Clinically manifest Zn deficiency in growing individuals is associated with various unspecific symptoms such as growth depression, feed refusal and impaired immunity( Reference Aggett 1 ), highlighting the ubiquitous essentiality of this certain trace metal. However, this phenotype marks the end point in response to long-term insufficient supply at which the animals’ mobile Zn stores are exhausted and the homoeostatic regulation is no longer capable of maintaining a stable equilibrium of body Zn. Moreover, this physiological state is rare in nature. Most likely, the predominant Zn malnutrition phenotype in men and animals is a subclinical deficiency associated with reduction of Zn status parameters without development of visible symptoms. Therefore, an experimental approach was recently developed that induces subclinical Zn deficiency in weaned piglets( Reference Brugger, Buffler and Windisch 2 ). It has been shown that Zn status parameters in plasma, bone and liver respond very sensitively to graduations in analysed dietary Zn supply. At the same time, no overt signs of Zn deficiency were evident throughout the entire study. Using this approach, it is now possible to investigate the early and truly Zn-dependent metabolic adaptions in the development of a clinically manifest Zn deficiency.
The pancreas is essential for the regulation of feed digestion and energy homoeostasis( Reference Klein 3 ). Moreover, significant amounts of endogenous Zn are secreted via the pancreatic duct into the gastrointestinal tract (GIT) in order to maintain a basal body Zn level( Reference Holt, Uiu-Adams and Keen 4 , Reference Oberleas 5 ). Hence, it can be hypothesised that the regulation of pancreatic Zn metabolism and exocrine pancreatic digestive function could be somehow connected. Feed digestion is an important biological function, especially for growing livestock. In this context, the first few weeks after weaning are critical with regard to the maintenance of gut health and integrity( Reference Lallés, Bosi and Smidt 6 ). Hence, dietary fluctuations that negatively influence the GIT have to be avoided. Indeed, there are already reports on the effects of clinically manifest Zn deficiency on digestive function( Reference Pallauf and Kirchgessner 7 , Reference Roth, Schülein and Kirchgessner 8 ). However, as stated above, these findings may not be related to Zn homoeostatic adaption but a result of secondary metabolic imbalances arising in the course of clinical Zn deficiency. Data on the pancreatic response and related measures of digestive capacity under subclinical Zn deficiency are currently scarce.
Therefore, the goal of the present study was to investigate the effects of subclinical Zn deficiency on exocrine pancreatic enzyme activity in weaned piglets. Furthermore, coefficients of apparent faecal digestibility of crude nutrients were estimated in order to monitor the effects on digestive capacity.
Methods
This animal study was registered and approved by the responsible animal welfare authorities (district government of Upper Bavaria, federal state of Bavaria (Germany) (Az. 55·2·1·54-2532·3·63-11).
Animals and diets
The experimental conditions of the present study, including animal housing conditions, diet design and trial conduction, have already been published in detail( Reference Brugger, Buffler and Windisch 2 ). In brief, forty-eight individually housed weaned piglets from six litters (eight animals per litter; 50 % castrated male, 50 % female, initial average body weight 8·5 (sem 0·27) kg, 28 d of age) were fed a Zn-adequate (added Zn amount from ZnSO4.7H2O (analytical grade; Merck 108883; Merck KGaA): +60 mg Zn/kg diet; analysed dietary Zn content: 88 mg Zn/kg diet) basal diet ad libitum. The basal diet consisted mainly of maize and soyabean meal, and was fed for 2 weeks before the study for acclimatisation purposes and to ensure full body Zn stores at day 1 of the experimental period (Table 1). The basal diet met all the recommendations for sufficient nutrient supply of growing piglets as published by the National Research Council( 9 ) (Table 1). Following the acclimatisation period, all animals were assigned to eight dietary treatment groups according to a complete randomised block design. Each block represented eight animals from the same litter (six litters per blocks in total) consisting of eight castrated male and eight female piglets. An animal from each block was assigned to one of eight treatment groups (yielding six animals per treatment group). Thereby, it was possible to establish a balanced distribution of life weight, genetics (litter mates) and sex over treatment groups. The total experimental period consisted of 8 d, during which all treatment groups received the same basal diet from the acclimatisation period restrictively (450 g diet/d; all animals consumed the total amount of feed) but with varying analysed dietary Zn contents as modulated by varying supplementation with ZnSO4.7H2O (added Zn amounts: +0, +5, +10, +15, +20, +30, +40, +60 mg Zn/kg diet; analysed dietary Zn contents: 28·1, 33·6, 38·8, 42·7, 47·5, 58·2, 67·8, 88·0 mg Zn/kg diet). The group receiving 88·0 mg Zn/kg diet was considered to serve as control as it represented the feeding situation during the acclimatisation phase, from which the Zn contents of all other groups were gradually reduced. TiO2 was admixed to all diets (3 g/kg diet) to serve as an indigestible marker for assessing apparent faecal crude nutrient digestibility.
