Introduction
Multiple herbicide-resistant Palmer amaranth in corn, cotton, and soybean fields is difficult to control and can drastically reduce yields if not controlled (Culpepper et al. Reference Culpepper, Webster, Sosnoskie, York and Nandula2010; Fast et al. Reference Fast, Murdock, Farris, Willis and Murray2009; Forseth et al. Reference Forseth, Ehleringer, Werk and Cook1984; Klingaman and Oliver Reference Klingaman and Oliver1994; Massinga et al. Reference Massinga, Currie, Horak and Boyer2001; Ward et al. Reference Ward, Webster and Steckel2013). In the United States alone, Palmer amaranth has evolved resistance to herbicides from eight site-of-action (SOA) groups: inhibitors of 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS), acetolactate synthase (ALS), 4-hydroxyphenylpyruvate dioxygenase, photosystem II, protoporphyrinogen oxidase (PPO), synthetic auxins, very-long chain fatty acid, and microtubule assembly, and many of these accessions exhibit multiple resistance mechanisms (Heap Reference Heap2019). In Arkansas and surrounding states, Palmer amaranth accessions resistant to both EPSPS- and ALS-inhibiting herbicides are common (Burgos et al. Reference Burgos, Kuk and Talbert2001; Norsworthy et al. Reference Norsworthy, Griffith, Scott, Smith and Oliver2008), and this widespread infestation has caused a dynamic shift in weed control programs (Hoffner et al. Reference Hoffner, Jordan, Chandi, York, Dunphy and Everman2012; Neve et al. Reference Neve, Norsworthy, Smith and Zelaya2011). For example, after the spread of multiresistant Palmer amaranth in Georgia cotton fields, Sosnoskie and Culpepper (Reference Sosnoskie and Culpepper2014) reported a 10-fold increase in use of PPO-inhibiting herbicides, such as flumioxazin and fomesafen.
Consequently, the increased reliance on PPO inhibitors for Palmer amaranth control selected for PPO resistance. Fomesafen resistance in Palmer amaranth was initially identified in an accession collected in 2011 (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). Since then PPO-resistant Palmer amaranth accessions have been positively identified in 18 of the 29 agricultural counties in Arkansas and in three states (Arkansas, Tennessee, and Illinois) (Heap Reference Heap2019; Varanasi et al. Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018). The major resistant mechanisms are the ΔG210 (210) glycine amino acid deletion, and the Arg-128-Gly/Met (R128) amino acid substitution, which are both target-site mechanisms and confer broad-spectrum resistance to PPO-inhibiting herbicides (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; Salas et al. Reference Salas, Burgos, Rangani, Singh, Refatti, Piveta, Tranel, Mauromoustakos and Scott2017; Varanasi et al. Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018). These mechanisms are well known, with the 210 deletion also identified in waterhemp [Amaranthus tuberculatus (Moq.) J.D. Sauer] and the R128/R98 mutation present in common ragweed (Ambrosia artemisiifolia L.). Both mechanisms are widespread in Palmer amaranth in Arkansas and have even been found in the same locations together (Varanasi et al. Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018). In fact, Varanasi et al. (Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018) reported that of the 167 accessions screened in Arkansas in 2017, 28% harbored the R128 mutation and 49% harbored the 210 deletion.
PRE control of PPO-resistant Palmer amaranth can still be achieved in part with specific PPO-inhibiting herbicides. Umphres et al. (Reference Umphres, Steckel and Mueller2017) concluded that flumioxazin and sulfentrazone would still provide some residual control of PPO-resistant Palmer amaranth as seen previously for waterhemp (Wuerffel et al. Reference Wuerffel, Young, Tranel and Young2015). Even so, reliance on these two specific herbicides could lead to control failures and difficulty in achieving zero-tolerance weed control programs (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012; Norsworthy et al. Reference Norsworthy, Griffith, Griffin, Bagavathiannan and Gbur2014). Unlike residual PRE activity, effective control of PPO-resistant Palmer amaranth with PPO inhibitors POST is only achievable at much higher-than-labeled rates (Schwartz-Lazaro et al. Reference Schwartz-Lazaro, Norsworthy, Scott and Barber2017). Fomesafen, a commonly used POST herbicide in soybean, did not control Palmer amaranth progeny originating from Crittenden County, AR, when applied at a higher-than-labeled rate (420 g ha−1) (Schwartz-Lazaro et al. Reference Schwartz-Lazaro, Norsworthy, Scott and Barber2017).
