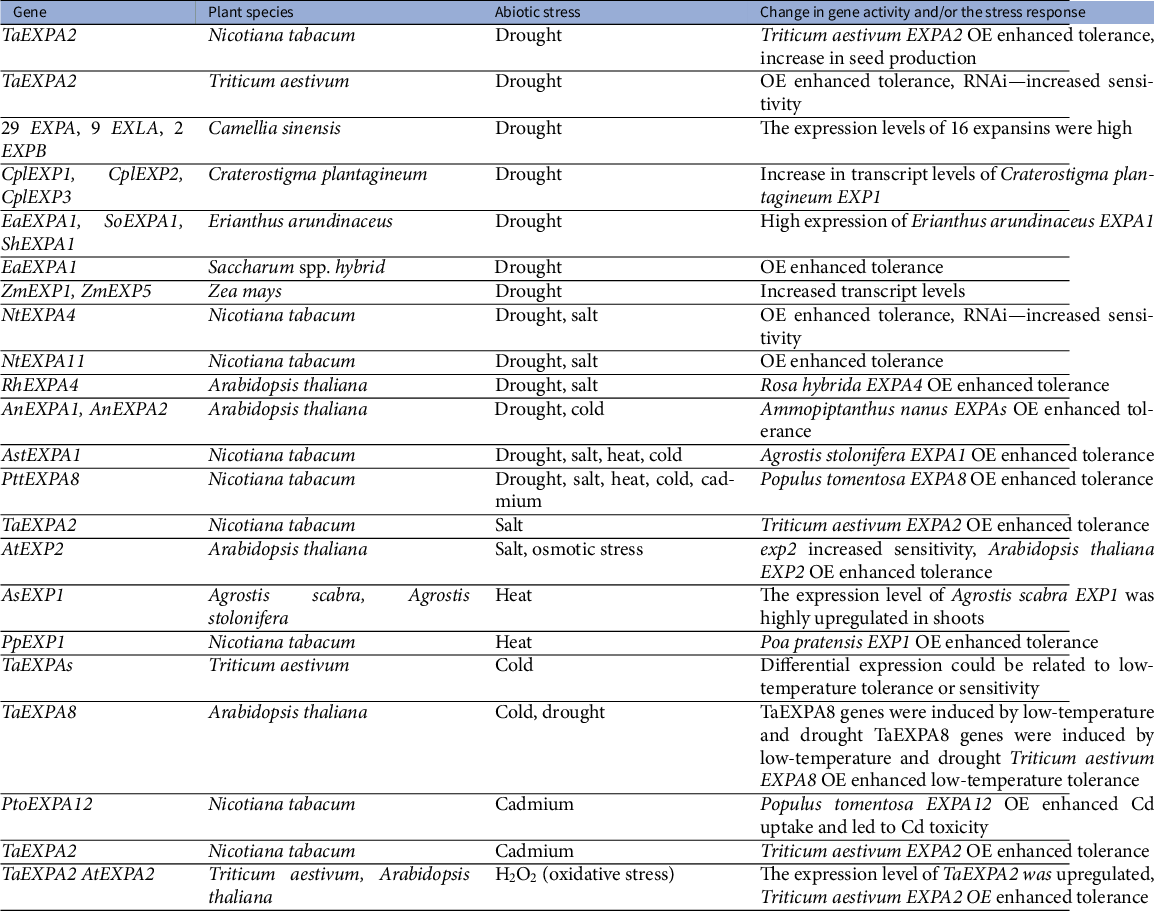
Note: TaEXPA8 genes were induced by low-temperature and drought.
The mechanism of expansin action in mediating stress resistance is still rather unclear. Investigating CW biomechanics under abiotic stresses is often challenging, so the focus has predominantly remained at the molecular level on genes involved in CW remodelling and on transcriptional and proteomic changes. Concerning changes in the composition and structure of CWs, loss of water can cause enhanced bonding among individual wall components which can impact the biosynthesis and deposition of newly formed CW polymers. This can be seen, for example, during salt stress, when sodium ions might influence pectin cross-links and disrupt microtubule stability, which consequently influence cellulose deposition (Wang et al., Reference Wang, McFarlane and Persson2016b).
Reactive oxygen species (ROS) and peroxidases may also play an important role in the process of CW remodelling. ROS production occurs under many different stress conditions, but it is also necessary for normal growth and development (Mittler, Reference Mittler2017) hence their production and quenching must be tightly controlled (Castro et al., Reference Castro, Citterico, Kimura, Stevens, Wrzaczek and Coaker2021 and references therein). ROS are responsible for the initial cross-linking of phenolic compounds and CWs glycoproteins resulting in stiffening. On the other hand, wall polysaccharides might be directly cleaved by hydroxyl radicals and weaken plant CWs (Fry, Reference Fry1998; Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009; Schopfer, Reference Schopfer2001; Schweikert et al., Reference Schweikert, Liszkay and Schopfer2000). Tenhaken (Reference Tenhaken2015) proposed a simplified model in which he suggests that plant organ growth under stress is a conflict between the two processes. According to this model, growth arrest under abiotic stresses is possibly caused by ROS- and peroxidase-induced cross-linking of glycoproteins and phenolics esterified with hemicellulose polymers, resulting in a dense network in which expansins and XTH do not have access to the xyloglucan substrate. If ROS production (stress) continues and all peroxidase substrates are depleted, ROS accumulation might lead to the formation of hydroxyl radicals, inducing the opposite effect, that is, cleavage of polymer chains. This results in CW weakening that enables further growth, comparable to growth under non-stress conditions. However, the experimental evidence for the model (Figure 1c) remains to be provided.