Table 1 Composition, metabolisable energy and crude nutrient contents of the basal diet( Reference Brugger, Buffler and Windisch 2 )
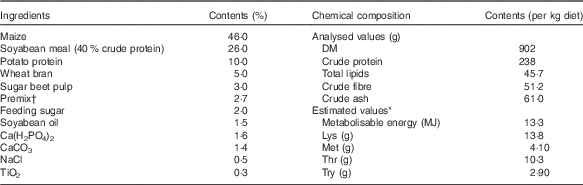
Vit., vitamin.
* The contents of metabolisable energy and essential pre-caecal digestible (synonymous with ‘ileal digestible’) amino acids were estimated according to feed table information (http://datenbank.futtermittel.net/).
† Premix: 2·8 % MgO; 0·08 % CuSO4.5H2O; 2·0 % FeSO4.7H2O; 0·20 % MnSO4.H2O; 0·002 % Na2SeO3.5 H2O; 0·002 % KI; 0·05 % Vit. A; 0·007 % Vit. D3; 0·2 % Vit. E; 0·002 % Vit. K3; 0·01 % Vit. B1; 0·03 % Vit. B2; 0·1 % niacin; 0·02 % pantothenic acid; 0·02 % Vit. B6; 0·15 % Vit. B12; 0·03 % biotin; 0·002 % folic acid; 6·7 % choline chloride; 77·6 % maize meal. Vit. and trace element contents (except Zn) met the requirements according to the National Research Council( 9 ).
Sampling conditions
Feed samples were stored at −20°C in air-tight polyethylene bottles, whereas faeces samples from the last 3 experimental days (days 6–8) were pooled animal-wise in plastic bags and stored at −20°C until freeze-drying before chemical analyses.
At experimental day 8, all animals were killed without fasting and the pancreases were removed. Pancreatic samples for the measurement of enzyme kinetics and tissue Zn contents were immediately snap-frozen in liquid N2 and stored at −80°C until further use.
Chemical analyses
In order to assess treatment-dependent shifts in digestive capacity, chemical analyses included Zn, crude nutrient and TiO2 contents in feed and faeces, pancreatic Zn content as well as pancreatic exocrine enzyme activities (trypsin, chymotrypsin, carboxypeptidase A, carboxypeptidase B, elastase, α-amylase).
Total zinc contents
Analyses of total Zn contents in feed, faeces and pancreatic tissue were performed as described earlier( Reference Brugger, Buffler and Windisch 2 ) using atomic absorption spectrometry (AAS) (novAA 350; Analytik Jena AG) after wet digestion via microwave (Ethos 1; MLS GmbH). Calibration was based on certified AAS standard solutions (Merck 109953; Merck Millipore).
Contents of DM, crude nutrients and TiO2 in feed and faeces
DM, crude nutrients and TiO2 in feed and faeces were assessed by standard procedures( Reference Naumann and Bassler 10 , Reference Brandt and Allam 11 ). In brief, all samples were milled through a 0·5-mm screen before analysis (faeces samples were freeze-dried before analysis). DM contents were assessed using 5 g of sample material by re-weighing after drying with heat (103°C) for 4 h. Crude ash (CA) was determined in 5 g of sample material by mineralising in platinum dishes at 550°C for 2 d. Crude protein (CP) contents were determined using 1 g of sample material according to the method of Kjeldahl, in which the sample was oxidised with H2SO4 (1·84 g/ml). After addition of 0·5 m-NaOH, the released ammonia was measured through titration. In order to estimate the CP content of the sample, the amount of ammonia-associated N was multiplied with factor 6·25, assuming average N contents in protein of 16 %. Total lipid (TL) contents were analysed in 2·5 g of sample material by treating all samples with hot 3 m-HCl for 3 h. The remaining solid residue was washed with double-distilled water, dried (1·5 h, 100°C) and used for lipid extraction with petroleum diethyl ether for 6 h in the Soxhlet apparatus. Crude fibre (CF) contents were assessed by treating 1 g of sample material for 30 min successively with boiling 0·13 m-H2SO4 and boiling 0·23 m-KOH. The solid residue was mineralised in Pt dishes at 475°C for 30 min. The difference between the residue weight and the remaining ash represented the CF content.
Determination of TiO2 contents was performed by extracting 1 g of sample in a hot solution consisting of 30 ml 96 % H2SO4, 9·75 g K2SO4 and 0·25 g CuSO4.5H2O for 3 h. The supernatant (10 ml) was treated with 1 ml of an aqueous solution consisting of 350 µl 96 % H2SO4, 150 µl 85 % H3PO4, 100 µl 35 % H2O2 and 400 µl double-distilled H2O and was incubated for 1 h at room temperature. The resulting yellow product was measured at 405 nm on the UV MC2 spectrophotometer (SAFAS Scientific Instruments) using plastic semi-micro cuvettes.
Pancreatic enzyme activities
Pancreatic tissue samples (100 mg frozen sample/animal) were placed in 1-ml, ice-cold 154 mm-NaCl and homogenised using the FastPrep® System with Matrix Green Beads (MP Biomedicals). The homogenate was centrifuged at 13 800 g for 20 min at 4°C. The supernatant was removed and stored at −80°C until further usage.
