INTRODUCTION
Over the past decade, burden of disease estimates have become of increasing significance in allocating medical resources, in targeting interventions, and for monitoring possible effect. These studies can use monetary units to quantify the burden of disease, so-called cost-of-illness studies, or can assess the disease burden in a population by utilizing the disability adjusted life years (DALYs) metric [Reference Murray and Lopez1].
The DALY integrates the effect of mortality as well as disease and disability, which allows them to be considered at the same time. This simplifies comparisons between distinct disease outcomes and subgroups of a population. A prerequisite for these comparisons, either in monetary units or in DALYs, is that the burden of disease comprises a complete assessment of the health effect due to a certain pathogen. This means for infectious intestinal disease (IID) that, apart from the direct consequence of gastroenteritis, all sequelae should be considered. In previous studies on the burden of IID [Reference Kemmeren2–Reference Havelaar4], we included outcomes, such as Guillain–Barré syndrome, reactive arthritis, inflammatory bowel disease and haemolytic uraemic syndrome, leading to chronic renal failure to complement the burden of acute gastroenteritis.
Previous studies have shown that the prevalence of irritable bowel syndrome (IBS) in Western populations is between 10% and 20% [Reference Hungin5, Reference Mertz6]. There is growing evidence of persisting gastrointestinal symptoms after bacterial IID, and these patients will, at least partly, meet the diagnostic criteria for (post-infectious) irritable bowel syndrome (PI-IBS) [Reference DuPont7–Reference Thabane, Kottachchi and Marshall11]. Although not life-threatening or severely debilitating, IBS symptoms may have a severe effect on daily life, affecting work, school and social life, and decreasing health-related quality of life over long periods [Reference Glia and Lindberg12, Reference Pare13]. These periods of decreased health-related quality of life on the one hand and its high prevalence in society on the other might add up to a substantial burden of disease, which is currently not considered in estimates of the burden of IID.
Nonetheless, when PI-IBS is considered in such studies, the background IBS population prevalence and the percentage attributable to IID should be indisputably clear. For this purpose, the attributable risk (AR) has been established as the preferred measure of association.
In the current study, we first aimed to assess the AR of developing PI-IBS for bacterial pathogens. Second, we estimated the disease burden in DALYs of PI-IBS due to a particular group of pathogens, i.e. (thermophilic) Campylobacter spp., Salmonella spp., and Shigella spp. in The Netherlands. These pathogens were chosen because they are the most frequent causes of bacterial IID and have been demonstrated to be responsible for the largest burden of IID [Reference Kemmeren2]. Third, we compared this burden with other outcomes associated with these pathogens.
METHODS
Literature review
We based our study on the recently published meta-analysis by Thabane et al. [Reference Thabane, Kottachchi and Marshall11]. From this paper we selected four studies (Table 1) in which:
• IID patients were considered who had no previous history of IBS or other bowel disorders.
• Appropriate control groups were included.
• A bacterial aetiology was confirmed by stool culture from at least a proportion of the patients with IID.
In an additional search in the recent literature, we identified one study [Reference Jung14] that met the same selection criteria. This study presented follow-up data of a previously reported cohort [Reference Ji15, Reference Kim16], and we therefore only used the most recent of these two studies in our evaluation. If available, we used data after a follow-up period of 1 year, or as close to this time point as possible. Studies involving patients with traveller's diarrhoea were excluded because it has been suggested that these are predominantly caused by enterotoxigenic E. coli, which has a milder course and a lower risk of developing PI-IBS [Reference DuPont17].
Table 1. Population attributable risk of developing IBS following gastroenteritis, per study

IBS, Irritable bowel syndrome; AR, attributable risk.
AR
The AR was calculated as the additional incidence rate (IR) of exposed (IID) cases compared to unexposed (non-diseased) controls:
The uncertainty in the AR was modelled by employing a Bayesian approach [Reference Vose18]. Both IRexposed and IRunexposed cases were considered as binomial fractions in which the uncertainty was modelled as a Beta(s, f) distribution, in which s is the number of persons in the exposed or unexposed group who developed IBS and f is the number of persons who did not develop IBS. With this approach, the 95% confidence interval (CI) is similar to that estimated by frequentist statistics, with the advantage that now a full uncertainty distribution can be simulated.
DALYs
The incidence of gastrointestinal infectious disease due to Campylobacter, Salmonella and Shigella was derived from national incidence data on foodborne disease, based on a method reported by Havelaar et al. [Reference Havelaar19]. The estimates were updated to the year 2006 (for details, see [Reference Kemmeren2]). These incidence data relate to all cases in the population and are based on data from Sensor, a Dutch community-based cohort study [Reference de Wit20]. By combining the incidence of gastrointestinal infectious disease associated with the selected pathogens and a weighted mean AR, the incidence of PI-IBS was calculated. The estimated incidence of PI-IBS was then used to calculate the burden of PI-IBS in DALYs. Uncertainty in incidence and DALY estimates was quantified by Monte Carlo simulation.