Interestingly, the action of expansins may result in enhancing the activity of CW-bound peroxidases in order to mitigate oxidative stress; however, the mechanism remains unknown (Han et al., Reference Han, Chen, Yin, Zhang and Wang2015). The increased activity of covalently bound CW peroxidases was observed in transgenic plants over-expressing TaEXPB23 and Arabidopsis expb2 mutant showed a reduction in the activity and a decrease of oxidative stress tolerance (Han et al., Reference Han, Chen, Yin, Zhang and Wang2015). Furthermore, expansin-mediated heat stress tolerance also seems to involve increased antioxidative capacity, photosynthesis rate and reduction of structural damage (Xu et al., Reference Xu, Xu, Shi, Xu and Huang2014).
According to Wu et al. (Reference Wu, Sharp, Durachko and Cosgrove1996; Reference Wu, Thorne, Sharp and Cosgrove2001)), root cell elongation is maintained at low water potential following enhanced expansin expression that enables plants to withstand drought conditions. This adaptive response, enabling roots to continue growing despite reduced turgor pressure, increases the root: shoot ratio allowing roots to explore the soil for water while limiting the water loss through leaves (Cosgrove, Reference Cosgrove2021). Furthermore, expansins were also proposed to be involved in increasing CW flexibility during the de- and rehydration processes in the resurrection plant Craterostigma plantagineum (Jones & McQueen-Mason, Reference Jones and McQueen-Mason2004).
8. Conclusions and future outlines
In contrast to the long-standing perception that considered the CW a rather static structure, passively delimiting the plant cell shape and providing mechanical support to plant bodies, the CW is a complex and highly dynamic structure, whose biomechanical properties have key consequences for a number of responses. Expansins are among the factors that allow plants to selectively change CW biomechanics, thus controlling plant growth and morphogenesis. As it is clear from our brief overview of the rich literature on the topic, there are several aspects of expansin action that are worth emphasising.
First, expansins seem to act in a manner that is dependent on both their dose and the particular developmental context. Second, CW sensitivity to expansin action seems to be actively controlled during the plant life cycle and in a location-specific fashion, and this is mediated by other factors including apoplastic pH. Third, expansins seem to control CW biomechanical properties not only by inducing creep but also by influencing CW remodelling, possibly through the modulation of substrate availability to other CW remodelling factors and/or CWI signalling. These effects might have important but different consequences for the downstream developmental regulations. It is therefore obvious that in order to comprehend the importance of expansin-regulated plant development and abiotic stress responses we will need a detailed understanding of the spatiotemporal specificity of expansin expression and its localization in living plant tissues. The existence of feedback regulatory loops between expansin activity/levels and expansin-modulated CW biomechanics might explain the dose-dependent and sometimes contradictory expansin effects. Moreover, functional redundancy among members of the expansin family is highly likely, and this may require phenotype assays of multiple mutants in expansin genes. Further, understanding the expansin structure (either using experimental or structure prediction algorithms, see Figure 1 and the text above) and binding specificity will be necessary to elucidate the possible importance of expansins in regulating CW composition by interfering with CW remodelling factors. However, it should be emphasised that most of the experimental evidence on the possible role of expansins in CW remodelling originates from overexpression studies. Thus, more detailed studies employing, for example, cell type-specific endogenous promoters will be necessary to assess the possible role of expansins in CW remodelling.
Finally, developing tools allowing in vivo assays of quantifiable CW biomechanical properties at (sub)cellular resolution will be critical. Approaches combining biology, physics and mathematical modelling are particularly salient in order to integrate the vast array of complex observations that is expected from state-of-the-art visualisation methods, molecular biology/biochemistry and genetics studies.
Glossary of used biomechanical terms

Modified from: Chebli and Geitmann (Reference Chebli and Geitmann2017), Cosgrove (Reference Cosgrove1993; Reference Cosgrove2018a) and Zhang et al. (Reference Zhang, Tang, Vavylonis and Cosgrove2019a).
Acknowledgements
We are grateful to Prof. Olivier Hamant for his kind invitation and for the opportunity to provide our view on this highly interesting and dynamically developing topic. We thank the anonymous reviewers for their constructive and helpful comments.
Financial support
This work was supported by the Ministry of Education, Youth and Sports of CR from the European Regional Development Fund Project ‘Centre for Experimental Plant Biology’: No. CZ.02.1.01/0.0/0.0/16_019/0000738, LTAUSA18161 and the Czech Science Foundation (19-24753S and 22-17501S).