The protein concentration within pancreatic tissue lysates was determined using a bicinchoninic acid assay( Reference Smith, Krohn and Hermanson 12 ). This information was subsequently used to normalise the respective enzyme kinetics for the mg content of proteins within the reaction mix.
Activation of pancreatic trypsinogen to trypsin was performed by in-vitro incubation with exocrine enteropeptidase (E0885; Sigma-Aldrich)( Reference Glazer and Steer 13 ). All other zymogens (chymotrypsinogen, pro-carboxypeptidase A, pro-carboxypeptidase B, pro-elastase) were activated by in-vitro incubation with exocrine trypsin (T4549; Sigma-Aldrich)( Reference Glazer and Steer 13 – Reference Lainé, Beattie and LeBel 16 ).
Enzyme kinetics were measured using either ninety-six-well microplates (Rotilab®-microtest plates; Carl Roth GmbH & Co. KG) on the LEDETECT 96 system (Deelux Labortechnik GmbH) (trypsin, chymotrypsin, elastase) or quartz cuvettes (carboxypeptidase A and B) and plastic semi-micro cuvettes (α-amylase), respectively, on the UV MC2 spectrophotometer.
Pancreatic enzyme activity was determined colourimetrically by monitoring the increase in absorption due to hydrolysis of respective substrates (trypsin: N α -benzoyl-l-arginine 4-nitroanilide hydrochloride (B3133; Sigma-Aldrich) to p-nitroaniline at 405 nm; chymotrypsin: N-glutaryl-l-phenylalanine p-nitroanilide (G2505; Sigma-Aldrich) to p-nitroaniline at 405 nm; carboxypeptidase A: hippuryl-l-phenylalanine to hippuric acid at 254 nm; carboxypeptidase B: hippuryl-l-arginine to hippuric acid at 254 nm; elastase: N-succinyl-ala-ala-ala-p-nitroanilide (S4760; Sigma-Aldrich) to p-nitroaniline at 405 nm; α-amylase: reduction of 3,5-dinitrosalicylic acid by reducing groups of soluble starch (S9765; Sigma-Aldrich) at 540 nm). Enzyme activities were defined as units (U) per mg protein and minute reaction time (U/mg per min); 1 U represented 1 nmol (trypsin, chymotrypsin, elastase) or 1 µmol (carboxypeptidase A, carboxypeptidase B, α-amylase) of released product, respectively. Reaction conditions for trypsin, chymotrypsin, carboxypeptidase A, carboxypeptidase B, elastase and α-amylase were pH 7·8/37°C, pH 7·6/25°C, pH 7·5/25°C, pH 7·65/25°C, pH 7·9/25°C and pH 6·9/25°C, respectively.
Estimation of apparent faecal digestibility coefficients for DM and crude nutrients
The percentage apparent faecal digestibility coefficients of DM, CP, diethyl ether extract (subsequently referred to as total lipids; TL), CA and CF were calculated on the basis of the respective ratios of DM and crude nutrients to TiO2 in feeds and faeces using the following formula:
 $$\eqalign{ & {\rm Apparent}\,\,{\rm digestibility}\,(\,\%\,){\equals}\cr &100{\,\minus}\left(\!\!{{{{\rm \,\%\,}\,{\rm TiO}_{{\rm 2}} \,\,{\rm in}\,\,{\rm feed}} \over {{\rm \,\%\,}\,{\rm TiO}_{{\rm 2}} \,\,{\rm in}\,\,{\rm faeces}}}{\,\,\,\times\,}{{{\rm \,\%\,}\,{\rm DM}\,\,{\rm or}\,\,{\rm nutrient}\,\,{\rm in}\,\,{\rm faeces}} \over {\,\%\,\,{\rm DM}\,\,{\rm or}\,\,{\rm nutrient}\,\,{\rm in}\,\,{\rm feed}}}{\,\times}100} \right).$$
$$\eqalign{ & {\rm Apparent}\,\,{\rm digestibility}\,(\,\%\,){\equals}\cr &100{\,\minus}\left(\!\!{{{{\rm \,\%\,}\,{\rm TiO}_{{\rm 2}} \,\,{\rm in}\,\,{\rm feed}} \over {{\rm \,\%\,}\,{\rm TiO}_{{\rm 2}} \,\,{\rm in}\,\,{\rm faeces}}}{\,\,\,\times\,}{{{\rm \,\%\,}\,{\rm DM}\,\,{\rm or}\,\,{\rm nutrient}\,\,{\rm in}\,\,{\rm faeces}} \over {\,\%\,\,{\rm DM}\,\,{\rm or}\,\,{\rm nutrient}\,\,{\rm in}\,\,{\rm feed}}}{\,\times}100} \right).$$
Statistical analyses
All procedures were performed using SAS 9.4 (SAS Institute Inc.). The individual animal represented the experimental unit. All assessed parameters were subject to a one-way ANOVA (independent variables: analysed dietary Zn content with block as covariate) using the general linear model procedure. Significantly different treatment means were identified using the Student–Newman–Keuls test. Furthermore, an orthogonal contrast was estimated with the function CONTRAST between the groups with <58 and ≥58 mg Zn/kg diet, in order to highlight potential differences in response between insufficiently and sufficiently supplied Zn treatment groups. This threshold was chosen in light of an earlier study in which the point of sufficient Zn supply under the present experimental conditions was recognised at 58 mg Zn/kg diet using broken-line response analysis of apparently digested feed Zn data( Reference Brugger, Buffler and Windisch 2 ).
