Management Implications
The global reputation of Hydrilla verticillata (L. f.) Royle (hydrilla), an invasive submerged aquatic plant, is well established. Upon the discovery of Hydrilla verticillata ssp. lithuanica (northern hydrilla), a new subspecies in the United States, its distribution and abundance were quantified. It was found to cover more than 344 ha along a 113-km section of the Connecticut River, extending from Agawam, MA, to about 9 km north of the Long Island Sound, exhibiting high to very high levels of abundance. There was concern this subspecies would spread from the river to waterbodies throughout the Northeast. Here, we report the first documented spread from the Connecticut River to other waterbodies within the states of Connecticut and Massachusetts. Of the eight sites where H. verticillata observations were made, 75% (n = 6) were confirmed to be H. verticillata ssp. lithuanica and 25% (n = 2) to be H. verticillata ssp. peregrina. Except for one site, all six locations infested with this novel subspecies provide watercraft access through public or private boat ramps. Significantly, three of these sites are well regarded for their fishing opportunities and are frequented by fishing tournaments from the Connecticut River. To minimize the risk of H. verticillata ssp. lithuanica spread and establishment, agencies overseeing public boat ramps for aquatic invasive species should give priority to monitoring anglers and other watercraft with previous activity in the Connecticut River.
Introduction
The aquarium and ornamental trades have led to the introduction of numerous aquatic invasive species in the United States, including hydrilla [Hydrilla verticillata (L. f.) Royle] (Gettys and Enloe Reference Gettys and Enloe2016; June-Wells et al. Reference June-Wells, Vibrancy, Gibbons and Bugbee2012; Padilla and Williams Reference Padilla and Williams2004). Before 2016, two subspecies within clade B (Tippery et al. Reference Tippery, Bugbee and Stebbins2020) were present in the United States: southern hydrilla (Hydrilla verticillata ssp. verticillata) and wandering hydrilla (Hydrilla verticillata ssp. peregrina). Hydrilla verticillata ssp. verticillata was discovered in Crystal River, FL, and in a canal near Miami, FL, in the United States in the 1960s (Blackburn et al. Reference Blackburn, Weldon, Yeo and Taylor1969), and H. verticillata ssp. peregrina was discovered in Delaware, USA, in the 1980s (True-Meadows et al. Reference True-Meadows, Haug and Richardson2016; Steward et al. Reference Steward, Van, Carter and Pieterse1984; Tippery Reference Tippery2023). In Connecticut, the first report of H. verticillata was recorded in 1989 from a pond located in Mystic (Les et al. Reference Les, Mehroff, Cleland and Gabel1997). Initially, tests indicated that this population was H. verticillata ssp. verticillata, but subsequent examinations from the same pond confirmed that the plants were H. verticillata ssp. peregrina. (Madeira et al. Reference Madeira, Van, Steward and Schnell1997, Reference Madeira, Jacono and Van2000, Reference Madeira, Van and Center2004).
In 2016, scientists from the Connecticut Agricultural Experiment Station (CAES) Office of Aquatic Invasive Species (OAIS) discovered a third subspecies, northern hydrilla (Hydrilla verticillata ssp. lithuanica), in the Connecticut River in Glastonbury, CT (Tippery Reference Tippery2023; Tippery et al. Reference Tippery, Bugbee and Stebbins2020). Upon the initial recognition of this subspecies, it was determined that H. verticillata ssp. lithuanica was already widely distributed and prevalent throughout the river. From 2017 to 2019, CAES OAIS surveyed the Connecticut River to quantify the distribution and abundance of this new intruder. Survey data indicated that its presence extended across more than 344 ha, spanning from Agawam, MA, to approximately 9 km from the Long Island Sound, where it is believed to be restricted by a lack of salt tolerance (Steward and Van Reference Steward and Van1987). Hydrilla verticillata ssp. lithuanica was found in high abundances predominantly in shallow coves, shoals, shorelines, and adjoining tributaries at depths less than 3 m (Bugbee and Stebbins Reference Bugbee and Stebbins2021, Reference Bugbee and Stebbins2022).
While many biological attributes (reproductive biology, nutrient requirement, etc.) of this subspecies remain unknown, it exhibits phenological differences from the other two subspecies, most notably a lack of tubers, greater turion production, a greater number of leaves per whorl (5 to 11), and increased robustness (Tippery Reference Tippery2023). Nonetheless, based on morphology alone, distinguishing the three subspecies present in the United States from one another can still be challenging due to significant overlap in leaf length, leaf width, the ratio between leaf length and width, and the number of leaves per node (Tippery Reference Tippery2023).
