Older people are frequently characterised by a high clinical complexity resulting from multiple chronic morbidities and mutually interacting syndromes. Furthermore, the ageing process exposes the individual to the onset of clinical conditions challenging to frame and define, such as fatigue.
Fatigue is a highly prevalent symptom in older people, responsible for the inability to properly function at the expected activity level. Despite its high prevalence and clinical impact, fatigue is often overlooked and underestimated, often considered an unavoidable result of ageing. In this context, the absence of a gold standard for its measurement and the subjectivity of the symptom hamper the routine assessment in the clinical setting. It is also noteworthy that the multiple operationalisations of fatigue existing in literature (i.e. tiredness, exhaustion, lassitude, anergia) also contribute to its poor understanding(Reference Zengarini, Ruggiero and Pérez-Zepeda1).
Fatigue is included in the diagnostic and statistical manual of mental disorders-fifth edition, where it is defined as ‘a state usually associated with a weakening or depletion of one's physical and/or mental resources, ranging from a general state of lethargy to a specific, work-induced burning sensation within one's muscles’(2). It is a complex symptom with multiple potential causal mechanisms. Among the many proposed in the literature, the most promising pathways at the basis of fatigue are probably those related to sleep disorders, autonomic nervous system abnormalities, frailty and malnutrition(Reference Zengarini, Ruggiero and Pérez-Zepeda1). Nevertheless, the biological substratum of fatigue remains unclear and difficult to disentangle. This gap of knowledge negatively affects the management of the symptom for which, for example, symptomatic interventions are currently missing.
The recent COVID-19 pandemic has further enhanced the need for research to advance in the field, given the high and long-lasting prevalence of fatigue among patients infected by the SARS-CoV-2(Reference Rudroff, Fietsam and Deters3,Reference Carfì, Bernabei and Landi4) . We have previously indicated nutrition as a critical factor underlying the manifestation of fatigue (Table 1). In the present review, we provide an overview of the role that nutrition may play as a determinant of fatigue in older people, also in the context of the COVID-19 pandemic.
Table 1. Potential determinants of fatigue in older people and in the context of COVID-19 pandemic
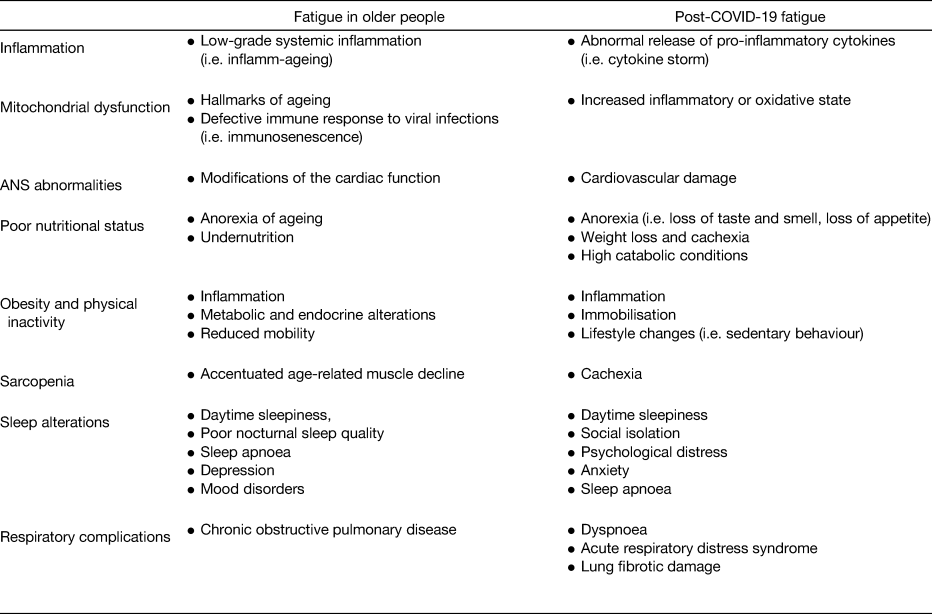
ANS, autonomic nervous system.
Adapted from Azzolino and Cesari(Reference Azzolino and Cesari87) under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives License (CC BY NC ND).
