The loss of integrity over large areas of skin as a result of injury or illness may lead to major disability or even death. The primary goals of wound treatments are rapid wound closure and a functionally and aesthetically satisfactory scar(Reference Singer and Clark1). Wound healing is a complicated and highly regulated series of cellular and biochemical events involving input from, and the interplay between, many cell types. Reactive oxygen species are regulators of this process; they are required for protection against invading pathogens(Reference Singer and Clark1), and low levels of reactive oxygen species are also essential mediators of intracellular signalling(Reference D'Autreaux and Toledano2). The process of wound healing is divided into three phases: inflammation; granulation tissue formation; remodelling(Reference Broughton, Janis and Attinger3). Cytokine synthesis is necessary for wounds to heal; however, excessive amounts of inflammatory cytokines may disturb the process (as observed in chronic non-healing wounds such as ulcers)(Reference Cooney, Iocono and Maish4, Reference Trengove, Bielefeldt-Ohmann and Stacey5). Various products of white adipose tissue (WAT) are linked specifically to inflammation(Reference Tilg and Moschen6), suggesting the participation of these factors in the transition from acute to chronic wounds.
A prolonged healing process represents a complication that contributes to morbidity and mortality. Obesity is a condition in which adipose tissue excess is associated with chronic low-grade inflammation, indicated by leucocytosis(Reference Perfetto, Mancuso and Tarquini7), increased plasma levels of inflammatory cytokines(Reference Dandona, Weinstock and Thusu8, Reference Yudkin, Kumari and Humphries9) and increased abundance of macrophages in adipose tissue(Reference Zeyda and Stulnig10). Overweight and obesity are problems of epidemic proportions among humans and are associated with an increased incidence of many persistent conditions, such as chronic wounds(Reference Wilson and Clark11). Data from a previous study in our laboratory(Reference Nascimento and Costa12) showed that an overweight phenotype induced by a high-fat (HF) diet, without correlation with comorbidity or the metabolic syndrome, delayed the cutaneous wound healing process through elongation of the inflammatory phase.
The ideal model to study adipose tissue increase is the one that mimics the symptoms observed in human subjects; rodents fed a HF diet are an excellent model of obesity in which the dietary environment is a major contributor(Reference Bullo, Casas-Agustench and Amigo-Correig13). Human subjects and other mammals show considerable inter-individual variation in susceptibility to weight gain in response to obesogenic diets. Rats fed a HF diet exhibit a bimodal pattern in body-weight gain similar to that observed in human subjects. Approximately, half of the rats gain weight rapidly compared with standard chow-fed rats and develop diet-induced obesity (DIO), whereas the other half gain body weight at a rate similar to or lower than that of standard chow-fed rats and are thus characterised as diet-resistant (DR)(Reference Lauterio, Bond and Ulman14). This model has been of great interest because the genesis of obesity proneness and obesity resistance within the same rat strain without genetic or surgical modification is similar to that observed in human subjects and enables one to dissociate between the factors related to diet and obesity per se.
Although it is known that overweight induced by a HF diet delays cutaneous wound healing, the pattern of wound healing in individuals who eat an obesogenic diet but are resistant to developing obesity is not known. We hypothesise that the consumption of an obesogenic diet, without inducing overweight, can affect cutaneous wound healing; thus the aim of the present study was to evaluate the cutaneous wound healing of DIO-prone and DR rats 7 and 14 d after wounding.
Materials and methods
Experimental animals
The present study was approved by the Ethical Committee for Animal Use of the State University of Rio de Janeiro (CEA/160/2006). After weaning, male Wistar rats weighing between 30 and 60 g started diet protocols with free access to food and water throughout the experimental period. Animals were kept in a room with controlled temperature (22°C) on a 12 h light–12 h dark cycle. The rats were divided into three groups of at least seven animals each and were studied at two different time points (fifty-seven rats began the experiment; fifty rats were included in the study and seven rats were excluded from the study as explained later).
Diet protocol
The rats were randomly divided into control (n 17) and diet (n 40) groups. The control group was fed with commercial pellets (23 % protein, 71 % carbohydrate and 6 % fat (percentage of dietary weight), 18 kJ/g; Nuvilab®; Nuvital, Colombo, PR, Brazil), and the diet group was fed with a high-saturated fat diet known to induce obesity in susceptible animals (15 % protein, 55 % carbohydrate, 30 % fat (percentage of dietary weight), 23 kJ/g, about 45 % of energy intake as fat)(Reference Nascimento and Costa12, Reference Aoyama, Fukui and Takamatsu15). The source of fat was shortening, a semi-solid fat composed of hydrogenated soyabean oil that contains 30 % trans-fatty acids (Primor®; Bunge Alimentos S. A., Florianópolis, SC, Brazil). Food intake was measured daily, and body weight was recorded weekly. Rats on the obesogenic diet were separated on the basis of body-weight gain at the end of week 20; rats with a high weight gain were designated as DIO rats (n 17, body-weight gain ≥ 350 g), and rats with a low weight gain were designated as DR rats (n 16, body-weight gain ≤ 300 g). The remaining rats (n 7) were excluded from the study because they were between the two limits of weight gain (Fig. 1). The DIO and DR rats remained on the obesogenic diet until the end of the experiment.