Following establishment, H. verticillata quickly proliferates throughout the waterbody through the production of turions, tubers, drifting fragments, root crowns, and stoloniferous growth (Haller and Sutton Reference Haller and Sutton1975). Hydrilla verticillata infestations are challenging to manage and if left unchecked quickly lead to numerous ecological and economic concerns. Hydrilla verticillata ssp. verticillata forms dense canopies within the water column and, as a result, can hinder recreational activities such as boating, fishing, and swimming, potentially leading to diminished property values in the affected areas (Milon et al. Reference Milon, Yingling and Reynolds1986). Extensive populations of H. verticillata result in changes to the local ecology through the plant’s ability to outcompete and displace native species (Balciunas et al. Reference Balciunas, Grodowitz, Cofrancesco, Shearer, van Driesche, Lyon, Blossey, Hoddle and Reardon2002). Ecological alterations following invasion of H. verticillata occur through changes in water chemistry and intensified competition among plants for space and nutrient resources. Additionally, it serves as a host to a potentially harmful epiphytic cyanobacterium (Aetokthonos hydrillicola) (Wilde et al. Reference Wilde, Murphy, Hope, Habrun, Kempton, Birrenkott, Wiley, Bowerman and Lewitus2005, Reference Wilde, Johansen, Wilde, Jiang, Bartelme and Haynie2014). Aetokthonos hydrillicola produces a neurotoxin that has been implicated as a causal agent of avian vacuolar myelopathy, a fatal disease of waterfowl and bald eagles (Haliaeetus leucocephalus) (Wilde et al. Reference Wilde, Johansen, Wilde, Jiang, Bartelme and Haynie2014).
The discovery of H. verticillata ssp. lithuanica in the Connecticut River and the breadth of the infestation represent a significant ecological invasion event with potentially far-reaching implications. Hydrilla verticillata is considered one of the world’s worst aquatic weed species (Gettys and Enloe Reference Gettys and Enloe2016). In North America, the annual management cost is estimated to be more than $2,471 ha−1 (Langeland Reference Langeland1996). In Florida alone, the Florida Wildlife Commission spends an estimated $5 to $15 million yr−1 on H. verticillata management (Hiatt et al. Reference Hiatt, Serbesoff-King, Lieurance, Gordon and Flory2019; Weber et al. Reference Weber, Wainger, Harms and Nesslage2020).
Once H. verticillata ssp. lithuanica was discovered in the Connecticut River, there was great concern this subspecies would spread outside the Connecticut River and degrade the native plant communities of previously uninvaded waterbodies. In 2023, the CAES OAIS received reports (OAIS, personal communication) of H. verticillata outside the Connecticut River in close proximity to boat ramps. Each site was visited and searched to document the extent of the infestation, collect samples for genetic analysis, and inform stakeholders (i.e., lake associations and marinas). In addition to receiving reports of potential H. verticillata ssp. lithuanica infestations, the authors also discovered apparent populations through regular invasive aquatic plant surveys on waterbodies throughout the state.
Materials and Methods
By October 2023, six individual reports of H. verticillata infestations in previously uninvaded waterbodies were received by CAES OAIS (Table 1). Amos Lake, a previously unidentified location, was found to have an H. verticillata infestation following routine aquatic plant surveys conducted by CAES OAIS. Additionally, Congamond Lake was identified as another H. verticillata–infested site due to suspicions raised by angling activity originating from the Connecticut River. Following reports or sightings, sites were visited to estimate the population density and collect specimens. Specimens were mounted and sub subsampled for genetic determination. Herbarium mounts are housed at the CAES OAIS and can also be found online at https://portal.ct.gov/CAES/OAIS/Herbarium.
Table 1. Waterbody locations, including report and site visit dates, Hydrilla verticillata subspecies, distance from the Connecticut River to the site location, and the distance from the boat ramp to the infestation site.

At the University of Wisconsin–Whitewater, genomic DNA was extracted using a cetyl trimethylammonium bromide (CTAB) method (Doyle and Doyle Reference Doyle and Doyle1987), modified to use an initial buffer volume of 600 μl of pure chloroform (without isoamyl alcohol) at the extraction step and ethanol (instead of isopropanol) at the DNA precipitation step. Polymerase chain reaction (PCR) was conducted to amplify the internal transcribed spacer (ITS) region using the p5F and p4R primers (Baldwin Reference Baldwin1992; Cheng et al. Reference Cheng, Xu, Lei, Li, Zhang and Zhou2016) and a 55 C annealing temperature, with the Phire Hot Start II DNA polymerase (Thermo Fisher Scientific, Waltham, MA). PCR products were cleaned using the Exonuclease and FastAP alkaline phosphatase enzymes (Thermo Fisher Scientific). Sanger sequencing (Sanger et al. Reference Sanger, Nicklen and Coulson1977) was performed by Eurofins Genomics (Louisville, KY). DNA sequences were compared visually against previously published sequences for H. verticillata (Tippery et al. Reference Tippery, Bugbee and Stebbins2020) in the program Mesquite v. 3.81 (Maddison and Maddison Reference Maddison and Maddison2023).