In order to investigate potential relationships between pancreatic Zn metabolism and exocrine enzyme activity, an orthogonal contrast between animals with the 50 % lowest pancreatic Zn contents compared with those with the 50 % highest Zn contents was calculated.
In order to assess the mathematical relationships between feed Zn supply and the measured pancreatic and faecal parameters, broken-line (y=a+bx+cx) regression analysis was conducted using the procedure NLMIXED (nonlinear mixed model).
A threshold of P≤0·05 was considered as indicator of statistical significance.
Results
Effects of the dietary treatment on analysed pancreatic zinc and exocrine pancreatic activity
Table 2 presents the results of one-way ANOVA and orthogonal contrasting with the data on analysed pancreatic Zn and pancreatic enzyme activities relative to dietary Zn supply. Reduction of dietary Zn supply reduced the analysed pancreatic Zn contents. The minimum was achieved already at 38·8 mg Zn/kg diet. Significant differences have been determined between groups receiving 38·8 and 42·7 mg Zn/kg diet compared with the highest supplied group (P=0·01). All other groups expressed no significant differences. Orthogonal contrasting revealed significantly lower pancreatic Zn contents in groups fed <58 mg Zn/kg diet (P<0·001). The activity of all investigated pancreatic exocrine enzymes numerically declined with fine-graded reduction in alimentary Zn supply. This relationship occurred to be significant for chymotrypsin and α-amylase as indicated by ANOVA (P=0·05 and P=0·01, respectively) and orthogonal contrasting (P<0·05 and P<0·01, respectively) as well as for elastase based on orthogonal contrasting only (P=0·05). However, the Student–Newman–Keuls test was not able to identify significant differences between treatment groups, except for α-amylase (significant difference between groups receiving 33·6 and 38·8 mg Zn/kg diet compared with the control group receiving 88·0 mg Zn/kg diet).
Table 2 Two-factorial ANOVA and orthogonal contrast of pancreatic enzyme activities relative to dietary zinc supply (Mean values and pooled standard errors of the linear model)

a,b Mean values with unlike superscript letters were significantly different (P≤0·05).
* Orthogonal contrast between groups of animals receiving <58 or ≥58 mg Zn/kg diet.
† Pancreatic enzyme activity is expressed as units of activity change per minute reaction time normalised to the total protein content within the sample; P values≤0·05 were considered as indicators of statistical significance.
Table 3 shows the results of broken-line regression analyses of analysed pancreatic Zn and pancreatic enzyme activities relative to dietary Zn supply. A highly significant break point in pancreatic Zn response was evident at 39 mg Zn/kg diet as indicated by significant parameter estimates for the X and Y intercept of the break point (P<0·0001, respectively). After a significant decrease of 0·41 mg pancreatic Zn/mg dietary Zn (P<0·0001) over doses ≥39 mg Zn/kg diet, the behaviour changed towards a replenishment of pancreatic Zn contents by 0·38 mg/mg dietary Zn at doses ≤39 mg Zn/kg diet. However, the latter slope in response missed the threshold of statistical significance. The suitability of the model to explain the response of pancreatic Zn to dietary Zn was indicated by a high R 2 of 0·92. All investigated pancreatic enzyme activities also exhibited highly significant break points in parameter response to variations in dietary Zn levels. This was evident by significant parameter estimates for the respective X and Y intercepts of the break points in response to varying dietary Zn supply (P=0·0001 and P<0·0001 for trypsin, P=0·01 and P<0·0001 for chymotrypsin, P<0·0001 and P<0·0001 for carboxypeptidase A, P<0·0001 and P<0·0001 for carboxypeptidase B, P=0·002 and P<0·0001 for elastase, P<0·0001 and P<0·0001 for α-amylase). Trypsin and α-amylase declined by 2·29 U/mg per min and 1·92 mU/mg per min per mg reduction in dietary Zn, respectively, until a break point in response of 58·0 mg Zn/kg diet. Below this threshold, the slopes in response decreased to 0·52 U/mg per min and 1·53 mU/mg per min with every mg further reduction in dietary Zn supply, respectively. All other assessed enzyme activities exhibited plateaus in response above the respective dietary thresholds of 58·0, 41·2, 47·5 and 57·7 mg Zn/kg diet for chymotrypsin, carboxypeptidases A and B as well as elastase, respectively. Below these thresholds, a reduction in enzyme activities occurred by 4·69 U/mg per min, 6·50 mU/mg per min, 0·04 U/mg per min and 1·97 U/mg per min per mg reduction in dietary Zn supply, respectively. In most cases, the estimated slopes in response over dietary Zn doses were significant (P=0·005 for b 2 of trypsin, P=0·008 for b 1 of carboxypeptidase A, P=0·006 for b 1 of carboxypeptidase B, P=0·05 and P=0·03 for b 1 and b 2 of α-amylase, respectively) except for b 1 of trypsin, chymotrypsin and elastase, respectively. Estimated broken-line models for trypsin, carboxypeptidases A and B, elastase as well as α-amylase were of high precision (R 2 0·90, 0·82, 0·63, 0·61, 0·77, respectively) apart from chymotrypsin (R 2 0·35).