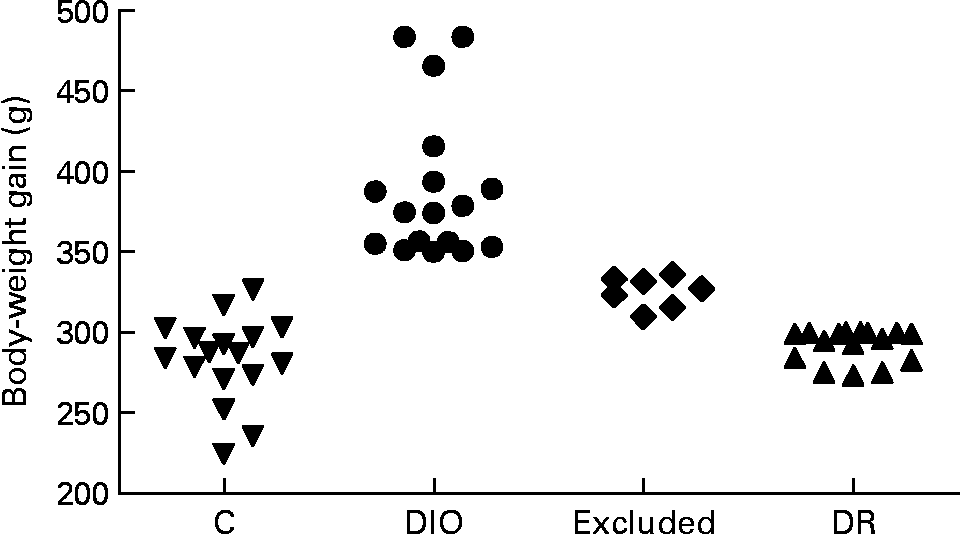
Fig. 1 Distribution of body-weight gain of all animals at the end of 20 weeks of ad libitum access to food. The control (C), diet-induced obese (DIO) and diet-resistant (DR) groups were composed of seventeen, seventeen and sixteen animals, respectively, and seven animals were excluded from the experiment.
Wounding model and macroscopic analyses
After 20 weeks of the diet (day 0), the rats were intraperitoneally anaesthetised with ketamine (5 mg/kg) and xylazine (2 mg/kg). After shaving the dorsum, a full-thickness excision wound (1 cm2) was made to the level of the panniculus carnosus. The wound was not sutured or covered and healed by second intention. The discomfort induced by this wound protocol was minimal, so no analgesics were required. To evaluate wound contraction and re-epithelialisation, a transparent plastic sheet was placed over each wound and its margins were traced(Reference Nascimento and Costa12, Reference Amadeu, Seabra and de Oliveira16). The wound area was measured and photographed soon after wounding, and weekly thereafter until euthanasia, without scab removal. Re-epithelialisation was measured 7 and 14 d after wounding. After digitisation, the wound area was measured using KS400 image software (Zeiss-Vision, Oberkochen, Germany). Data are expressed as a percentage of the initial wound area and as a percentage of the re-epithelialised wound area.
Oral glucose tolerance test
At the end of 20 weeks of diet, following a 12–14 h fast but before wounding, glucose concentration from the tail tip was determined (time 0) in six randomly selected animals per group, and then each rat received an oral administration of glucose (2 g/kg). Blood samples were collected from the tail tip at 15, 30, 60 and 120 min after the glucose challenge(Reference Anwer, Sharma and Pillai17). Glucose concentration in the blood was assessed using a reagent strip (Accu-Chek Advantage; Roche Diagnostics, Mannheim, Germany). Results of the oral glucose tolerance test are expressed as integrated areas under the curves (AUC) over 120 min for glucose calculated using the trapezoid rule(Reference Bhatt and Makwana18):
where C 1 and C 2 are blood glucose at times 0 (t 1) and 15 min (t 2), respectively, after the glucose load.
Tissue harvesting and microscopic analyses
Rats were killed by CO2 exposure 7 d (control, n 7; DIO, n 8; DR, n 7) or 14 d (control, n 10; DIO, n 9; DR, n 9) after wounding. A fragment of the lesion and adjacent normal skin was formalin-fixed and paraffin-embedded, and another fragment of the lesion was frozen ( − 70°C). WAT from the retroperitoneal pad was carefully removed, weighed and frozen ( − 70°C).
Paraffin sections were stained with haematoxylin–eosin to quantify the number of polymorphonuclear (PMN) leucocytes in the granulation tissue; ten random fields (0·02 mm2) per animal were analysed using a 100 × objective (Zeiss Primo Star; Zeiss-Vision). Sections were also stained with toluidine blue to evaluate the number of mast cells; between six and ten random fields per animal (0·15 mm2) were analysed in the deep region of the granulation tissue using a 40 × objective (Zeiss Primo Star; Zeiss-Vision). Results are expressed as cells/mm2, and the quantifications were performed blindly and repeated without significant differences between them.
Additionally, sections were stained with Sirius red and observed under polarisation to evaluate the organisation of collagen fibres. Thick collagen fibres, characteristic of tissues with high densities of collagen, appeared strongly birefringent and yellow to red in colour, whereas thin collagen fibres, indicative of tissues with a low density of collagen fibres, were weakly birefringent and greenish.