Nutrition as a major determinant of fatigue
Several age-related physiological, pathological and psycho-social changes (Table 2) have been indicated as responsible for the so-called anorexia of ageing (i.e. the loss of appetite and/or decreased food intake in late life(Reference Landi, Calvani and Tosato5)), a risk factor for malnutrition, sarcopenia, frailty, morbidity and mortality(Reference Cox, Morrison and Ibrahim6–Reference Pilgrim, Robinson and Sayer8). Interestingly, some determinants of the anorexia of ageing (i.e. inflammatory cytokines, sleep disorders, poor dentition, depression) are strongly associated with fatigue(Reference Zengarini, Ruggiero and Pérez-Zepeda1,Reference Avlund, Schultz-Larsen and Christiansen9,Reference Lacourt, Vichaya and Chiu10) . Indeed, poor nutritional status may represent a mediator (if not a causal factor) in the clinical expression of the symptom.
Table 2. Major changes occurring with ageing

Adapted from Azzolino et al.(Reference Azzolino, Arosio and Marzetti52) under the Creative Commons Attribution 4⋅0 International license (CC BY 4⋅0).
Fatigue can be envisioned as a disorder of energy balance, a sort of alert launched by the organism in the presence of limited and decreasing reserves. When energy and protein intakes become inadequate to meet the demands, catabolic pathways are activated, explaining (at least, in part) the onset of fatigue(Reference Amorim, Coppotelli and Rolo11). Interestingly, fatigue is one of the most common symptoms of hypothyroidism, which is characterised by metabolic derangements, myalgias and muscle weakness(Reference Chaker, Bianco and Jonklaas12).
We recently explored the association between malnutrition (assessed using the mini nutritional assessment short form) and the lack of energy in a population of 570 nursing home (NH) residents(Reference Azzolino, Marzetti and Proietti13). In both univariate and multivariate logistic regression analyses, the mini nutritional assessment short form score was inversely associated with the lack of energy. In particular, decreased food intake, reduced mobility and stressful conditions were strongly associated with anergia.
Inflammation and mitochondrial dysfunction
As mentioned earlier, malnutrition can influence fatigue via direct and indirect mechanisms, influencing critical biological pathways such as inflammation and mitochondrial dysfunction. Mitochondria represent the hub of energy production through oxidative phosphorylation, in which nutrients are processed to generate ATP(Reference Wesselink, Koekkoek and Grefte14). Ageing is characterised by a state of low-grade systemic inflammation (i.e. the so-called inflamm-ageing) and mitochondrial dysfunction(Reference López-Otín, Blasco and Partridge15). In particular, inflammation, along with other predisposing factors (e.g. inadequate intake of energy and proteins), could lead to mitochondrial dysfunction with the production of mitochondrial damage-associated molecular patterns(Reference Wesselink, Koekkoek and Grefte14,Reference Picca, Fanelli and Calvani16,Reference Marzetti, Calvani and Cesari17) . Consequently, there is an increased production of cytokines, chemokines, nitric oxide and reactive oxygen species, further promoting mitochondrial damage and establishing a vicious circle(Reference Picca, Fanelli and Calvani16) with the enhancement of wasting syndromes(Reference Marzetti, Calvani and Cesari17,Reference Cruz-Jentoft and Sayer18) .
The mitochondrial dysfunction caused by critical illness and physiological ageing increases lactate levels and decreases ATP production, leading to reduced stamina and onset of fatigue(Reference Lacourt, Vichaya and Chiu10,Reference Wesselink, Koekkoek and Grefte14,Reference Filler, Lyon and Bennett19) . The acidosis occurring at the skeletal muscle level resulting from the increased mitochondrial lactate production can manifest as muscular fatigue(Reference Avlund20). An inflammatory state and mitochondrial dysfunction are common in a variety of diseases characterised by the presence of fatigue, including type 2 diabetes, cardiovascular conditions, chronic fatigue syndrome or amyotrophic lateral sclerosis(Reference Amorim, Coppotelli and Rolo11,Reference Pieczenik and Neustadt21,Reference Ferrucci and Fabbri22) . Indeed, mitochondrial dysfunction may represent a marker of ageing and a consequence of the age-related deterioration of the organism(Reference López-Otín, Blasco and Partridge15).