The widespread distribution of EPSPS-, ALS-, and PPO-resistant Palmer amaranth has severely limited PRE and POST options for Palmer amaranth control in soybean, corn, and cotton. Therefore, PRE and POST fallow experiments were conducted to determine how to control multiresistant Palmer amaranth accessions harboring both the 210 and R128 PPO resistance mechanisms.
Materials and Methods
Field experiments were conducted in 2016 and 2017 near Crawfordsville (35.227029°N, 90.344904°W) and Marion, AR (35.209203°N, 90.187199°W) (Crittenden County), at on-farm sites on a Dundee silt loam soil (fine-silty, mixed, active, thermic Typic Endoaqualfs) and a Dubbs silt loam soil (fine-silty, mixed, active, thermic Typic Hapludalfs), respectively. The soil near Marion had a pH of 5.8 with 1.6% organic matter, and the soil near Crawfordsville had a pH of 5.3 with an organic matter content of 1.8%. Each site-year contained a PRE-only and a POST-only experiment to determine herbicide efficacy on multiresistant Palmer amaranth. All experiments were established into a crop-free environment. Herbicides labeled for use in soybean, cotton, and corn production were applied at the typical field-use rates listed in the herbicide index (Table 1). Visible control ratings and density reduction data were gathered in both experiments, each on a 0% to 100% scale relative to the nontreated control, with 0% being no control and 100% being complete weed mortality. Nontreated controls were included in each replication to assess relative control.
Table 1. Herbicides, rates, timing, and manufacturer details for PRE and POST experiments.
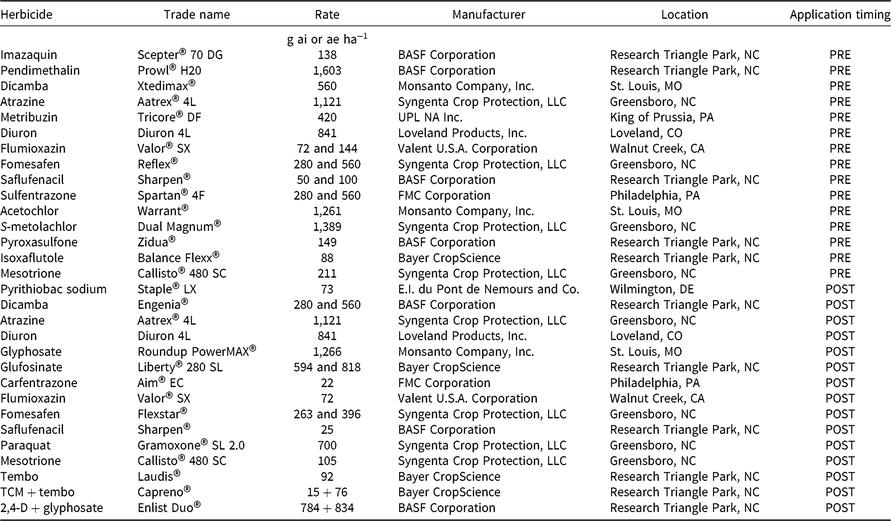
a Abbreviations: TCM, thiencarbazone-methyl; tembo, tembotrione.