Immunohistochemistry and quantifications
For the quantification of CD68-positive macrophages, 4-hydroxynonenal-positive cells (4-HNE), myofibroblasts and blood vessels, sections were incubated with mouse anti-CD68 (1:500; Serotec, Inc., Raleigh, NC, USA), goat anti-4-HNE (1:4000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and mouse anti-α-smooth muscle actin (1:1000; DAKO, Carpinteria, CA, USA), respectively. For antigen retrieval, the sections were digested with 0·1 % trypsin (Difco Laboratories, Detroit, MI, USA) before labelling. Subsequently, the sections were incubated with 3 % H2O2 in methanol to inhibit endogenous peroxidase activity and then incubated with the primary antibody. After washing, the primary antibodies anti-CD68 and anti-α-smooth muscle actin were detected using the EnVision system® (DAKO), and anti-4-HNE was detected using a biotinylated donkey anti-goat IgG antibody (Santa Cruz Biotechnology, Inc.) and streptavidin–horseradish peroxidase (DAKO). Diaminobenzidine was used as a chromogen. The sections were counterstained with haematoxylin. The negative controls on sections, where the primary antibodies were omitted, exhibited no labelling.
To quantify CD68-positive macrophages, fifteen random fields (3636 μm2) per animal were analysed in the granulation tissue using a 40 × objective. For this, a video microscope system (Axiostar Plus Zeiss microscope (Zeiss, Oberkochen, Germany), Pixelink video camera (PixeLINK, Ottawa, Canada) and Intelbras monitor (Intelbras, Santa Catarina, Brazil)) was used. Data are presented as the average of CD68-positive macrophages/mm2. To quantify the number of 4-HNE-positive cells, as 4-HNE is a major product of endogenous lipid peroxidation(Reference Esterbauer, Schaur and Zollner19), fifteen random fields (0·02 mm2) per animal were analysed using a 100 × objective (Zeiss Primo Star; Zeiss-Vision). Results are expressed as cells/mm2.
Because stereological methods are precise tools that allow for the acquisition of information about three-dimensional structures based on observations made in two-dimensional sections(Reference Baddeley, Gundersen and Cruz-Orive20, Reference Gundersen, Bendtsen and Korbo21), the quantitative distribution of myofibroblasts and blood vessels (expressing α-smooth muscle actin) was evaluated using this method (point counting)(Reference Nascimento and Costa12). Vessels were identified by the presence of blood cells within the lumens or positive staining for α-smooth muscle actin in the wall (to confirm the presence of smooth muscle cells or pericytes)(Reference Nascimento and Costa12). For this quantification, eight random fields per animal were analysed using a 40 × objective for myofibroblasts, and five random fields per animal were analysed in both the superficial and deep regions of granulation tissue using a 20 × objective for blood vessels. For analyses, a video microscope system (Axiostar Plus Zeiss microscope, Pixelink video camera and Intelbras monitor) was used. Data are expressed as volume density (Vv) of myofibroblasts (Vv(myofibroblasts) %) and blood vessels (Vv(blood vessels) %) in the superficial and deep regions.
All quantifications were performed blindly and were reproduced without significant differences.
Biochemical analyses
Collagen deposition was quantified by the hydroxyproline assay in frozen lesions(Reference Souza, Santos and Costa22). Dry and defatted tissue (3·5–11·0 mg) was hydrolysed in 6 m-HCl for 18 h at 118°C. Hydrolysate was then diluted with distilled water, neutralised with 6 m-NaOH and centrifuged at 3000 rpm for 15 min. Hydroxyproline levels were measured in this hydrolysate as described previously(Reference Woessner23). Briefly, aliquots of hydrolysate (80 μl) were mixed with 40 μl chloramine-T (0·05 m; Merck & Company, Inc., Whitehouse Station, NJ, USA) and incubated for 20 min at 25°C. Then, 40 μl perchloric acid (3·17 m; Vetec, Rio de Janeiro, Brazil) and 40 μl 4-dimethylamino benzaldehyde (Merck & Company, Inc.) were added and incubated for 20 min at 60°C. The developed colour was read spectrophotometrically at 550 nm. Hydroxyproline concentrations were determined from a standard curve generated by different concentrations of l-4-hydroxyproline (Sigma-Aldrich, St Louis, MO, USA). Data are expressed as ng hydroxyproline/mg tissue.
Levels of nitrite in wound lysates were determined by a spectrophotometric method based on the Griess reaction(Reference Green, Wagner and Glogowski24). The detection limit for nitrite using this method is 1·0–5·0 μm. An aliquot of each sample (100 μl) was mixed and incubated with 100 μl of Griess reagent (1 % sulphanilamide in 5 % phosphoric acid and 0·1 % naphthalene diamine dihydrochloride in water) at room temperature for 10 min. The developed colour was then read spectrophotometrically at 550 nm. Nitrite concentrations in the samples were determined from a standard curve generated by different concentrations of sodium nitrite (0·1–100 μm). Data are expressed as μmol nitrite/μg total protein.
All analyses were performed in triplicate and were reproduced without significant differences.
ELISA
Frozen retroperitoneal fat tissue fragments (0·5 g) were homogenised in 1 ml of PBS solution containing 0·5 % Tween 20 and protease inhibitors. Retroperitoneal fat samples were subjected to ELISA analyses for TNF-α (PeproTech, Rocky Hill, NJ, USA) according to the manufacturer's instructions. The TNF-α ELISA development kit contains key components required for the quantitative measurement of TNF-α within the range of 32–2000 pg/ml. The samples were quantified using a standard curve (0–2 ng/ml). Data are expressed as pg of TNF-α/g of retroperitoneal fat tissue.