Table 3 Broken-line regression analysis of analysed pancreatic zinc and pancreatic enzyme activity relative to dietary zinc supply (Parameter estimates with their standard errors)

R 2, coefficient of determination of the respective broken-line regression model; X B , X intercept of the respective break point in parameter response; Y B , Y intercept of the respective break point in response; b 1, slope of the respective broken-line regression curve over dietary Zn doses lesser than or equal to the respective break point in parameter response; b 2, slope of the respective broken-line regression curve over dietary Zn doses greater than or equal to the respective break point in parameter response.
* Pancreatic enzyme activity is expressed as units of activity change/min reaction time normalised to the total protein content within the sample; P values≤0·05 were considered as indicators of statistical significance.
Comparing exocrine enzyme activities between animals with the 50 % lowest pancreatic Zn contents and their counterparts with the 50 % highest pancreatic Zn contents indicated a decline in activity with reductions in tissue Zn (164 (SD 93) v. 190 (SD 68) U/mg per min for trypsin, 687 (SD 155) v. 719 (SD 157) U/mg per min for chymotrypsin, 204 (SD 78) v. 226 (SD 76) mU/mg per min for carboxypeptidase A, 28 (SD 7) v. 32 (SD 5) U/mg per min for carboxypeptidase B, 284 (SD 26) v. 324 (SD 50) U/mg per min for elastase and 147 (SD 56) v. 186 (SD 67) mU/mg per min for α-amylase) (data are not further shown in a table). However, these results were only significant for carboxypeptidase B and α-amylase (P=0·04 and P=0·03, respectively).
Effects of the dietary treatment on measures of digestive capacity
Table 4 presents the results of one-way ANOVA and orthogonal contrasting of apparent faecal digestibility coefficients relative to changes in dietary Zn supply.
Table 4 Two-factorial ANOVA and orthogonal contrast of apparent faecal DM and crude nutrient digestibility relative to dietary zinc supply (Mean values and standard errors of the linear model)

a,b,c Mean values with unlike superscript letters were significantly different (P≤0·05).
* Orthogonal contrast between groups of animals receiving <58 or ≥58 mg Zn/kg diet.
† Coefficients of apparent faecal digestibility are expressed as percentage of feed intake; P values≤0·05 were considered as indicators of statistical significance.
Except for CF (average percentage faecal digestibility: 66·9 (SD 1·64) %, CF data are not further shown in a table), all coefficients of apparent faecal digestibility exhibited a significant relationship with the analysed dietary Zn. This was characterised by a decline in response with fine-graded reduction in alimentary Zn supply. However, reductions were only significant for the lowest supplied group (28·1 mg Zn/kg diet) according to ANOVA and post hoc testing in all assessed digestibility coefficients except for CF (P<0·01, P=0·01, P<0·0001 and P<0·01 for DM, CP, TL and CA, respectively). Orthogonal contrasting revealed significantly lower faecal digestibility coefficients for DM, CP, TL and CA in the groups receiving <58 mg Zn/kg diet (P<0·001, P<0·01, P<0·001 and P<0·001, respectively). Again, CF digestibility was not affected in a significant manner.
Table 5 presents results on broken-line regression analysis of apparent faecal digestibility coefficients relative to dietary Zn supply. Except for CF digestibility (data not shown), all assessed parameters revealed significant thresholds in parameter response as indicated by highly significant parameter estimates of X and Y intercepts of the respective break points (P<0·0001 for X and Y intercepts of DM, CP, TL and CA, respectively). Above respective dietary break points of 54·7, 45·0, 46·9 and 58·2 mg Zn/kg diet, DM, CP, TL and CA digestibility coefficients exhibited a plateau in response to dietary Zn supply. Below these break points, significant reductions in digestibility by 0·07, 0·18, 0·54 and 0·12 %/mg reduction in dietary Zn were evident for DM, CP, TL and CA digestibility coefficients, respectively (P=0·003, P=0·006, P=0·001 and P=0·002, respectively). All broken-line models were of high precision as indicated by R 2 values of 0·88, 0·80, 0·90 and 0·74 for DM, CP, TL and CA, respectively. In the case of faecal CF digestibility, no significant regression model could have been estimated.
Table 5 Broken-line regression analysis of apparent faecal DM and crude nutrient digestibility relative to dietary zinc supply (Parameter estimates with their standard errors)
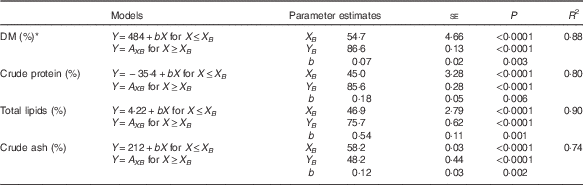
R 2, coefficient of determination of the respective broken-line regression model; X B , X intercept of the respective break point in parameter response; Y B , Y intercept of the respective break point in response; b, slope of the respective broken-line regression curve over dietary Zn doses lesser than or equal to the respective break point in parameter response.