The DALY aggregates mortality, expressed in years of life lost (YLL) and morbidity, expressed in years lived with disability (YLD), according to the following calculation:
where YLL represents the time lost due to premature mortality. For PI-IBS the mortality component of the DALY is not taken into account as IBS is not associated with increased mortality. YLD represents the time in good health lost while living with a disease or disability and is calculated with the following formula:
where n l is the number of cases with health outcome l, t l the duration of the health outcome and dw l the disability weight assigned to health outcome. The disability weight is a value ranging from 0 to 1 that is assigned to living with a medical condition. This value reflects the effect of a specific health condition on health-related quality of life and is commonly based on the preferences of an expert or lay panel. We adopted the IBS disability weight from the Mild Diseases and Ailments Study, a Dutch study that generated disability weights for 52 health conditions from panels of medical experts and laymen recruited from the general population. In this study a renewed methodology was applied that focused especially on obtaining and improving disability weights for functional losses of a temporary and complex nature [Reference Janssen, Birnie and Bonsel21]. This can also be applied to IBS symptoms, because they tend to subside and exacerbate with periods of partial remission. To align with the societal point of view of the DALY, we used the IBS disability weight derived from the population panel (n=105) which had a value of 0·042. For the duration of PI-IBS, several long-term follow-up studies were assessed [Reference Ji15, Reference Kim16, Reference Gwee22–Reference Neal, Barker and Spiller25]. Based on these studies, we assumed for PI-IBS provoked by bacteria an average duration of 5 years [Reference Jung14].
RESULTS
Table 1 presents an overview of the results of the five case-control studies that analysed the incidence of PI-IBS following IID. In these studies, the index group was a cohort of gastroenteritis patients with confirmed bacterial aetiology. Controls in these studies were either volunteers, patients from the same general practice or siblings or spouses of the patient. The PI-IBS incidence rate in these five studies ranged from 4% [Reference Rodriguez and Ruigomez26] up to 17% [Reference Parry27]. The calculated AR is also shown in Table 1. These data do not suggest that PI-IBS is different for various bacterial pathogens (χ2 test for homogeneity, P=0·95 [Reference Fisher and van Belle28]). We therefore pooled the studies considering patients with bacterial aetiology, weighted by the number of cases that experienced an episode of IID. This resulted in a mean weighted AR for bacterial infections (10–12 months post-IID) of 8·8% (90% CI 7·2–10·4; Fig. 1).

Fig. 1. Uncertainty distribution of the attributable risk of post-infectious irritable bowel syndrome in infectious intestinal disease patients with bacterial aetiology.
In The Netherlands, about 124 000 cases of IID due to the three pathogens were considered in 2006 (Table 2). Most of these IID cases were caused by Campylobacter (78 000), and Salmonella (43 000). About 27 000 GP visits were registered due to these three pathogens.
Table 2. Incidence of infectious intestinal disease due to three pathogens in The Netherlands (population 16·3 million), 2006
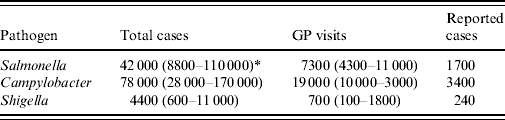
GP, General Practitioner.
* Mean (5th–95th percentile).
The three pathogens were estimated to be associated with about 11 000 new cases of PI-IBS per year (Table 3), in this way contributing 55 000 cases to the overall prevalence of IBS in The Netherlands; which is estimated to be 330 000 formally diagnosed patients [Reference Hungin5].
Table 3. Incidence and prevalence of post-infectious irritable bowel syndrome due to three pathogens in The Netherlands, 2006
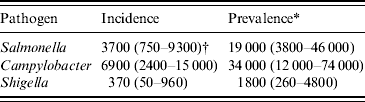
* Incidence×5 years.
† Mean (5th–95th percentile).
The disease burden of PI-IBS is shown in Table 4. In 2006, an estimated 2302 DALYs were lost due to PI-IBS following IID induced by the three selected pathogens, with 63% of DALYs due to Campylobacter, 34% to Salmonella and 3% to Shigella. Including PI-IBS in this calculation increased the burden of disease (Fig. 2) for Salmonella by 86% (from 905 to 1686 DALYs), for Campylobacter by 92% (from 1564 to 3008 DALYs) and for Shigella by 151% (from 51 to 128 DALYs).