Statistical analyses
Data were analysed using a one-way ANOVA with Bonferroni's post-test (body mass, retroperitoneal fat, wound contraction, re-epithelialisation and blood glucose levels), unpaired t test (nitrite and hydroxyproline levels, and ELISA) or Mann–Whitney test for data that were not normally distributed (inflammatory cell and 4-HNE-positive cell quantifications and stereological analyses) using the software GraphPad InStat version 3.01 (GraphPad Software, Inc., San Diego, CA, USA). Values of P < 0·05 were considered statistically significant. The results are shown as means with their standard errors.
Results
Body weight and retroperitoneal fat-pad weight
The initial mean body weight of the control, DIO and DR groups did not differ (Table 1). At week 6, the DIO group (219·4 (sem 6·3) g) presented a higher average body weight than the DR group (188·5 (sem 6·6) g; P < 0·01, data not shown), and the body weight remained higher throughout the experimental period (Table 1). At week 11, the body weight of the DIO group (347·5 (sem 14·7) g) was greater than that of the control group (306·5 (sem 3·7) g; P < 0·01, data not shown) and remained higher until the end of the experiment (Table 1). On the day the lesion was performed (week 20, day 0), the body weight was 28·8 and 24·6 % higher in the DIO group compared with the control and DR groups, respectively. The DIO group consumed more food than the DR group; however, there was no difference in the food intake between the control and obesogenic diet (DIO and DR) groups (Table 1). Furthermore, the DIO group consumed significantly more energy per day than the control and DR groups throughout the study (Table 1), and the DR group consumed significantly more energy per day than the control group (Table 1).
Table 1 Body weight (BW), daily food intake, retroperitoneal fat pad, wound contraction and re-epithelialisation in the control (C), diet-induced obesity (DIO) and diet-resistant (DR) groups*
(Mean values with their standard errors of at least seven animals per group)

Day 7, 7 d after wounding; day 14, 14 d after wounding.
a,b,c Mean values within a row with unlike superscript letters were significantly different (one-way ANOVA with Bonferroni's post-test; P < 0·05).
* Body weight, daily food intake, retroperitoneal fat pad (C = 17, DIO = 17, DR = 16); wound contraction and re-epithelialisation after day 7 (C = 7, DIO = 8, DR = 7); wound contraction and re-epithelialisation after day 14 (C = 10, DIO = 9, DR = 9).
As shown in Table 1, retroperitoneal fat mass was higher in the DIO group than in the control and DR groups (518·2 and 91·5 %, respectively, P < 0·05). The retroperitoneal fat mass was higher in the DR group than in the control group (222·7 %, P < 0·05). The retroperitoneal fat percentage showed the same pattern of results (Table 1).
Fasting glucose and oral glucose tolerance test
There was no difference between the average fasting blood glucose of the groups (Fig. 2(A)). The blood glucose level of the DR group was lower than the glucose levels of the control and DIO groups (P < 0·05; Fig. 2(A)) 15 min after the oral glucose tolerance test. After the glucose load, blood glucose levels over a period of 120 min in the DIO group were higher than in the control and DR groups. The glucose area under curve was significantly higher in the DIO group compared with the control and DR groups (P < 0·05; Fig. 2(B)).
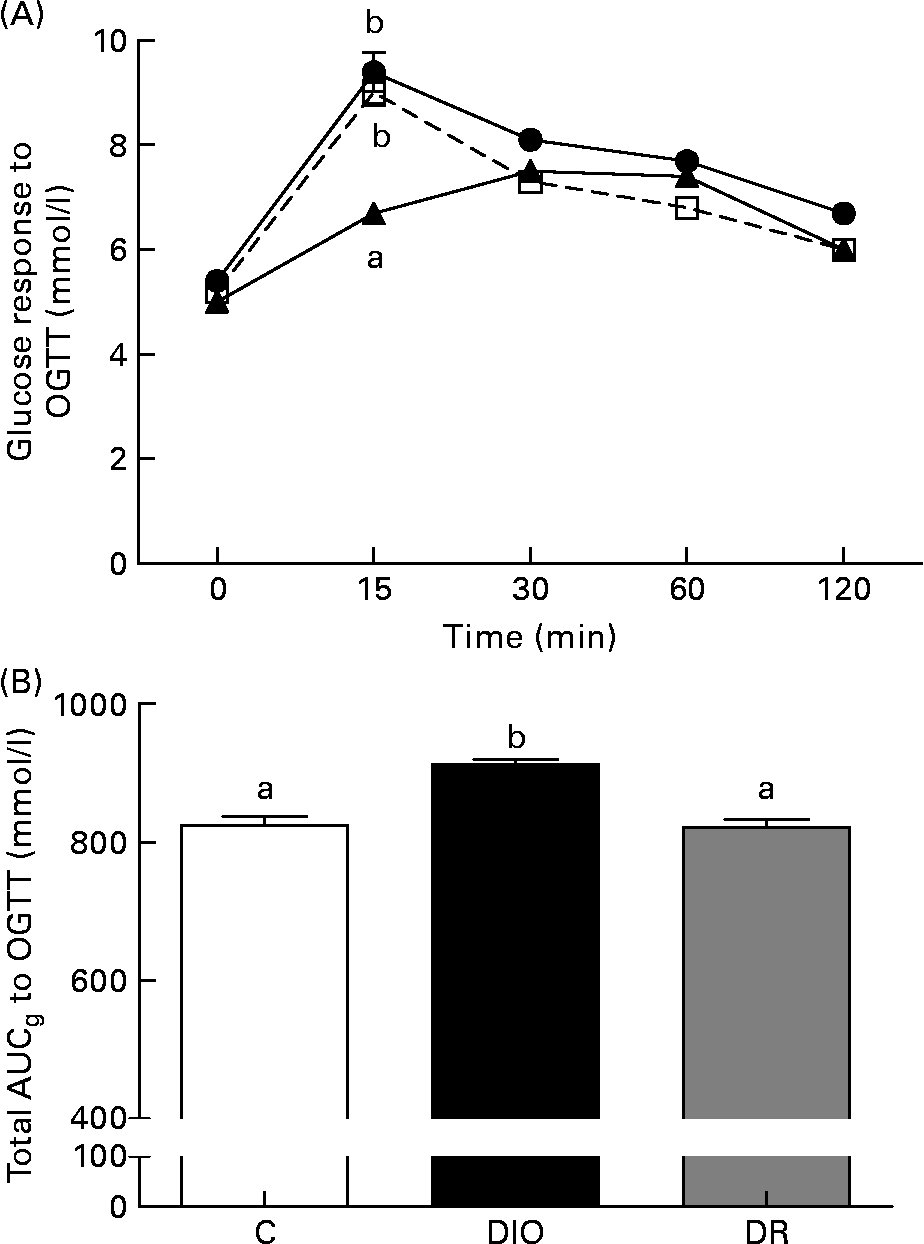
Fig. 2 (A) Fasting glucose and blood glucose response to the oral glucose tolerance test (OGTT), (B) total area under curveglucose (AUCg) to the OGTT after 20 weeks of diet in the control (C, ![]() ), diet-induced obese (DIO,