Results and Discussion
Once a species is introduced to an area outside its native range, it may experience a sharp population expansion within its new environment (e.g., a waterbody). However, even in such cases, there is typically a temporal gap or lag phase between the time when the species was introduced to the new area and the subsequent secondary expansion into areas outside its original establishment zone. The specimens sequenced for this study were exact matches from previously published sequences for each of the respective subspecies. Of the eight H. verticillata infestation reports received by or discoveries made by CAES OAIS, 75% (n = 6) were confirmed to be H. verticillata ssp. lithuanica, and the remaining 25% (n = 2) to be H. verticillata ssp. peregrina. Before 2022, H. verticillata ssp. lithuanica had not been detected beyond the confines of the Connecticut River. However, by the end of 2023, there was a 6-fold increase in the number of locations where H. verticillata ssp. lithuanica infestations were observed compared with the preceding 7 yr, when this strain was initially identified in 2016 (Figure 1). The rate at which these discoveries are being made is cause for concern and is likely higher than the results presented here.
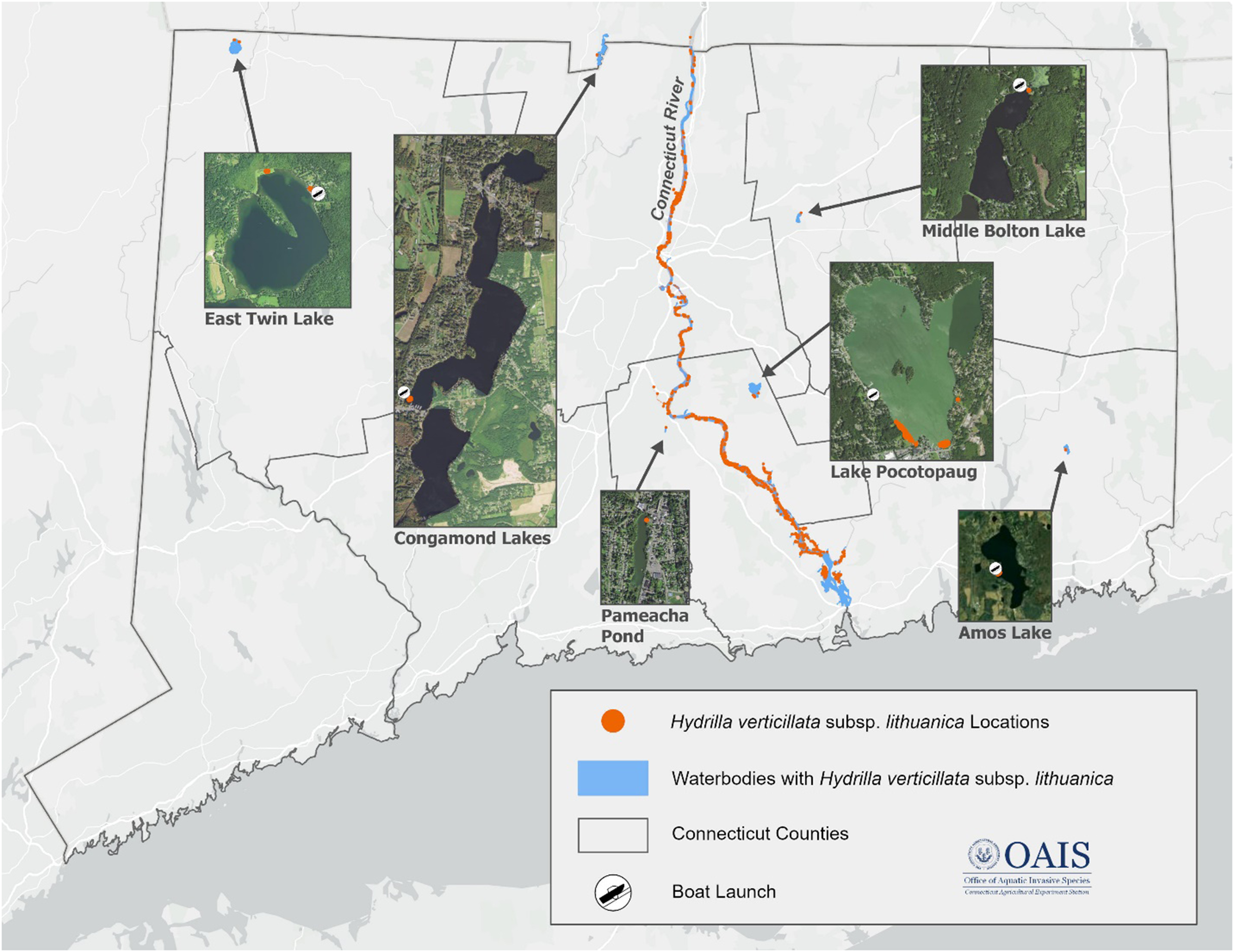
Figure 1. Distribution of Hydrilla verticillata ssp. lithuanica in the Connecticut River and the six new infestations in waterbodies within or bordering Connecticut, USA.