PRE Trials
PRE trials were conducted in a randomized complete block design with four replications. Plots were 3.9-m wide by 7.6-m long on 97-cm-wide beds. Herbicide treatments were applied to weed-free ground that had been sprayed for winter annual weed control and tilled using standard production practices. Fifteen different herbicides were applied at standard in-crop labeled use rates (Tables 2 and 3). In addition, the four PPO-inhibiting herbicides were applied at two times the recommended rate (2X) to evaluate the effect of increased dose. A nontreated control was included for comparison. At Marion, herbicides were applied in spray solution at 112 L ha−1 with a Bowman MudmasterTM (Bowman Manufacturing, 2450 Jackson 36, Newport, AR, 72112) sprayer. At Crawfordsville, herbicides were applied in spray solution at 140 L ha−1 using a CO2-pressurized backpack sprayer. All treatments were applied with a four-nozzle boom equipped with 110015 TeeJet® air induction extended-range nozzles at an application speed of 4.8 km h−1. Density data were not available for Crawfordsville in 2016.
Table 2. PRE Palmer amaranth control and density reduction averaged over experiments in Crawfordsville and Marion, AR, in 2016 and 2017.
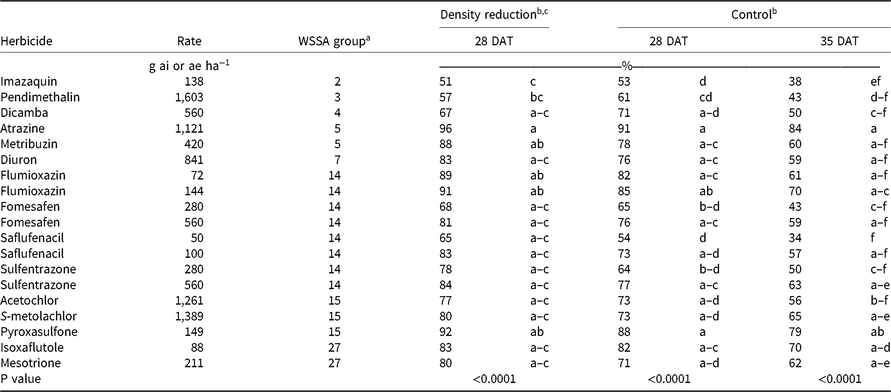
a Abbreviations: DAT, days after treatment; WSSA, Weed Science Society of America.
b Means within a column followed by the same lowercase letter are not different based on Tukey honestly significant difference test (P = 0.05).
c Density ratings were not available for Crawfordsville, AR, in 2016.
Table 3. Comparing sites-of-action, referenced by WSSA group number, for control of Palmer amaranth PRE in Crawfordsville and Marion, AR in 2016 and 2017.

a For contrasts, P < 0.05 was considered significant.
b Means for contrasts are shown in parentheses.
c Low is equivalent to the labeled rate of the respective Group 14 herbicides and the high is twice the labeled rate.
Because irrigation was not available at either of these sites, rainfall was the only source of water to incorporate herbicides into soil solution. Rainfall data for all experiments were recorded from in-field and local weather stations and are shown in Figures 1 and 2. At each location, a 2-cm or more rainfall event occurred within 1 to 2 weeks after herbicide treatments were applied in both years.
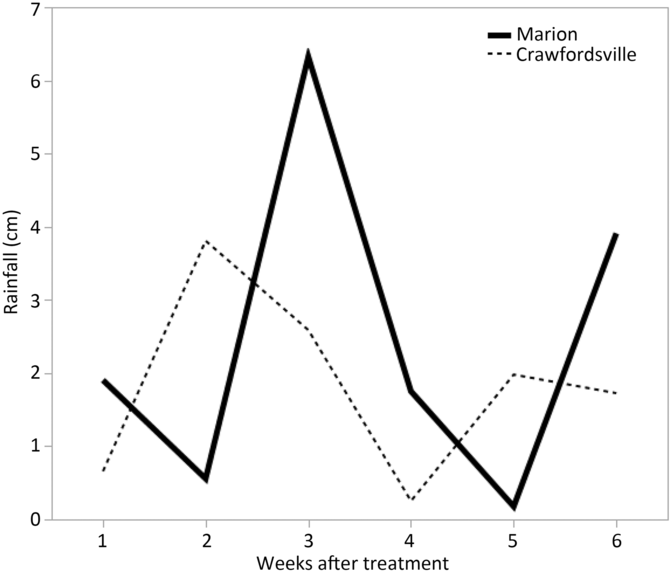
Figure 1. Rainfall data for Marion and Crawfordsville, AR, in 2016 each week after PRE treatment.