), diet-induced obese (DIO, ![]() ) and diet-resistant (DR,
) and diet-resistant (DR, ![]() ) rats. Values are means of six randomly selected animals per group, with their standard errors represented by vertical bars. a,b Mean values of groups with unlike letters were significantly different (one-way ANOVA with Bonferroni's post-test; P < 0·05).
) rats. Values are means of six randomly selected animals per group, with their standard errors represented by vertical bars. a,b Mean values of groups with unlike letters were significantly different (one-way ANOVA with Bonferroni's post-test; P < 0·05).
Macroscopic analyses
The wound area was greater in the DIO (55·0 %, P < 0·05) and DR (50·6 %, P < 0·05) groups than in the control group 7 d after wounding (Table 1). However, there was no difference in the area between the groups 14 d after wounding (Table 1).
The DIO group showed no sign of re-epithelialisation 7 d after wounding, whereas the control and DR groups presented re-epithelialisation at a low rate. The percentage of the re-epithelialised wound area was lower in the DIO ( − 18·0 %, P < 0·05) and DR ( − 20·3 %, P < 0·05) groups compared with the control group 14 d after wounding (Table 1).
Quantification of inflammatory infiltrate
The inflammatory infiltrate was intense in the DIO and DR groups 7 d after wounding. The number of PMN leucocytes was greater in the DIO (33·4 %, P < 0·05) group than in the DR group (Table 2). In both groups, mast cells were localised in the deep region, but this was especially prominent in the DIO group, where the mast cells were near the adipocytes (Fig. 3(A)). The number of mast cells was greater in the DIO group (59·3 %, P < 0·05) than in the DR group (Table 2; Fig. 3((A) and (B))). The number of CD68-positive macrophages was similar in both groups (Table 2).
Table 2 Microscopic and biochemical analyses of wounded areas 7 and 14 d after wounding*
(Mean values with their standard errors of at least seven animals per group for microscopic analyses and of six animals per group randomly selected for biochemical analyses)

DIO, diet-induced obese group; DR, diet-resistant group; PMN, polymorphonuclear; day 7, 7 d after wounding; day 14, 14 d after wounding; HNE, 4-hydroxynonenal-positive cells; Vv, volume density.
a,b Mean values within a row with unlike superscript letters were significantly different (Mann–Whitney test for microscopic analyses and unpaired t test for biochemical analyses; P < 0·05).
* Microscopic analyses after day 7: DIO = 8, DR = 7; microscopic analyses after day 14: DIO = 9, DR = 9; biochemical analyses after days 7 and 14: DIO = 6, DR = 6.

Fig. 3 (A, B) Distribution of mast cells and (C, D) CD68-positive macrophages 7 and 14 d after wounding. The diet-induced obese (DIO (A, C)) and diet-resistant (DR (B, D)) groups are shown. Mast cells were analysed in toluidine blue-stained sections, and macrophages were identified by immunohistochemistry against CD68 in sections. To evaluate the number of mast cells, between six and ten random fields per animal were analysed (at least seven animals per group). To quantify CD68-positive macrophages, fifteen random fields per animal were analysed (at least seven animals per group). All quantifications were performed blindly and repeated. Scale bar = 50 μm. Mobilisation of mast cells was higher in (A) the DIO group than in (B) the DR group. The amount of CD68-positive macrophages was higher in (C) the DIO group than in (D) the DR group. In detail (C), CD68-positive macrophages around adipocytes in the DIO group can be observed. Microscopic analyses after day 7 (DIO = 8, DR = 7); microscopic analyses after day 14 (DIO = 9, DR = 9).