East Twin Lake
East Twin Lake was the first site reported to have H. verticillata. East Twin Lake is one of two lakes in the Twin Lakes area, in Salisbury, CT, located approximately 62 km from the Connecticut River, in the northwest corner of Connecticut (Figure 1). East Twin is one of the most managed and studied coldwater lakes in the state and spans 230 ha with a maximum depth of 25 m and a watershed covering 1,068 ha.
On June 27, 2023, Northeast Aquatic Research (NEAR), a private company specializing in aquatic plant surveys, identified populations of H. verticillata in the vicinity of the O’Hara’s Landing private boat ramp. The East Twin Lakes Association and CAES OAIS were promptly notified of this discovery. Three days later, CAES OAIS carried out a site visit and collected plant samples for herbarium mounts and genetic analysis. The analysis confirmed that the H. verticillata strain found in East Twin Lake belonged to clade C and was H. verticillata ssp. lithuanica. Subsequent visits to the lake were conducted by CAES OAIS and NEAR to document the extent of the infestation.
East Twin was the first waterbody discovered to have H. verticillata ssp. lithuanica outside the Connecticut River. Altogether, a minimum of 19 separate populations were located in the vicinity of the boat ramp, marina, and an area 0.7 km to the north of the boat ramp where they were mixed in with emergent and submerged vegetation (Figure 1). After obtaining the necessary state herbicide application permits, efforts were made to address the infestations in and around the marina by employing florpyrauxifen-benzyl (ProcellaCOR® SePRO, Carmel, IN 46032). The outcomes of this treatment are currently under assessment.
Amos Lake
Amos Lake, spanning 47 ha, is located in Preston, CT, and is positioned approximately 37 km east of the Connecticut River, in the central-southern region of eastern Connecticut. This lake has a maximum depth of roughly 14 m and an average depth of approximately 6 m and features a public boat ramp on its western shoreline. Notably, Amos Lake is a popular recreational destination, highly regarded as a prime location for fishing enthusiasts seeking trophy bass and trout.
During standard aquatic plant assessments conducted by CAES OAIS, H. verticillata was detected on August 14, 2023. Genetic analysis was conducted on sampled populations, confirming the presence of H. verticillata ssp. lithuanica. The H. verticillata ssp. lithuanica population was found approximately 7 m away from the public boat ramp and was intertwined with emergent (e.g., swamp loosestrife [Decodon verticillatus (L.) Elliott], pickerelweed [Pontederia cordata L.], white water lily [Nymphaea odorata Aiton], and yellow water lily [Nuphar variegata Engelm. ex Durand]) and submerged (e.g., coontail [Ceratophyllum demersum L.], western waterweed [Elodea nuttallii (Planch.) H. St. John], and purple bladderwort [Utricularia purpurea Walter] native plants.
Middle Bolton Lake
Middle Bolton Lake is in Vernon, CT, approximately 17 km east of the Connecticut River, and spans 49 ha. With a maximum depth of 6 m and an average depth of 3.7 m, this lake offers a state-managed public boat ramp, a town beach, and a camp for recreational activities.
On September 4, 2023, a report issued by NEAR identified a population of H. verticillata near the northern end of the lake, approximately 24 m from the public boat ramp (Figure 1). The CAES OAIS visited the location on September 8, 2023, and gathered samples for genetic analysis that confirmed the presence of H. verticillata ssp. lithuanica. The population was relatively small and limited in distribution, with only a few mature plants found. Efforts were made to remove the population through manual removal via hand pulling, and monitoring is ongoing.