* Coefficients of apparent faecal digestibility are expressed as percentage of feed intake; P values≤0·05 were considered as indicators of statistical significance.
Discussion
Methodological explications
At present, there is a lack of comprehensive data sets regarding the effects of subclinical Zn deficiency on the organism, as appropriate experimental approaches to induce this phenotype are missing. Therefore, an experimental model was recently developed to induce fine-graded differences in Zn supply status of weaned piglets, ranging from deficient states to mild oversupply (28·1–88 mg Zn/kg diet) under practical feeding conditions( Reference Brugger, Buffler and Windisch 2 ). The basis of this approach was the short experimental period of 8 d during which the organism was able to respond to the insufficient alimentary Zn supply without exceeding its capacities in terms of bone Zn mobilisation. This time frame has been chosen on the basis of earlier published data, which stated that the mammalian Zn homoeostasis needs between 3 and 5 d to adapt to changes in dietary Zn supply( Reference Windisch and Kirchgessner 17 , Reference Windisch and Kirchgessner 18 ). Otherwise, the time during which growing piglets under practical feeding conditions develop clinically manifest Zn deficiency is approximately 10–12 d from which on the first visible symptoms (e.g. reduced feed intake) are evident( Reference Schlegel and Windisch 19 – Reference Windisch 21 ). Previously published data obtained under the present experimental conditions clearly indicate fine-graded differences in the examined Zn supply status parameters (e.g. bone Zn, blood plasma Zn parameters, etc.) without promotion of Zn-deficiency symptoms( Reference Brugger, Buffler and Windisch 2 ). This was evident by complete absence of any changes in zootechnical performance data of all experimental groups( Reference Brugger, Buffler and Windisch 2 ). Moreover, the point of gross Zn requirement was recognised to lie at 58 mg Zn/kg diet as indicated by broken-line response analysis of, for example, apparent Zn digestion( Reference Brugger, Buffler and Windisch 2 ). This value corresponds to the gross Zn requirement threshold of growing pigs under practical feeding conditions as stated by the National Research Council (60 mg Zn/kg diet)( 9 ).
However, it might be questioned whether an experimental approach that used restrictive feeding is comparable with an ad libitum feeding situation. Obviously, too intense feed restrictions could cause a stronger depletion of body Zn over time, and hence might change measurements of the gross Zn requirement. However, under the present experimental conditions, the level of feed restriction was adapted on the basis of the average ad libitum feed intake of all animals through the last 3 d of the acclimatisation period (450 g diet/animal and day). Thereby, it was possible to adjust the amount of diet provided per animal and day very closely to the average amount consumed under non-restrictive conditions. The success of this approach was highlighted by the fact that all animals were able to fully exploit their potential in terms of growth development during the experimental phase. Another possible point of criticism refers to the fact that none of the administered Zn doses met the recommendations for piglets within the life weight range monitored in the present investigation (100 mg Zn/kg diet)( 9 ). In light of the considerably long acclimatisation phase of 2 weeks, one might argue that the control group (88 mg Zn/kg diet) was insufficiently supplied, and hence all the animals were challenged with alimentary Zn deficiency. However, it has to be clearly different between net/gross Zn requirements and feeding recommendations. The first represents the amounts of Zn that have to be present behind the gut barrier and within complete feed, respectively, to enable the animal maintenance of its developmental stage and biological performance (growth, lactation, reproduction, etc.). The latter is the net/gross Zn requirement extended by a safety margin, which, in case of weaned piglets, represents a surplus of approximately 67 % of the gross Zn requirement. This is a practical tool for pig feeders in order to establish a dietary Zn content that is always high enough to fulfill the animal’s Zn demand also in times of higher requirements (e.g. increased immune activity). The extent of the safety margins is a result of uncertainties regarding feed Zn bioavailability as data on the matter are scarce. Given the good biological performance of all animals throughout the whole study and the response of Zn status parameters (especially the broken-line response of apparently digested feed Zn), it leads to the conclusion that five groups received insufficient alimentary supply (28·1–47·5 mg Zn/kg diet), and hence expressed fine-graded differences in the development of subclinical Zn deficiency. In contrast, the groups receiving ≥58 mg Zn/kg diet were considered as sufficiently Zn supplied.
Therefore, it is now possible to investigate the early metabolic shifts before the development of clinically manifest Zn deficiency during which the organism is still capable of compensating deficiency symptoms by mobilisation of body Zn stores. In a first attempt, the early effects of varying alimentary Zn supply on the exocrine pancreas and related measures of digestive capacity have been studied.