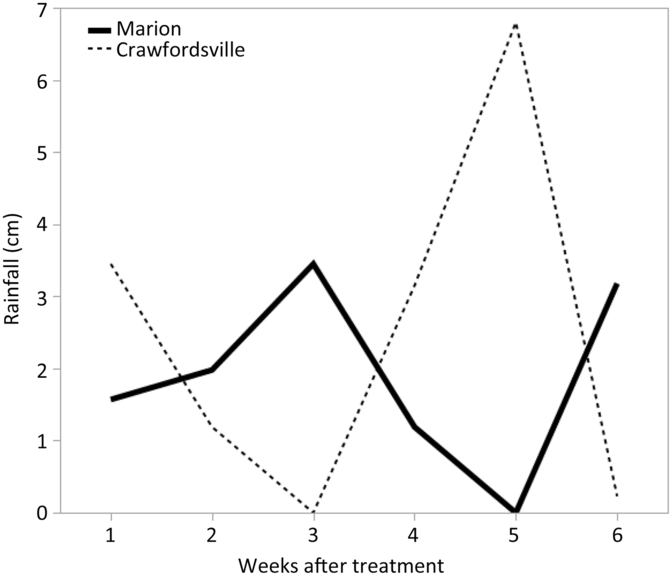
Figure 2. Rainfall data for Marion and Crawfordsville, AR, in 2017 each week after PRE treatment.
POST Trials
POST trials were conducted in a randomized complete block design with plots 2-m wide by 7.6-m long on flat, bed-absent ground. Trials at Marion contained three replications, whereas trials in Crawfordsville contained four replications each year. POST herbicide treatments were applied when Palmer amaranth reached 7 to 10 cm in height. S-metolachlor was applied at 1,064 g ha−1 across each trial at the time of POST application to prevent emerging Palmer amaranth from becoming a factor in density reduction and visible control ratings. All POST herbicides were applied at labeled crop use rates and included crop oil concentrate at 1% vol/vol, except for treatments containing glufosinate and glyphosate (Table 4). POST applications were made at 140 L ha−1 spray volume with a four-nozzle boom at 4.8 km h−1 attached to a pressurized backpack. Treatments containing dicamba or 2,4-D were made with 110015 turbo Teejet® induction nozzles; all other treatments were applied with 110015 TeeJet® air induction extended-range nozzles.
Table 4. POST Palmer amaranth control and density reduction averaged over experiments in Crawfordsville and Marion, AR, in 2016 and 2017.

a All treatments, excluding those containing glyphosate or glufosinate, were applied with a crop oil concentrate at 1% vol/vol.
b Herbicide Group 4 vs. Group 10 contrast (P < 0.05 was considered significant; means for contrasts are shown in parentheses): Control: 7 DAT, P < 0.0001 (58 vs. 76) 14 DAT P = 0.1195 (62 vs 53). Density reduction 14 DAT, P = 0.7795 (85 vs 90).
c All treatments, excluding those containing glyphosate or glufosinate, were applied with a crop oil concentrate at 1% vol/vol.
d Means within a column followed by the same lowercase letter are not different based on Tukey honestly significant difference test (P = 0.05).
e Crawfordsville, AR in 2017 and Marion, AR in 2016 density reduction data not available.
f Abbreviations: DAT, days after treatment; WSSA, Weed Science Society of America.