Lake Pocotopaug
Lake Pocotopaug, located in East Hampton, CT, is approximately 6 km east of the Connecticut River, spans over 207 ha, and has an average depth of 3.4 m and a maximum depth of 7.3 m. The town-owned boat ramp is located on the western side of the lake. On September 4, 2023, CAES OAIS received a report and a sample of H. verticillata from the lake. Following genetic analysis, H. verticillata ssp. lithuanica was confirmed. On October 13, 2023, CAES OAIS carried out a field visit to record the presence and density of H. verticillata ssp. lithuanica in the southern area of this lake (Figure 1). The northern and eastern shorelines of the lake were not included in the survey. The infestation was identified approximately 400 m south of the town-owned boat ramp, covering an area of about 1 ha, representing the most significant known infestation outside the Connecticut River. Currently, we have no information regarding the extent, if any, of H. verticillata ssp. lithuanica infestations on the lake’s northern and eastern sides. The Lake Pocotopaug Project was informed of the infestation, and discussions surrounding the best management approach are currently ongoing.
Congamond Lakes
The Congamond Lakes are a natural lake system in Southwick, MA, with part of the shoreline following the Massachusetts–Connecticut border (Figure 1). Congamond Lakes consist of three connected basins: Northen, Middle, and Southern, totaling 188 ha. Within this lake system are two boat ramps, both in the middle basin. The Congamond Lakes have gained a strong reputation among anglers due to their renowned fish populations, which include regular trout stocking and the annual organization of fishing tournaments. Upon the suspicion of H. verticillata ssp. lithuanica presence, prompted by the schedule of angling tournaments that had previously taken place in the Connecticut River, a survey of the site was conducted on October 12, 2023. Hydrilla verticillata populations were found within 18 m of the southern boat ramp in the middle basin. Again, H. verticillata ssp. lithuanica was confirmed. Upon subspecies confirmation, the Massachusetts Department of Conservation and Recreation and related lake associations and authorities were notified. To date, the presence of H. verticillata ssp. lithuanica in the Congamond Lakes is the first and only known location in Massachusetts, outside the Connecticut River, to have this subspecies.
Pameacha Pond
Pameacha Pond is a relatively small lake (0.7 ha), 1.6 km west of the Connecticut River, in Middletown, CT. Pameacha Pond has limited recreational activity and does not have a public or private boat ramp. During pretreatment surveys of aquatic fauna by the Pond and Lake Connection on October 7, 2023, H. verticillata ssp. lithuanica was observed, sampled, and transported to CAES OAIS for genetic analysis. It was subsequently confirmed to be H. verticillata ssp. lithuanica. A follow-up site visit by CAES OAIS was also conducted on October 19, 2023, to determine the extent of the infestation. The H. verticillata population was limited in distribution and abundance and was located on the northeastern shore intermixed with dense populations of C. demersum.
The introduction of invasive plants and invertebrates into new aquatic environments is frequently linked to recreational activities such as boating (Johnson et al. Reference Johnson, Bossenbroek and Kraft2006; Johnstone et al. Reference Johnstone, Coffey and Howard-Williams1985; Rothlisberger et al. Reference Rothlisberger, Chadderton, McNulty and Lodge2010). Understanding the importance of these transmission pathways provides valuable insights into how regulatory agencies and volunteers can collaborate to mitigate the rate of expansion (Haight et al. Reference Haight, Kinsley, Kao, Yemshanov and Phelps2021). Notably, East Twin Lakes, Amos Lake, and Congamond Lakes serve as hosts for numerous annual angling tournaments, drawing participants from across the state. Of particular concern are tournaments that involve travel from infested waterbodies to noninfested ones.
Except for Pameacha Pond, all six H. verticillata ssp. lithuanica–infested sites offer watercraft access through public or private boat ramps. Given the variable distances of H. verticillata ssp. lithuanica infestation sites from the Connecticut River, the limited size of the infestations, and the distance from the boat access points to the locations of the infestations, avian dispersal seems unlikely. Consequently, the primary mechanism for the spread of H. verticillata ssp. lithuanica from the Connecticut River to these waterbodies appears to be inadequate adherence to decontamination protocols when transporting recreational equipment, especially watercraft (Mohit et al. Reference Mohit, Johnson and Arnott2021; Rothlisberger et al. Reference Rothlisberger, Chadderton, McNulty and Lodge2010).
Further research should focus on assessing the relative contribution of angling tournaments to vectoring this highly invasive species from one waterbody to another. Understanding and addressing these vectors are critical for developing effective strategies to prevent and manage the spread of H. verticillata ssp. lithuanica in these diverse aquatic ecosystems.
Acknowledgments
The authors would like to thank the East Twin Lakes Association, Amos Lake Association, Northeast Aquatic Research, the Pond and Lake Connection, and Benjamin Burpee for their assistance and technical help and for allowing us to use their properties. No conflicts of interest have been declared.