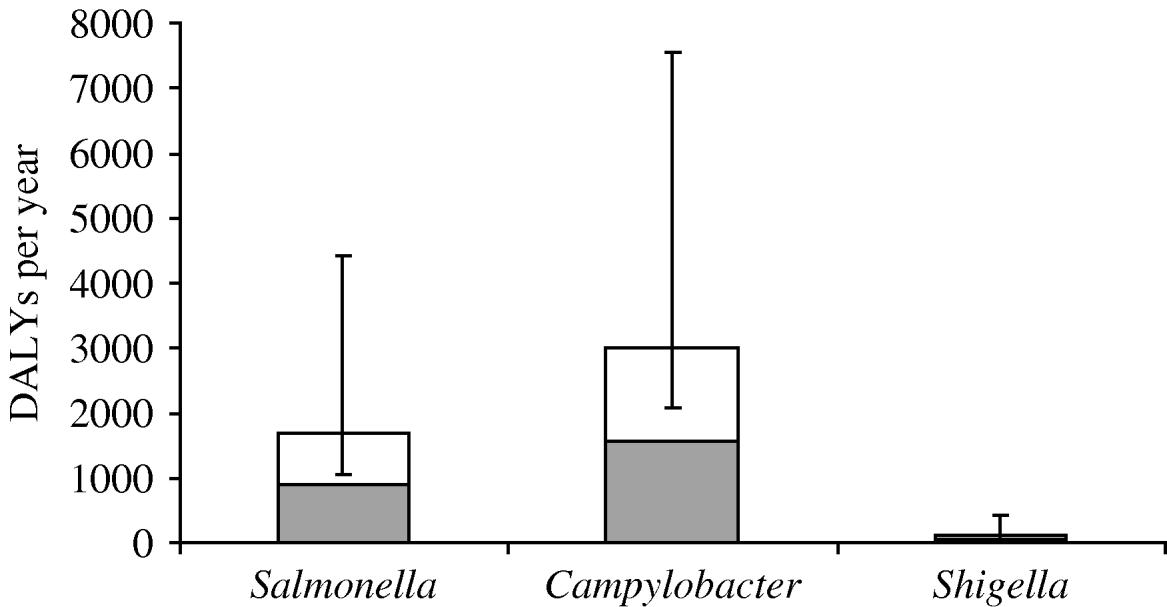
Fig. 2. Disease burden in disability adjusted life years (DALYs) per year due to three pathogens in The Netherlands, 2006, including (□) and excluding (![]() ) post-infectious irritable bowel syndrome. Error bars express the 5th and 95th percentiles resulting from Monte Carlo simulation.
) post-infectious irritable bowel syndrome. Error bars express the 5th and 95th percentiles resulting from Monte Carlo simulation.
Table 4. Disease burden of post-infectious irritable bowel syndrome due to three pathogens in The Netherlands, 2006
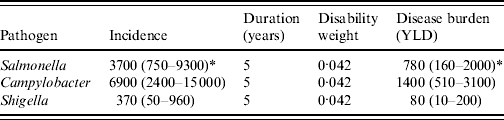
YLD, Years lived with disability.
* Mean (5th–95th percentile).
DISCUSSION
Based on recent findings in the literature, we estimated that about 9% of patients with bacterial IID will develop PI-IBS 1 year after infection [Reference Thabane, Kottachchi and Marshall11]. We also estimated that about 55 000 prevalent cases of PI-IBS in the Dutch population will actually result from an infection with Salmonella, Campylobacter or Shigella. This is about 17% of the total prevalence of formally diagnosed PI-IBS. Others [Reference Spiller and Garsed29] have estimated a similar range of 6–17%.
Our study used data from case-control studies to assess the incidence and disease burden of PI-IBS. Over the past few years, the evidence that an episode of IID is a risk factor for developing IBS has increasingly been recognized, reinforced by a growing understanding of the pathophysiology of IBS [Reference Crowell30–Reference Houghton, Foster and Whorwell32]. In brief, mucosal injury and inflammation are assumed to increase the number of enterochromaffin cells, thus increasing the release of serotonin (5-hydroxytryptamine, 5-HT). 5-HT affects gastrointestinal motility, enterocyte secretion and visceral sensation. Increased levels of pro-inflammatory cytokines and lymphocyte counts in intestinal epithelium and lamina propria have indeed been documented in PI-IBS patients [Reference DuPont7].
In a recent follow-up study, 3 years after a case-control study on laboratory-confirmed cases of Campylobacter and Salmonella, an increased risk of IBS could not be confirmed [Reference Doorduyn33]. In contrast, the results of this study suggested that patients with IBS or with another chronic intestinal condition were more susceptible to IID. The studies identified in our literature review excluded cases with pre-existing symptoms; hence, we only included cases in which the IID episode triggered a new case of IBS. Patients with already existing IBS or another intestinal illness were not included in our study.