Effects of the dietary treatment on exocrine pancreatic activity
Exocrine proteases are present within the pancreatic tissue as inactive forms (so-called zymogens) to prevent the tissue from uncontrolled self-digestion( Reference Klein 3 ). In order to assess the activities of these enzymes within a pancreatic tissue lysate, in-vitro activation of zymogens had to be conducted before the enzymatic assay. The activity of digestive enzymes (activated zymogens as well as α-amylase) within tissue homogenates should serve as an indicator of the amount of enzymes within the pancreas. In the present study, a decline in the respective enzyme activities within tissue homogenates was evident in response to changes in dietary Zn supply. These findings are in line with earlier published data( Reference Hove, Elvehjem and Hart 22 – Reference Perez-Jimenez, Bockman and Singh 27 ). Therefore, it can be proposed that one of the first pancreatic responses during the early states in the development of Zn deficiency is an increased degradation of exocrine enzymes and maybe enzyme-containing granules themselves in order to decline zymogen secretion to reduce endogenous Zn losses. It has yet to be evaluated whether this is accompanied by reduced transcription and/or translation of exocrine enzyme mRNA.
All assessed enzyme activities followed a broken line over dietary treatment groups, with significant break points in response between 41·2 and 58 mg Zn/kg diet, below which their activities declined in a linear fashion. This is in line with earlier findings under the present experimental conditions regarding apparent Zn digestion, liver Zn as well as hepatic metallothionein gene expression( Reference Brugger, Buffler and Windisch 2 ). On the basis of these data, the point of gross Zn requirement was estimated to lie at 58 mg Zn/kg diet. This corresponds precisely to the threshold of 60 mg Zn/kg diet published by the National Research Council( 9 ). Therefore, the assessed response patterns of enzyme activities indicate a relationship between the amount of pancreatic zymogens as well as α-amylase and the Zn supply status of the organism. In summary, a reduction in dietary Zn supply below the point of gross Zn requirement impaired pancreatic zymogen and α-amylase activity. Furthermore, this is to our knowledge the first report of a Zn-dependent regulation of pancreatic elastase activity.
Response of the analysed pancreatic zinc content and its potential role as a mediator of exocrine pancreatic activity
Regarding the response of exocrine enzyme activity over the range of dietary treatment groups, an involvement of Zn homoeostatic regulation would represent a possible explanation for the basic mode of action. Indeed, in the present study, a reduction in analysed dietary Zn supply caused a decline in the response of pancreatic Zn. Furthermore, based on the broken-line analysis, a replenishment of tissue Zn stores in groups fed dietary doses <39·9 mg Zn/kg diet was evident. Interestingly, comparable response patterns regarding the analysed Zn contents of other tissues (heart, muscle, lymph nodes, thymus) were observed within the same experiment (D Brugger and WM Windisch, unpublished results). Furthermore, cardiac stress metabolism was investigated, and a decrease in antioxidative capacity accompanied by an increase in stress-responsive pro-apoptotic gene expression became evident (D Brugger and WM Windisch, unpublished results). Therefore, replenishment of certain tissue Zn stores at the expense of body Zn stores in the lowest supplied groups might represent a compensation mechanism to counteract increased stress levels in order to maintain tissue integrity. Indeed, whether comparable adaptions with regard to pancreatic stress metabolism are evident has to be investigated in follow-up studies. In order to prove whether decreased pancreatic Zn contents had an effect on the above-discussed measures of exocrine pancreatic enzyme activity, potential differences in response of enzyme activity between samples in the range of 50 % higher and 50 % lower pancreatic Zn contents have been investigated. On the basis of these secondary statistical analyses, a decrease in pancreatic Zn load reduced pancreatic enzyme activities. However, considerable variation within the data set was evident by the fact that only a few enzyme responses to changes in pancreatic Zn turned out to be statistically significant (carboxypeptidase B, α-amylase). This might indicate that there was some interference with other regulative stimuli. It has been shown that the small intestine communicates with the exocrine pancreas via a cholecystokinin-dependent signalling in order to increase its synthesis and secretory activity in times of reduced digestive capacity – for example, in the presence of enzyme inhibitors within the GIT( Reference Bragado, Tashiro and Williams 28 ). Presumably, there is a conflict of interest between Zn homoeostasis-dependent suppression and cholecystokinin-dependent stimulation of exocrine pancreatic activity. These potential interconnections should be addressed in appropriate follow-up studies.
Effects of the dietary treatment and exocrine pancreatic activity on measures of digestive capacity
The response of coefficients of apparent faecal digestion of DM, CP, TL and CA followed a broken line over the whole range of analysed dietary Zn doses, which is in good context to the response of pancreatic exocrine enzyme activity. Again, clear break points in response ranging between 47 and 58 mg Zn/kg diet were evident. This further confirms clear differences in the response of animals fed sufficient amounts of dietary Zn (≥58 mg Zn/kg diet) and insufficiently supplemented diets (<58 mg Zn/kg diet). Moreover, these findings are in concordance with earlier published results, which suggest a decrease in apparent feed digestibility under the terms of clinically manifest Zn deficiency( Reference Pallauf and Kirchgessner 7 , Reference Roth, Schülein and Kirchgessner 8 ). The clear relationship with certain pancreatic enzyme activities suggests a loss in luminal catalytic activity as a major cause of increased faecal DM and crude nutrient losses under the terms of short-term subclinical Zn deficiency. The efficiency by which the feed matrix is digested within the intestinal lumen determines the amount of soluble and hence absorbable, nutrients at the gut barrier. Therefore, impairment of digestive capacity would foster a translocation of indigested feed components to lower intestinal segments. Indeed, concerning the TL digestibility, the activity of pancreatic lipase has not been assayed in the present study, as it was not possible to establish a suitable method in an appropriate amount of time at the laboratory responsible for these analyses. However, it has been shown that clinically manifest Zn deficiency can affect pancreatic lipase activity( Reference Koo and Turk 29 ). Therefore, in light of our TL digestibility data, it seems plausible that pancreatic lipase was affected by the treatment similar to all other assessed enzymes.