Interestingly, fatigue has been suggested as a clinical manifestation of underlying abnormal ageing. A recent consensus statement proposed the so-called accelerated ageing and cellular decline condition to identify persons expressing accelerated/accentuated ageing with fatigue representing a dominant phenotypic characteristic(Reference Cesari, Cherubini and Guralnik23).
Sarcopenia, frailty and fatigue
Ageing is associated with a progressive loss of muscle mass and strength leading to a poor physical function, the so-called ‘sarcopenia’(Reference Cruz-Jentoft, Bahat and Bauer24). Conversely, frailty is defined as ‘a clinical state in which there is an increase in an individual's vulnerability for developing an increased dependency and/or mortality when exposed to a stressor’(Reference Morley, Vellas and van Kan25). Malnutrition, sarcopenia and frailty frequently show a remarkable overlap, especially in the physical domain(Reference Ligthart-Melis, Luiking and Kakourou26–Reference Gingrich, Volkert and Kiesswetter28). Of note, fatigue is included (more or less explicitly) in some instruments to screen for frailty(Reference Ensrud, Ewing and Taylor29–Reference Morley, Malmstrom and Miller31).
Fatigue is strongly associated with poor physical function(Reference Vestergaard, Nayfield and Patel32) which is recognised as a core component of both sarcopenia and frailty. In particular, according to the European working group of sarcopenia in older people, poor physical function (i.e. low gait speed) is used to determine sarcopenia severity(Reference Cruz-Jentoft, Bahat and Bauer24). Recently, Justine et al.(Reference Justine, Latir and Noor33) reported associations of fatigue severity (assessed with the fatigue severity scale) with the SARC-F questionnaire (acronym for the poor Strength, need of Assistance with walking, impaired Rising from a chair, difficulty at Climbing stairs and history of Falls), calf circumference, muscle strength and gait speed. Wyness et al.(Reference Wyness, Lemmon and Arvanitidou34) found an association of low gait speed with higher scores for physical fatigue, reduced activity and reduced motivation. They also reported that the presence of sarcopenia was related to reduced motivation.
The reduction of muscle strength, a key characteristic of sarcopenia, is another aspect that should be considered. In fact, after the fourth decade of life there is a progressive reduction in muscle strength of about 1⋅5 % per year(Reference Hughes, Frontera and Roubenoff35). The European working group of sarcopenia in older people, in the defining algorithm of sarcopenia(Reference Cruz-Jentoft, Bahat and Bauer24), has given priority to the assessment of muscle strength over the muscle mass quantification both to promote the concept of sarcopenia in clinical practice and for its strong predictive capacity for adverse clinical outcomes(Reference Cruz-Jentoft, Bahat and Bauer24,Reference Delmonico, Harris and Visser36) . Patino-Hernandez et al.(Reference Patino-Hernandez, David-Pardo and Borda37) found an association among low gait speed and handgrip strength (i.e. two out of three sarcopenia-defining variables) with fatigue. To date, muscle fatigue can be defined as ‘the inability of the muscle to produce or maintain force’(Reference Edwards38). In other words, the force developed by the muscle necessary to produce fatigue is the result of the maximum force that the skeletal muscle can develop. Accordingly, any factor reducing the maximum muscle force can result in fatigue and the consequent reduced muscle function(Reference Aubier39). As previously mentioned, the diagnostic and statistical manual of mental disorders-fifth edition defines fatigue (i.e. physical fatigue) as ‘a specific, work-induced burning sensation within one's muscles’ leading to the inability to continue functioning at the normal level of activity(2). It is therefore clear that skeletal muscle abnormalities play a major role in the manifestation of the symptom. It has been also suggested that fatigue without disability may represent an early stage of frailty(Reference Avlund, Schultz-Larsen and Christiansen9), representing a limitation to performance(Reference Ball and Maughan40) and impacting the capacity to conduct regular physical activities in older persons(Reference Gill, Desai and Gahbauer41). Therefore, fatigue may be envisioned as the clinical manifestation of the reduction of the homeostatic reserves of the older individual. In other words, given that poor physical function has been strongly associated with fatigue and is a core component of some instruments to early identify older people at risk for frailty and sarcopenia, the presence of fatigue may represent an early clinical indicator of the organism exhaustion.