Conflict of interest
The authors declare no conflict of interest.
Authorship contributions
M.S., E.G. and J.H. performed the literature search, conceived the review structure and wrote the manuscript. J.H. drew Figure 1.
Data availability statement
All the data discussed in the review were obtained from the referenced papers. The AtEXPA1 (AT1G69530) structural prediction was downloaded from AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/).





Comments
Comments to Author: The manuscript ‘Expansin-mediated developmental and adaptive responses – a matter of cell wall biomechanics?’ reviews the current knowledge on the expansins proteins in the context of the current cell wall models.
The expansins although being studied for a long time, have often proven to be tricky in the interpretation of their direct phenotypes. The effects are often subtle, probably very local, and specific function often masked due to redundancy. The link to a clear mechanism of action of these enigmatic proteins still remains a big question in the field. The review is timely in view of the increasing understanding of the ultrastructure and composition of the cell wall and techniques for measurement of biomechanical properties. The review would be of broad interest to the cell wall and biomechanics community among others.
The authors highlight these aspects in the review and extensively cover several recent advances in cell wall models and expansin in this context. The authors further discuss the role played by expansins in different developmental processes and in response to abiotic stresses. They detail approaches employed to study these proteins and highlight the challenges with unravelling the mechanism of expansin action with the current tools available.
Overall, this review is well very structured and written. Only a few comments to consider:
1. Overall, The link between pH and expansins is an important one that can be highlighted more in the text. Line 13 describes the loosening theory linking the turgor pressure and wall stress aspect well. The review could benefit from a more critical discussion on the link between auxin – pH and expansins in the context of acid growth theory and its fit with the current cell wall biomechanical models. Particularly to support the statement in Line 77 and 107. This might help delineate the role of expansin on cellulose, pectin and wall components further. Some useful references: Brummel et al., 1999; Arsuffi and Braybrook, J.Exp.Bot, 2018; Dunser and Kleine-Vehn, Curr. Op in Plant Bio., 2015, Fendrych et al., eLife 2016.
2. Line 154: The section could benefit from a few lines on the difference in the grass wall compared to eudicots and β-expansins in this context. Some useful references: Wang et al., Plant Phys., 2016; Valdivia et al., Sexual Plant Reproduction, 2009; Yennawar et al., PNAS., 2006)
3. Line 265: It would be interesting to extend the discussion initiated on the link between auxin – microtubules and SAM in line 246 in the context of lateral root initiation. A few recent works in this context by Vilches Barro et. al., Current Biology, 2020; Review: Robinson, New Phytologist, 2021; Chebil et al., Current Biology, 2021. The recurrence of expansin-mediated wall loosening associated with asymmetrically dividing cells, suggests an interesting link between expansins and i) their ability to respond to unique mechanical stress asymmetry sensed in these meristematic tissues; ii) potential for polar auxin flux to influence local expansion.
4. Line 308: Referen in the text to the commentary on overexpression of α-expansin in conference of drought tolerance in wheat – Cosgrove et al., 2021.
5. The Figure 1 has not been referenced in the main text. The scheme is rather minimal compared to the wealth of information covered in the review. The review would overall benefit if the figure could be expanded to present expansins in light of the current cell wall model and differences between cell wall loosening and remodelling covered in the review.
6. The abstract mentions that the review covers the role of expansins in stress response. The use of the terms “abiotic stress response” would be more appropriated here as it is the only stress covered in this review and will help distinguish from the term ‘stress’ in the biomechanical context.
7. Line 312: Additional references for methods and studies could be highlighted: Improved plant tissue friendly confocal Raman microscopy – combined view of wall chemistry and couple with AFM etc., for biomechanics: (Antreich et al., J.Exp.Bot., 2021, Gierlinger et al., 2012); Cellular force microscopy – Majda et al., Plant Cell Morphogenesis, 2019; Mechanoprobes – Michels et al., PNAS, 2020; single particle tracking (sptPALM)– Bayle et al., Nature Protocols., 2021; microfluidics – Yanagisawa et al., Plant & Cell Physiology., 2021.
Minor comments, some that might make it more accessible to non-cell wall specialists.
- As the terms such as wall creep, stress relation, wall loosening, wall softening, wall remodelling have been used extensively across the text, the readers could benefit from a Table or an Appendix defining these terms and linked into the manuscript early on.
- Line 25, The sentence ‘to the best of our knowledge’ would be better that ‘all’ plants species.
- Line 45: Would be worth including these references– cotton (Lv et al., BMC Plat Biology, 2020); brassica (Li et al., Plant Phys and Biochem, 2021).
- Line 113: The main message from the statement in line 113 is not very clear and could be simplified.
- Line 112-113: Please provide references on the model(s) the authors refer to in these lines.
- Line 133: Nuclear Magnetic Resonance and can abbreviated (NMR).
- Table 1 – Additional references for wheat and drought tolerance, Calderini et al., New Phytologist, 2020, sugarcane Narayan et al., 2021.