Data from both experiments were separately subjected to an ANOVA using JMP Genomics, version 8 (SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513). Data from both experiments were analyzed with a randomized site-year effect and randomized replications. Densities measured from each experiment were converted to a percentage of the nontreated control for the corresponding replication. Density data were not available in Marion in 2016 and Crawfordsville in 2017. Data from the nontreated control for each experiment were not included in analyses.
Before experimentation, there was particular interest in comparing the effects of application of the labeled rate (1X) and the 2X rate of PPO inhibitors and in determining the effectiveness of labeled PPO-inhibiting herbicides versus other SOAs. In addition, there was interest in comparing glufosinate, 2,4-D, and dicamba POST for potential efficacy differences when used for control of multiresistant Palmer amaranth. Thus, contrasts were used to test these specific interactions in both experiments. Data were separated using the Tukey honestly significant difference test at an α level value of 0.05.
Results and Discussion
PRE Trial
Timely and adequate rainfall occurred for incorporation of PRE herbicides into soil solution in both years at both locations. Precipitation was comparable across both years and locations in the first 2 weeks, averaging between 2.5 and 3 cm. Both locations also shared similar soil textures, field history, and management practices. The Marion and Crawfordsville locations were silt loam soil textures, previously in continuous soybean production, and were prepared by conventional tillage each year. The most notable difference in soil characteristics between the two locations was soil pH, but that only differed by 0.5 units. At both locations, PPO-resistant Palmer amaranth with target-site resistance mechanisms was confirmed previously (Varanasi et al. Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018).
Herbicide activity was evaluated at 28 and 35 DAT because POST herbicides typically need to be applied by 4 weeks after emergence of corn, cotton, or soybean to protect crop yields (Halford et al. Reference Halford, Hamill, Zhang and Doucet2001; Mulugeta and Boerboom Reference Mulugeta and Boerboom2000). Control of Palmer amaranth with the labeled rates of imazaquin, pendimethalin, fomesafen, saflufenacil, and sulfentrazone was less than 66% at 28 DAT and no more than 50% at 35 DAT (Table 2). Palmer amaranth has confirmed resistance to the SOAs of these herbicides at these locations (Heap Reference Heap2019; Varanasi et al. Reference Varanasi, Brabham, Norsworthy, Nie, Young, Houston, Barber and Scott2018). Interestingly, flumioxazin, another PPO-inhibiting herbicide, controlled Palmer amaranth 82% at 72 g ha−1 and 85% at 144 g ha−1. Of the 19 herbicide treatments evaluated, only atrazine at 1,120 g ha−1 (91%) and pyroxasulfone at 149 g ha−1 (88%) provided greater than 85% control at 28 DAT (Table 2). In crop systems without the presence of an effective POST option for multiresistant Palmer amaranth, such as conventional soybean, these PRE herbicide treatments would not be enough to ensure extended control alone. In this experiment, emphasis was placed on comparing the efficacy of multiple PRE PPO-inhibiting herbicides at a labeled rate versus a 2X rate and comparing the results with activity at other SOAs (Table 2).
Regardless of the variation in response between individual herbicides within a group, contrasts for control at 28 and 35 DAT identified separation in only three group pairings (Table 2). Contrasts indicated that the 2X rate of Group 14 herbicides was more effective than the 1X rate, based on density reduction and visible weed control ratings at 28 DAT and control at 35 DAT (Table 2). When comparing Palmer amaranth densities and control at 28 DAT and control at 35 DAT, Group 2, 3, and 4 herbicides were never more effective than Group 14 herbicides at a labeled rate, based on contrasts. Conversely, Group 5 and 7 herbicides, collectively along with Group 15 and Group 27 herbicides, were more effective in controlling multiresistant Palmer amaranth at 28 DAT than the labeled rate of Group 14 herbicides. The ineffectiveness of the Groups 2 and 3 SOAs is attributed to herbicide resistance within these populations, whereas the lack of effectiveness of dicamba (Group 4) is its low adsorption coefficient and the occurrence of rainfall soon after application in both years, likely moving the herbicide well below the depth that Palmer amaranth germination and emergence occurs (Figures 1 and 2).