The DIO group still had a high inflammatory infiltrate 14 d after wounding. The number of PMN leucocytes decreased in both groups, with no differences between them (Table 2). The number of mast cells remained higher in the DIO group than in the DR group (41·2 %, P < 0·05) (Table 2). In addition, the number of CD68-positive macrophages decreased slightly in the DR group but remained elevated in the DIO group. In the DIO group, the CD68-positive macrophages were localised around adipocytes, in close association with them (Fig. 3(C) inset). The number of CD68-positive macrophages was higher in the DIO group than in the DR group (13·7 %, P < 0·05; Table 2 and Fig. 3(C) and (D))).
Lipid peroxidation
To evaluate the tissue damage due to the inflammatory response in the DIO and DR rats, we assayed the 4-HNE levels in wound sections. 4-HNE is formed during lipid peroxidation and is present in higher levels during oxidative stress(Reference Esterbauer, Schaur and Zollner19). The number of 4-HNE-positive cells was higher in the DIO group than in the DR group (27·2 %, P < 0·05) 7 d after wounding (Table 2). There was no difference between the DIO and DR groups 14 d after wounding.
Development of granulation tissue and angiogenesis
Myofibroblasts were equally scarce in the DIO and DR groups 7 d after wounding (Table 2). However, 14 d after wounding, this pattern was reversed, with myofibroblasts found in all granulation tissues of the DIO and DR groups. The Vv of myofibroblasts was greater in the DIO group than in the DR group (61·1 %, P < 0·05; Table 2).
There was no difference in the volume occupied by blood vessels between groups at all time points in the superficial and deep regions of the wound. Furthermore, the Vv of blood vessels 14 d after wounding remained high in the DIO and DR groups compared with the Vv 7 d after wounding (Table 2).
Collagen deposition
In both groups, 7 d after wounding, most of the collagen fibres were yellow-greenish and a few were yellow-reddish, and the yellow-reddish were short and had a random arrangement. However, 14 d after wounding, most collagen fibres were yellow-reddish, elongated and arranged parallel to the surface. To confirm these histological observations, hydroxyproline levels were measured. In the DIO and DR groups, hydroxyproline levels were lower 7 d after wounding and increased slightly 14 d after wounding (Table 2). There was no difference in the levels between the groups.
Measurement of nitrite levels
Because nitrite is a stable molecule and accounts for more than 90 % of total measurable nitrite and nitrate(Reference Schaffer, Tantry and Efron25), the nitrite dosage was used as an index of NO synthesis. There was no difference between the groups 7 d after wounding; however, 14 d after wounding, the nitrite levels were lower in the DIO group ( − 340 %) than in the DR group (P < 0·05; Table 2).
Measurement of TNF-α release
Within the same group, no differences were observed in the TNF-α production from animals killed 7 or 14 d after wounding (data not shown). As shown in Fig. 4, the TNF-α amount in retroperitoneal fat was higher in the DIO group than in the DR group (130 %, P < 0·05) at both 7 and 14 d after wounding.
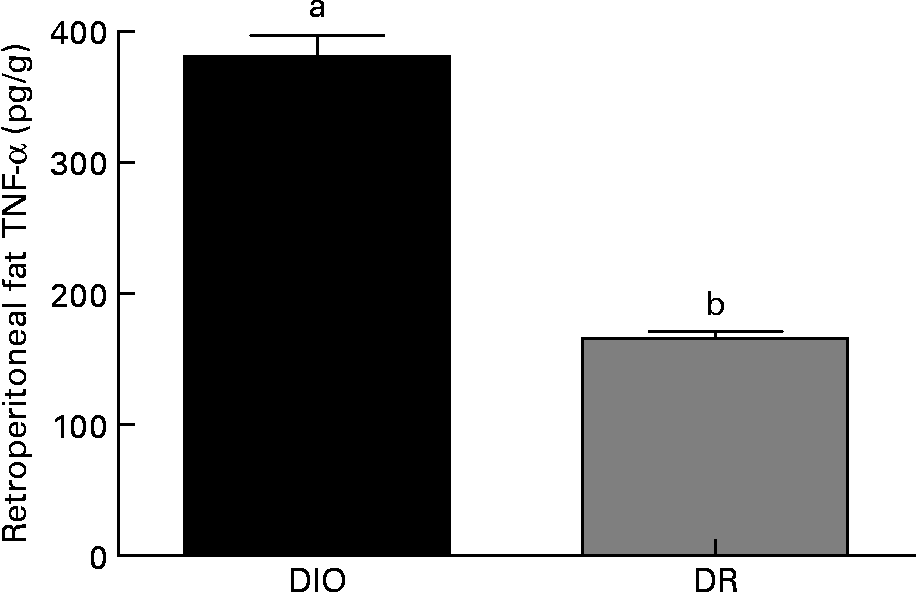
Fig. 4 TNF-α levels in the retroperitoneal fat of the diet-induced obese (DIO) and diet-resistant (DR) rats. Values are means of six randomly selected animals per group, with their standard errors represented by vertical bars. a,b Mean values of a group with unlike letters were significantly different (unpaired t test; P < 0·05).
Discussion
Overweight induced by a HF diet delays cutaneous wound healing in obesity-prone rats through the elongation of the inflammatory phase(Reference Nascimento and Costa12). Nevertheless, the pattern of wound healing of individuals who are resistant to an increase in body weight when fed an obesogenic diet is unknown. In the present study, both DIO and DR rats presented delayed wound healing; however, DIO rats had a more intense inflammatory phase and more oxidative damage.