Obesity and fatigue
Obesity has been consistently associated with increased levels of fatigue(Reference Resnick, Carter and Aloia42–Reference Theorell-Haglöw, Lindberg and Janson44). Also in this case, inflammation and mitochondrial dysfunction seem to play a relevant role(Reference Wesselink, Koekkoek and Grefte14,Reference Skuratovskaia, Komar and Vulf45–Reference Valentine, McAuley and Vieira47) . In fact, in those people with obesity, the presence of low-grade systemic inflammation as a consequence of the release of several pro-inflammatory mediators can result in insulin resistance and then mitochondrial dysfunction, with a disruption of energy production(Reference Skuratovskaia, Komar and Vulf45,Reference Vgontzas, Papanicolaou and Bixler46) . It is interesting to note that, with ageing, parallel to the muscle mass decline there is an increase in adiposity which can mask the presence of sarcopenia if nutritional status is assessed through anthropometric measures such as BMI(Reference Cruz-Jentoft and Sayer18,Reference Azzolino and Cesari48) . Furthermore, obesity is strictly associated with sleep disorders and vice-versa(Reference Beccuti and Pannain49). Sleep alterations are widespread among older people and are strongly interrelated with fatigue. In fact, according to the diagnostic and statistical manual of mental disorders-fifth edition of mental disorders, mental fatigue frequently manifests as somnolence (i.e. sleepiness)(2). Obesity and sleep disorders share some metabolic determinants (i.e. insulin resistance, decreased, glucose tolerance) as well as endocrine alterations (i.e. abnormal cortisol, leptin and ghrelin levels)(Reference Beccuti and Pannain49–Reference Azzolino, Arosio and Marzetti52). Also in this case, obesity-related inflammation may alter sleep parameters with the consequent manifestation of fatigue(Reference Vgontzas, Bixler and Chrousos53).
Nutrition, fatigue and COVID-19
Fatigue has been reported as the most complained and persistent symptom of the COVID-19 infection(Reference Rudroff, Fietsam and Deters3,Reference Carfì, Bernabei and Landi4) . Interestingly, some mechanisms potentially determining fatigue seem to be common to both older people and COVID-19 patients (Table 1). The COVID-19 infection is characterised by an increased inflammatory state (i.e. arriving up to the so-called ‘cytokine storm’ in the most severe cases)(Reference Bonnet, Cosme and Dupuis54). Indeed, COVID-19 infection results in a high catabolic response, predisposing to weight loss and muscle wasting, which may further explain the manifestation of fatigue(Reference Bauer and Morley55).
The COVID-19 infection is also characterised by loss of taste and smell (which can persist for several months) along with gastrointestinal alterations (i.e. nausea, vomiting, diarrhoea), enhancing the anorexia of ageing(Reference Morley, Kalantar-Zadeh and Anker56). At the same time, obesity has been evoked as a predisposing factor for COVID-19 infection and disease severity. Again, the mediating mechanisms might be indicated in inflammation and mitochondrial function abnormalities(Reference Azzolino and Cesari48,Reference Dietz and Santos-Burgoa57–Reference Sattar, McInnes and McMurray59) .
Finally, since muscle wasting and weakness occur as whole-body processes, they also involve muscles dedicated to breathing and swallowing, challenging their function(Reference Azzolino, Passarelli and D'Addona60). For example, the resulting weakness of respiratory muscles may contribute to the so-called ‘respiratory fatigue’(Reference Aubier39) characterised by a decrease in expulsive airway clearance tasks (i.e. coughing and sneezing), and increased risk of respiratory infections.