POST Trial
Visible control ratings for Palmer amaranth 7- to 10-cm tall at application ranged from 18% to 91% at 7 DAT (Table 3). Control with paraquat (91%) was equal to control with diuron at 841 g ha−1 (80%) and both rates of glufosinate (70% and 82%, respectively). Conversely, control with glyphosate, pyrithiobac sodium, tembotrione, or carfentrazone did not exceed 32%. As expected, contrasts between auxin herbicides and glufosinate at 7 DAT indicated glufosinate provided greater initial control. More rapid control of Palmer amaranth with glufosinate than with dicamba and 2,4-D at 7 DAT was not surprising, because glufosinate is a contact herbicide and the other two are systemic (Coetzer and Al-Khatib Reference Coetzer and Al-Khatib2001; Grossman Reference Grossman2010).
At the 14 DAT timing, no treatment provided greater than 75% control (Table 3). Glyphosate and pyrithiobac sodium at 14 DAT provided less than 7% Palmer amaranth control. The lack of control from treatments evaluated infer that reliance on POST herbicides alone for control of these resistant Palmer amaranth accessions is not an acceptable weed control program. No single application of an herbicide, including dicamba, glufosinate, and 2,4-D, provided 85% control, demonstrating their lack of viability as stand-alone options. Density reduction data at 14 DAT followed trends similar to those of visible control data (Table 3). Contrast at 14 DAT between auxin herbicides and glufosinate revealed the options were comparable for controlling these Palmer amaranth accessions (P = 0.1195). Although efficacy of dicamba and 2,4-D at 14 DAT was evident, Palmer amaranth control with these herbicides would likely have increased with continued ratings at 21 and 28 DAT.
Practical Implications
Suppression of multiresistant Palmer amaranth with Group 5, 7, 15, and 27 herbicides is possible at 28 DAT, but not as stand-alone options. Although flumioxazin still provided good control of PPO-resistant Palmer amaranth accessions, applying flumioxazin alone could result in escapes and high selection pressure for additional PPO-inhibitor resistance. Because of lack of control of multiresistant Palmer amaranth with ALS- and PPO-inhibiting herbicides, earlier POST applications may be necessary to ensure that herbicides are applied in a timely manner to small weeds. Even when using the most effective PRE herbicide tested, it was not possible to maintain a high level of Palmer amaranth control through 35 DAT. For this reason, POST herbicides should be applied no later than 28 days after PRE application and residuals should be overlapped to provide season-long control (Aulakh and Jhala Reference Aulakh and Jhala2015; Halford et al. Reference Halford, Hamill, Zhang and Doucet2001). Overlapping effective residuals is key for Palmer amaranth control, demonstrated by the lack of control options in the POST experiment. ALS-, EPSPS-, and PPO-inhibiting herbicides should not be relied on POST in areas with confirmed or suspected Palmer amaranth resistance to these SOAs, because poor weed control will result. Even though S-metolachlor was applied PRE to all plots in the POST experiment, one application of any POST herbicide was not found to be efficacious in controlling these Palmer amaranth accessions. Based on these data, an optimum herbicide program will likely require multiple effective residuals at planting followed by two applications of an effective herbicide POST. In addition to using multiple SOA for weed control, it will also delay the onset of herbicide resistance. Control of multiresistant Palmer amaranth is not possible with ALS-, EPSPS-, or PPO-inhibiting herbicides alone.
Author ORCIDs
Michael M Houstonl, https://orcid.org/0000-0001-8968-9892
Acknowledgements
Funding for this research was provided by the Arkansas Soybean Promotion Board. No conflicts of interest have been declared.