In the current study, only limited information was available on some other factors involved in PI-IBS, and this may have affected our results in the following way. First, the data used to estimate the incidence of PI-IBS were retrieved from the existing literature. Several case-control studies were used and the time interval chosen for follow-up of exposed and non-exposed participants varied from 3 months [Reference Ji15] to 5 years [Reference Jung14]. Overall, we found that the incidence of PI-IBS in exposed participants was higher with a shorter follow-up period [Reference Gwee22, Reference Marshall23, Reference Mearin34]. In order to present a pooled estimate we chose a follow-up period of 10–12 months. Using shorter follow-up periods would have resulted in an increased AR, and, subsequently, a higher estimated incidence of PI-IBS. As these additional cases had a disease duration of <1 year, they would have added little value to the total burden of PI-IBS.
Second, in this study we did not consider patient characteristics (such as gender, smoking or psychosocial comorbidity) as risk factors for developing PI-IBS. Similarly, we did not take into account the details of the gastroenteritis episode, such as duration or aetiology of the infection [Reference Spiller10, Reference Gwee22, Reference Parry27, Reference Neal, Hebden and Spiller35, Reference Cuomo, Savarese and Gargano36]. With regard to duration of gastroenteritis, previous studies revealed that the longer period patients have symptoms due to gastroenteritis or the larger effect these symptoms have, the higher the risk of having PI-IBS [Reference Cuomo, Savarese and Gargano36, Reference Wang, Fang and Pan37]. We were not able to adjust for IID duration or severity, due to a lack of comparison.
Third, the disability weight is based on the preferences of a population panel, which is in line with the societal perspective of the DALY metric. Nevertheless, the IBS disability weight is not able to capture the heterogeneous group of clinical symptoms that are experienced by patients. Several studies have shown that there is a wide variation in symptoms between patients [Reference Bijkerk38]. As a result, actual health states of patients with (PI-)IBS may differ considerably from the health state descriptions valued by the population panel. Moreover, the value of the disability weight seems to be rather low. Health-related quality-of-life studies performed in patients with IBS have shown that the loss of health-related quality of life was indeed considerable [Reference Pare13, Reference Brazier39]. This is not reflected by the value of the disability weights as applied in this study.
A case-control study by Marshall et al. has suggested that PI-IBS may also result from presumed viral IID [Reference Marshall23]. As many as 20% (95% CI 11–29) of PI-IBS cases developed in IID patients, although this risk was only statistically significant after 3 months. This shorter duration of PI-IBS due to viral IID is in line with the observations of Spiller & Garsed [Reference Spiller and Garsed29], who noted that recent studies have demonstrated that there is very little mucosal destruction in viral IID and, therefore, that the mucosal structure is likely to rapidly return to normal. When the shorter duration is taken into account, PI-IBS due to norovirus would still accumulate to a prevalence estimate of 32 000 cases in the population and a disease burden of 1300 YLD, which is comparable to the disease burden of PI-IBS due to Campylobacter infection. This implies that including PI-IBS in disease burden estimates of the selected bacterial pathogens might still underestimate the burden of disease of foodborne disease. Further follow-up studies are needed that provide more information on the risk of IBS after viral IID.
The results from the current study show that PI-IBS is a frequent sequel of IID and that the disease burden of PI-IBS is considerable compared to other outcomes of IID. The resulting annual non-fatal burden of disease of PI-IBS is over 2300 YLD, almost doubling the total non-fatal burden of disease for the selected pathogens. Ignoring PI-IBS in burden-of-disease studies results in an underestimation of the size of the burden of these types of gastrointestinal diseases. In order to satisfy the aspirations of burden-of-disease studies, i.e. identifying priorities in medical resource allocation, targeting of interventions and monitoring possible effect, all sequelae of IID should be included, given that the evidence on the association is sufficiently conclusive. There is definitely more research required on the duration of PI-IBS and related fluctuations in health-related quality of life, as well as on the association of viral gastroenteritis and PI-IBS.
ACKNOWLEDGEMENTS
The authors thank Yvonne van Duynhoven, Wilfrid van Pelt, Marion Koopmans, Erwin Duizer (National Institute for Public Health and the Environment, Bilthoven, The Netherlands), Claudia Stein (World Health Organization, Geneva, Switzerland), Martyn Kirk (Australian National University, Canberra, Australia) and Fred Angulo (Centers for Disease Control and Prevention, Atlanta, GA) for stimulating discussions and feedback on the draft manuscript.
DECLARATION OF INTEREST
None.