Overweight and obesity are problems of epidemic proportions and are associated with an increased incidence of many chronic conditions, such as chronic wounds(Reference Wilson and Clark11). In rodents, an obesogenic diet promotes obesity through hyperphagia(Reference Oscai, Brown and Miller26); this finding was corroborated by the results of the present study. The DIO group ingested more food and showed a higher body weight than the control and DR groups, although the latter was fed the same diet. Importantly, obesogenic DIO occurs in some but not all rats(Reference Lauterio, Bond and Ulman14). In the present study, the weight of retroperitoneal fat mass was higher in the DIO group, lower in the control group and intermediate in the DR group, indicating that not only hyperphagia, but also the fat contained in the diet, plays an important role in the increase in adipose tissue. This finding is confirmed by the fact that the control and DR groups consumed equivalent amounts of food, yet the DR group had a higher amount of retroperitoneal fat mass than the control group. According to the literature, various products of WAT are specifically linked to inflammation(Reference Tilg and Moschen6), and the inflammatory phase is disturbed during the cutaneous wound healing of obesity-prone rats(Reference Nascimento and Costa12). However, wound healing (and inflammatory phase duration) has never been studied in obesity-resistant rats.
Although obesity-induced chronic inflammation is related to insulin resistance and the development of type 2 diabetes(Reference Kahn, Zinman and Haffner27), no difference in fasting blood glucose was observed in the present study. Previous studies with Wistar rats fed a HF diet showed no metabolically significant hyperglycaemia(Reference Borst and Conover28). A mild fasting hyperglycaemia with a long-term (approximately 10 months) HF feed has been observed(Reference Chalkley, Hettiarachchi and Chisholm29); however, our diet protocol lasted only 20 weeks. Although the obesogenic diet did not cause an elevation in fasting glucose, the DIO group presented a small degree of glucose intolerance; the area under the glucose level curve was significantly elevated in the DIO group compared with the control and DR groups, indicating a possible early stage of insulin resistance development. Borst & Conover(Reference Borst and Conover28) reported that the induction of an insulin-resistant state by HF feeding is accompanied by increased adiposity and increased tissue expression of TNF-α protein, supporting a possible autocrine/paracrine role for TNF-α in obesogenic diet-induced insulin resistance. Importantly, glucose intolerance was only observed in the DIO group, which presented a substantial increase in retroperitoneal fat (sixfold) and of TNF-α protein in adipose tissue. However, the blood glucose concentration does not report all of the information regarding glucose metabolism, and further studies assessing the insulin concentration are required.
The resolution of the inflammatory response is a limiting factor in successful repair after tissue injury. Most non-healing wounds fail to progress through the normal phases of wound repair, remaining in a chronic inflammation state(Reference Cooney, Iocono and Maish4, Reference Trengove, Bielefeldt-Ohmann and Stacey5). The inflammatory response is necessary, because it induces the production of pro-inflammatory cytokines, recruits macrophages and stimulates the proliferation of resident cells and angiogenesis(Reference Eming, Krieg and Davidson30). In the present study, the DIO group presented a higher number of inflammatory cells and mast cells than the DR group. WAT from obese animals contain more mast cells than WAT from their lean counterparts, and these cells promote DIO and glucose intolerance(Reference Liu, Divoux and Sun31). This result indicates a more prominent inflammatory phase in the DIO group. A body of evidence suggests the presence of an overall, low-grade inflammation in obesity, with altered levels of several circulating factors, such as an increase in the plasma levels of C-reactive protein, TNF-α, IL-6 and other biological markers of inflammation(Reference Das32–Reference Mosca34). This association is meaningful, and several animal models have suggested that these inflammatory processes have a causal relationship with obesity and its comorbidities. Thus, the increase in TNF-α in adipose tissue of the DIO rats may explain the large number of inflammatory cells in this group. Furthermore, there is a strong positive correlation between the degree of adiposity and several obesity-associated disorders(Reference Janssen, Heymsfield and Allison35). This association was observed in the present study, as the DR group presented only an intermediate increase in retroperitoneal fat mass but did not develop obesity, did not increase levels of TNF-α and possessed a lower number of inflammatory cells than the DIO group. Although the DR group did not develop obesity, it showed delayed wound healing. A previous study has shown differential regulation of some inflammation-related genes in adipose tissue between the DIO and DR rats. DIO rats presented an increase in TNF-α, IL-6 and haptoglobin mRNA levels, whereas DR rats only presented an increase in IL-6 mRNA levels(Reference Perez-Echarri, Perez-Matute and Marcos-Gomez36), suggesting different regulation of the inflammatory healing phase. Regardless of these differences, the result is deleterious to wound repair. However, more studies are needed to define the inflammatory status of the DIO and DR rats during wound healing.
Reactive oxygen species are produced in response to cutaneous injury(Reference Gupta, Singh and Raghubir37) and may cause cellular damage by peroxidation of membrane lipids, inactivation of sulfhydryl enzymes, cross-linking of proteins and breakdown of DNA(Reference Russo, Longo and Vanella38). In most studies, reactive oxygen species levels at the wound site have been determined indirectly through analyses of oxidation products of lipids, proteins or DNA(Reference Urso and Clarkson39). A major product of lipid peroxidation, 4-HNE, can be detected by immunohistochemistry(Reference Ojha, Roy and He40). In the present study, the amount of 4-HNE-positive cells was greater in the DIO group than in the DR group. The number of inflammatory cells was also greater in the DIO group and was probably responsible for the increased lipid peroxidation.
