Introduction
Currently, for embryo culture systems for in vitro fertilization (IVF), the culture method represents a key factor to achieve successful embryo outcome. Culture methods mainly include individual culture, group culture and different embryo density culture. In the last two decades, there have been several studies on the effects of culture methods (individual or group culture vs. different embryo culture density) on embryo developmental outcome (Ebner et al., Reference Ebner, Shebl, Moser, Mayer, Arzt and Tews2010; Rebollar-Lazaro et al., 2010; Minasi et al., Reference Minasi, Fabozzi, Casciani, Lobascio, Colasante, Scarselli and Greco2015; De Munck et al., Reference De Munck, Santos-Ribeiro, Mateizel and Verheyen2015; Lehner et al., Reference Lehner, Kaszas, Murber, Rigo, Urbancsek and Fancsovits2017). However, none of these studies had adjusted covariates that might have affected embryo development. It has been known that women’s fertility declines with increasing age, and embryo quality and developmental potential would also be compromised by maternal age (Ziebe et al., Reference Ziebe, Loft, Petersen, Andersen, Lindenberg, Petersen and Andersen2001). Intracytoplasmic sperm injection (ICSI) could improve fertilization rate and the embryo quality for patients with infertility of non-male factors (Kim et al., 2013). An optimum number of retrieved oocytes is needed to achieve the best IVF outcome (Ji et al., Reference Ji, Liu, Tong, Luo, Ma and Chen2013). These findings indicate that the early development of human embryos would be affected by cofounding factors, including the patient’s demographic characteristics. It is important to adjust the cofounding factors when investigating the relationship between the culture method and the embryo developmental outcome.
According to previous reports, in mammalian animals, group culture would achieve obviously better developmental outcome compared with individual culture (Schini and Bavister Reference Schini and Bavister1988; Gardner et al., Reference Gardner, Lane, Spitzer and Batt1994; Salahuddin et al., Reference Salahuddin, Ookutsu, Goto, Nakanishi and Nagata1995; O’Doherty et al., Reference O’Doherty, Wade, Hill and Boland1997; Vutyavanich et al., 1997), and this has even been a prerequisite for achieving good embryo quality for several other species (Lehner et al., Reference Lehner, Kaszas, Murber, Rigo, Urbancsek and Fancsovits2017). However, for in vitro culture of human embryos, although benefits have been reported for group culture compared with individual culture (Moessner and Dodson, Reference Moessner and Dodson1995; Almagor et al., Reference Almagor, Bejar, Kafka and Yaffe1996; Ebner et al., Reference Ebner, Shebl, Moser, Mayer, Arzt and Tews2010; Rebollar-Lazaro et al., 2010), it has not become a common practice in the IVF laboratory worldwide (Christianson et al., Reference Christianson, Zhao, Shoham, Granot, Safran, Khafagy, Leong and Shoham2014). There may be three reasons. First, with the development of new IVF technologies, such as time-lapse microscopy (Rubio et al., Reference Rubio, Galán, Larreategui, Ayerdi, Bellver, Herrero and Meseguer2014;) and non-invasive embryo screening based on embryo culture medium metabolomics evaluation (Marhuenda-Egea et al., Reference Marhuenda-Egea, Martínez-Sabater, Gonsálvez-Alvarez, Lledó, Ten and Bernabeu2010; Seli et al., Reference Seli, Vergouw, Morita, Botros, Roos, Lambalk, Yamashita, Kato and Sakkas2010) and non-invasive preimplantation genetic testing (PGT) (Leaver and Wells Reference Leaver and Wells2020), the need for individual culture has been increasing. Second, there are no universally recognized and accepted protocols for other culture conditions concerning culture medium, oxygen concentration and total volume of microdrop (Reed Reference Reed2012), which may affect group culture in embryo development (Moessner and Dodson, Reference Moessner and Dodson1995; Almagor et al., Reference Almagor, Bejar, Kafka and Yaffe1996; Rijnders and Jansen, Reference Rijnders and Jansen1999; Spyropoulou et al., Reference Spyropoulou, Karamalegos and Bolton1999; Fujita et al., Reference Fujita, Umeki, Shimura, Kugumiya and Shiga2006). Finally, culture conditions have been gradually improved, resulting in significantly increased quality of human embryos (Sciorio and Smith, Reference Sciorio and Smith2019). Therefore, further studies are still needed to verify the beneficial effects of group culture under altered culture conditions (such as hypoxia culture).