The slope in response of TL digestibility to varying dietary Zn supply was approximately 3-fold higher compared with CP digestibility. This might be interpreted as an effect of Zn metabolism being stronger in case of fat compared with protein. However, apparent faecal digestibility reflects digestion of dietary protein only in part as most of faecal CP is of microbial origin( Reference Fuller 30 ). A higher influx of fermentable substrates into the hindgut promotes microbial growth, and hence reduces apparent faecal digestibility of CP( Reference Mosenthin, Sauer and Henkel 31 ). Reduction in faecal coefficients of CP digestibility is therefore indicative of impairment of pre-caecal nutrient digestion in general, including dietary protein and carbohydrates. Therefore, quantifying the effects of Zn metabolism on digestive capacity requires additional measurements such as pancreatic lipase activity, bile secretion, and, most importantly, in vivo experiments addressing pre-caecal (=ileal) digestion.
The reductions in faecal digestibility coefficients, although statistically significant, are minor in terms of absolute numbers, and might question the biological significance of the data. However, it should not be forgotten that these data were evaluated in animals that were subclinically Zn deficient compared with animals with satisfied Zn demands. This means that we compared differences in Zn status and related metabolic response between healthy animals, as subclinical states are defined by a total absence of pathological symptoms. Therefore, we did not expect to see drastic differences in response of certain biological functions. The monitored shifts in digestive capacity represent early adaptations that contribute to the development of clinical deficiency on a mid-term scale. Indeed, based on earlier published data( Reference Schlegel and Windisch 19 – Reference Windisch 21 ), we would expect the assessed differences to increase in the course of Zn deficiency and to promote the development of digestive depression.
Clinically manifest Zn deficiency has been associated with anorexia and decreased growth. The first intention would be to speculate that feed refusal promotes the reduction in growth development of Zn-deficient animals. Potential connections between Zn homoeostasis and leptin signalling, vagus nerve stimulation as well as reduced ghrelin synthesis/secretion have been discussed as possible modes of action in order to explain the anorexia( Reference Kwun, Cho and Lomeda 32 – Reference Yin, Li and Li 34 ). However, some authors suggest the growth depression occurs earlier than the reduction in feed consumption( Reference Roth, Schülein and Kirchgessner 8 , Reference O´Dell and Reeves 35 ). This indicates both symptoms develop to some extent independent of each other in the time course of clinical Zn deficiency. In the present study, declines in digestive capacity were evident before the onset of Zn deficiency symptoms. A decrease in digestive efficiency may lead to an enrichment of indigested feed within the GIT in the course of Zn deficiency, thereby establishing a higher filling level and associated response of mechanoreceptors of the gut. This may be another plausible explanation for the repeatedly reported Zn deficiency-associated anorexia. However, as we neither assessed the filling level of the GIT in the present study nor the response of gastrointestinal mechanoreceptors, this hypothesis has to be proven in further studies.
Conclusion
Significant reductions in exocrine pancreatic enzyme activities under the terms of subclinical Zn deficiency have been recognised in weaned piglets. Remarkably, these shifts were evident after just 8 d of insufficient Zn supply under practical feeding conditions. These effects may be related to pancreatic Zn metabolism as analysed pancreatic Zn contents were also affected by the treatment, and the activities of carboxypeptidase B and α-amylase exhibited a significant reduction in animals with the 50 % lowest pancreatic Zn contents. Furthermore, the reduction in exocrine pancreatic enzyme activities was accompanied by a decrease in faecal digestibility coefficients of DM, CP, TL and CA, indicating a direct impairment of feed digestion under the terms of subclinical Zn deficiency. The practical consequence of the present study is that even short periods of insufficient alimentary Zn supply have to be urgently avoided in order to maintain digestive function.
Acknowledgements
The authors express their deepest gratitude to Katharina Weiß, MSc, Dipl. Ing. (FH), Michael Gertitschke and Andrea Reichlmeir for excellent technical assistance. Furthermore, the authors thank their colleagues from the DÖF for valuable discussions and advice.
The authors thank the Bayerische Arbeitsgemeinschaft Tierernährung for the generous support for this study.
Contributions of the authors are as follows: D. B. wrote the manuscript, contributed to the study design, supervised the chemical analyses, performed the statistical analyses and contributed to the interpretation of the results; W. M. W. was the principal investigator of the study, contributed to the study design and interpretation of the results as well as writing of the manuscript. All authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.