Wound healing involves a variety of processes such as acute inflammation, cell proliferation and wound contraction(Reference Singer and Clark1). These phases overlap in time and duration, each depending on various factors such as tissue type, health status, nutritional status and the presence of infections. Several types of cells are recruited to an injury site to carry out the processes of repair. In rodents, wound contraction is the main mechanism of wound closure(Reference Davidson41), and myofibroblasts are the main cells responsible for wound contraction(Reference Desmouliere, Chaponnier and Gabbiani42, Reference Gabbiani43). In the present study, the DIO and DR groups presented delayed wound contraction 7 d after wounding. This result can be explained by the delay in myofibroblastic differentiation observed at this time point in the groups. A lack of apoptotic neutrophils at the wound site deprives the macrophages of their main stimulus to secrete transforming growth factor-β1, a key mediator involved in myofibroblast differentiation from fibroblasts(Reference Peters, Sindrilaru and Hinz44, Reference Tomasek, Gabbiani and Hinz45). Transforming growth factor-β1 derived from macrophages also acts as a strong inhibitor of the inducible isoform of NO synthase during wound healing(Reference Vodovotz, Bogdan and Paik46). This association was corroborated by the present results; 7 d after wounding, the number of PMN leucocytes and nitrite levels was elevated in the DIO and DR groups. These results indicate that the large number of PMN leucocytes may be related to the delay in myofibroblastic differentiation and increased NO synthesis in the DIO and DR groups.
Re-epithelialisation is the process of restoring the epidermis and consists of proliferation and migration of keratinocytes(Reference Li, Chen and Kirsner47). In the present study, the DIO and DR groups presented delayed re-epithelialisation. Keratinocyte proliferation and migration occurs, while the levels of immune cells and pro-inflammatory cytokines decrease(Reference Gurtner, Werner and Barrandon48), and the present results showed that the DIO and DR groups still presented a large number of macrophages 14 d after wounding. Although the proliferation is increased in overweight rats(Reference Nascimento and Costa12), the re-epithelialisation is still delayed, suggesting that the late, but considerable, presence of macrophages in the wounds of the DIO and DR groups compromised keratinocyte migration, consequently delaying re-epithelialisation.
When the levels of immune cells and pro-inflammatory cytokines decrease, fibroblasts and endothelial cells start to migrate into the wound and proliferate to initiate the second phase of healing, granulation tissue formation. Fibroblasts are attracted to the wound edge, proliferate and are responsible for the synthesis, deposition and remodelling of the extracellular matrix(Reference Singer and Clark1). In the granulation tissue, the fibroblasts differentiate into myofibroblasts, which are responsible for wound contraction and for the production of the extracellular matrix(Reference Desmouliere, Chaponnier and Gabbiani42, Reference Gabbiani43). Although we observed a delay in myofibroblastic differentiation and the development of inflammation in the DIO and DR groups, there was no difference in the deposition and organisation of collagen fibres. The delay in myofibroblastic differentiation could have been counteracted by the increased NO by increased NO synthesis (nitrite dosage) in the DIO and DR groups 7 d after wounding. Studies have demonstrated that NO is able to stimulate extracellular matrix (mainly collagen) synthesis and deposition, and the link between NO and collagen deposition has been described in vivo and in vitro (Reference Rizk, Witte and Barbul49, Reference Schwentker, Vodovotz and Weller50). Another study from our laboratory(Reference Amadeu, Seabra and de Oliveira16) has shown an increase in collagen deposition due to topical NO donor application in both inflammatory and proliferative phases.
To support the new tissue with oxygen and nutrients, blood vessels sprout at the edge of the wound, and the granulation tissue is continuously replenished by new blood vessels. Once the wound is filled with new granulation tissue, angiogenesis ceases and many blood vessels disintegrate as a result of apoptosis(Reference Singer and Clark1). In the present study, there was no difference in the volume occupied by blood vessels 7 and 14 d after wounding. However, 14 d after wounding, the volume occupied by blood vessels remained high in both groups. The high occupied volume was probably due to high cellularity, which was still observed in granulation tissue 14 d after wounding, suggesting that these vessels do not undergo apoptosis due to the high nutritional need still required by granulation tissue.
In conclusion, the obesogenic diet induced an increase in body weight and retroperitoneal fat mass in the DIO rats, whereas only an intermediate increase in retroperitoneal fat mass was observed in the DR rats. Although it did not induce weight gain, the obesogenic diet compromised (delayed) the wound healing process in the DR animals, highlighting the importance of diet composition in the wound healing process. More studies using purified diets are needed to distinguish the effects of fat level from the effects of fat type and/or effects of dietary protein level and quality on cutaneous wound healing of the DIO-prone and DR rats.
Acknowledgements
The present study was partially supported by FAPERJ. A. P. d. N. holds a postgraduate fellowship from FAPERJ. Neither of the authors has conflicts of interest with respect to the present study. The contributions of the authors were as follows: A. P. d. N. designed and conducted the experiment, and wrote the manuscript. A. M.-A.-C. designed the experiment, critically analysed the results and participated in the critical revision of the manuscript. The authors thank Claudia Benjamim and Ariane Brogliato for their help in performing the ELISA.