Nutritional strategies
Given that some determinants of fatigue are the same of anorexia of ageing and sarcopenia, nutritional strategies aimed at counteracting muscle loss could be beneficial against fatigue. In this context, it might be important to compensate the eventual deficits of energy and protein intake to meet the individual's specific needs. It is noteworthy that, according to the recommendations(Reference Deutz, Bauer and Barazzoni61,Reference Bauer, Biolo and Cederholm62) , older people need more protein to counteract muscle decline. In particular, the suggested amount of protein is 1–1⋅2 g/kg of body weight/day and up to 1⋅2–1⋅5 g/kg of body weight/day in the presence of acute or chronic illness(Reference Deutz, Bauer and Barazzoni61,Reference Bauer, Biolo and Cederholm62) . In relation to energy provision, it has been proposed a guiding value of about 125⋅52 kJ/kg (30 kcal/kg) of body weight/day(Reference Volkert, Beck and Cederholm63). In addition, the amount of energy intake has to be adjusted according to the person's nutritional status, physical activity and diseases(Reference Volkert, Beck and Cederholm63).
The ‘energy diet’, proposed by the National Health Service for sleep and tiredness, may represent an interesting strategy(64). Recommendations include eating at least five portions/day of fruit and vegetables and the consumption of starchy carbohydrates. The importance of iron-rich foods is also emphasised(64). Special attention is also paid to alcohol intake and food fortification (to increase energy and nutrient density).
It is important to note that no single food (including the so-called ‘superfoods’) can provide an energy boost. All the vitamins and minerals needed by the organism can be provided by eating a healthy, balanced diet. Some groups of people at risk of nutrient deficiencies might be advised to take a supplement. Fatigue is observed in some conditions characterised by micronutrient deficiencies(Reference Zengarini, Ruggiero and Pérez-Zepeda1), and some vitamins and minerals are pivotal for mitochondrial functioning(Reference Wesselink, Koekkoek and Grefte14). It has been hypothesised that antioxidant supplementation may reduce fatigue in animal models(Reference Lacourt, Vichaya and Chiu10), but the evidence in human subjects remains scarce(Reference Haß, Herpich and Norman65).
Most evidence for nutritional interventions against fatigue comes from the chronic fatigue syndrome. Several studies have demonstrated a potential benefit on fatigue from creatine supplementation, both stand-alone or in combination with exercise training(Reference Rawson and Venezia66). Creatine is mainly stored in muscles, where it is converted into phosphocreatine through the enzyme creatine kinase. The phosphocreatine acts as an energy shuttle, transferring a high-energy phosphate group to adenosine diphosphate to regenerate ATP during the muscle contraction(Reference Bemben and Lamont67,Reference Damanti, Azzolino and Roncaglione68) . β-hydroxy-β-methylbutyrate, a metabolite of leucine, has been also indicated as a promising agent. Several studies (mostly conducted in athletes) have reported that the β-hydroxy-β-methylbutyrate supplementation may promote muscle health and increase resistance to fatigue(Reference Holeček69). Beneficial effects of acetyl l-carnitine on mental and physical fatigue have been reported by Malaguarnera et al. (Reference Malaguarnera, Gargante and Cristaldi70). Low concentrations of vitamin D have also been associated with both mental and physical fatigue in older people(Reference Pennisi, Malaguarnera and Di Bartolo71).
Coenzyme Q plays an important role in the mitochondrial electron transport chain, and coenzyme Q10 has showed antioxidant and anti-inflammatory properties(Reference Abiri and Vafa72). NADH is also involved in the mitochondrial function(Reference Goody and Henry73) and regulation of inflammation(Reference Navarro, Gómez de Las Heras and Mittelbrunn74). Castro-Marrero et al. (Reference Castro-Marrero, Sáez-Francàs and Segundo75) have reported that NADH and coenzyme Q10 supplementations are able to reduce fatigue in patients with chronic fatigue syndrome.
Low concentrations of selenium have been associated with anxiety, depression and tiredness(Reference Benton and Cook76). Selenium may influence mitochondrial biogenesis(Reference Wesselink, Koekkoek and Grefte14) as well as the antioxidant defence, redox signalling and redox homeostasis(Reference Guillin, Vindry and Ohlmann77). With its antioxidant properties, selenium is involved in the dampening of the inflammatory responses by eliminating free radicals(Reference Fang, Yang and Wu78). Oral supplementation with coenzyme Q10 plus selenium has shown to improve fatigue through the modulation of oxidative stress and inflammatory status in chronic fatigue syndrome(Reference Castro-Marrero, Domingo and Cordobilla79). Other studies reported improved well-being and diminished fatigue in patients with autoimmune hypothyroidism from selenium supplementation(Reference Duntas, Mantzou and Koutras80–Reference Gärtner, Gasnier and Dietrich82). Some seleno-proteins (i.e. glutathione peroxidases, thioredoxin reductases) seem to be effective at controlling oxidative stress and inflammation(Reference Guillin, Vindry and Ohlmann77,Reference Hoffmann and Berry83,Reference Arthur, McKenzie and Beckett84) . Glutathione might also be of interest for reducing fatigue, given its immunomodulating capacity(Reference Lobo, Patil and Phatak85). n-3 PUFA have been indicated as potentially beneficial in inflammatory conditions (mainly for their effect on mammalian target of rapamycin and insulin resistance)(Reference Dupont, Dedeyne and Dalle86), and might be worth to be explored in the management of fatigue.
The fatigue in centenarians study
The fatigue in centenarians (FACET) pilot study has been established to preliminarily explore the complex mechanisms underlying fatigue in older persons. In particular, FACET focuses on three critical mechanisms (i.e. sleep patterns, nervous autonomic system, biological complexity) potentially at the basis of fatigue. It hypothesises possible interactions of fatigue with age and sleep, age and heart rate variability, age and biological complexity. In FACET, a total of thirty participants will be recruited. The sample will be composed of ten persons presenting extreme longevity (e.g. centenarians), ten direct offspring of them and ten apparently healthy older persons (matched with the offspring by age and sex). The main eligibility criteria applied in the recruitment of the three groups are: (1) inability/unwillingness to provide written informed consent, (2) clinician's perception that the participant may not adhere to the study protocol, (3) type 2 diabetes, (4) use of benzodiazepines and/or β-blockers. A comprehensive assessment of participants is conducted by trained personnel (Fig. 1). Analyses are planned to be conducted in the whole sample and separately in the three groups of participants. The interaction terms defining the three study groups will be tested in the associations of interest to capture possible differences and similarities across groups.

Fig. 1. Variables that will be collected in the FACET study. BIA, bioelectrical impedance analysis; CRP, C reactive protein; TNF-α, tumor necrosis factor α; IGF, insulin growth factor; BDNF, brain-derived neurotrophic factor. *Brief fatigue inventory(Reference Mendoza, Wang and Cleeland88), SF-36 vitality subscale(Reference Ware, Kosinski and Keller89), the multidimensional assessment of fatigue(Reference Belza, Henke and Yelin90), fatigue severity scale(Reference Krupp, LaRocca and Muir-Nash91).
FACET is a pilot experience to provide preliminary data to approach the fatigue symptom at the clinical and biological levels. It will be considered a successful experience if it will provide robust information for designing/optimising future research initiatives, particularly for the definition of targets of pharmacological and non-pharmacological interventions.
The FACET recruitment phase started on October 2021 and is expected to end in September 2022. The results will be released on spring 2023.
Conclusions
Fatigue is a complex and multidimensional symptom. Although older adults frequently report it, its underlying mechanisms are still incompletely understood. Consequently, fatigue is commonly considered an unavoidable result of ageing and remains often neglected in clinical practice. Nutritional status may play a critical role in explaining the fatigue manifestation. In particular, fatigue may represent the clinical expression of an abnormal ageing process caused or mediated by inadequate nutrition. Increased efforts should be made to better characterise fatigue at the biological and clinical level to support the development of specific interventions against such a burdening symptom.
Acknowledgements
The authors acknowledge the Scottish section of the Nutrition Society for inviting the present review paper as part of the Scottish Section Conference 2022: Nutrition, immune function and infectious disease.
Financial Support
The FACET project (principal investigator: Matteo Cesari) is funded by the Velux-Stiftung Foundation (Zurich, Switzerland).
Conflict of Interest
Matteo Cesari has served as consultant and member of scientific advisory boards for Nestlé Health Science. The other authors declare no conflict of interest.
Author Contributions
D. A. contributed to conceptualising and writing the manuscript. H. J. C. J., M. P., V. M. M. and M. C. edited and revised manuscript. All the authors approved the final version of manuscript.
Authorship
The authors had sole responsibility for all aspects of preparation of this paper.