Embryo density is defined as the ratio of microdrop volume to the number of cultured embryos, and can be modified by changing either the number of embryos or the microdrop volume. The direct effect of embryo density on embryo development should be taken into serious consideration regardless of group culture or individual culture. At this time, embryologists have not yet reached a consensus on the optimal embryo density, and the embryo density in most laboratories undertaking IVF embryo culture has been determined according to historical tradition or practical convenience (Reed, Reference Reed2012). For group culture under certain microdrop volumes, embryo density could be regulated by changing the number of cultured embryos. Although Gardner and Lane (2017) have recommended that maximum embryo density should not exceed 12.5 μl/embryo, the relationship between embryo density and the developmental outcome still needs to be studied with adjusted covariates. For individual culture, embryo density could be regulated by changing the microdrop volume. However, the findings of two recent studies concerning the optimal microdrop volume for individual culture were contradictory (De Munck et al., Reference De Munck, Santos-Ribeiro, Mateizel and Verheyen2015; Minasi et al., Reference Minasi, Fabozzi, Casciani, Lobascio, Colasante, Scarselli and Greco2015).
In this study, the relationship between embryo density and the developmental outcome of day 3 embryos was investigated, with the following covariates adjusted: maternal age, paternal age, antral follicles, level of anti-Müllerian hormone (AMH), type of infertility, controlled ovarian stimulation (COS) protocol, length of stimulation, number of retrieved oocytes, number of zygotes (two pronuclei) and insemination type. The embryos were cultured with different embryo densities, and the developmental outcomes of day 3 embryos were analyzed and compared between group culture and individual culture under hypoxia after adjusting for covariates.
Materials and methods
Study patients and embryo selection
In this retrospective study, in total, 1196 embryos from 206 cycles were collected; these embryos had been cultured in the Department of Reproductive Medicine Center, Peking University People’s Hospital (Peking, China), between June 2018 and December 2018 (during this period the culture medium and drop volume was unchanged). Patients who intended to undergo IVF and embryo transfer (IVF-ET) were included. Exclusion criteria were as follows: patients who did not retrieve any oocytes after controlled ovarian hyperstimulation; who did not have normally fertilized zygotes (with two pronuclei 16–18 h after insemination); or who only had degenerated oocytes. There were no PGT or donor cycles in our study. All embryos cleaved from normally fertilized zygote were non-selectively and consecutively included. Cycles with abnormally fertilized zygotes or with degenerated embryos were excluded. Every patient was informed in detail on the IVF treatment procedures and risks, and their consent was obtained. Additional participants’ informed consent was waived because of the retrospective cohort study.
Ovarian stimulation
Different COS regimens, i.e. long, antagonist and others (minimal stimulation, natural, luteal phase stimulation and progestin-primed stimulation), were selected for ovarian stimulation according to patients’ characteristics and responses during previous IVF cycles or ovulation induction. When the diameter of at least one leading follicle was 18 mm, ovulation was induced with 5000–10,000 IU of human chorionic gonadotropin (hCG; Choragon, Ferring, Switzerland), alone or in combination with 0.2 mg triptorelin acetate (Ferring). Transvaginal ultrasound-guided oocyte retrieval was performed at 36 h after hCG administration.
IVF and embryo culture
After retrieval, oocytes were incubated in fertilization medium in a 60-mm IVF dish (#353653; Falcon, Franklin Lakes, NJ, USA). In addition, fresh sperm samples were collected from the male partners by masturbation. After 80%/40% density gradient centrifugation (Sage, USA), sperm samples were kept in a 37°C, in a 6% CO2 in air incubator until insemination. Oocytes were inseminated using conventional IVF or ICSI at 40 h after hCG injection, based on the quality of the sperm sample, oocyte count and previous IVF cycle performance. Conventional IVF was performed by transferring the prepared sperm sample into the dish where oocytes were incubated. For ICSI, cumulus cells of oocytes were removed at 1 h after retrieval by gently pipetting, following a short enzymatic digestion in hyaluronidase (Sage). Denuded oocytes were incubated in the cleavage medium, and then subjected to sperm injection in gamete manipulation buffer at 40 h after hCG injection, finally these were transferred to a culture dish in cleavage medium immediately post-ICSI.
Embryo culture was performed in a Cook incubator with mixed gas of 5% O2, 6% CO2 and 89% N2. Embryos were cultured in 35-mm polystyrene dishes (#150255; Nunc, USA). Six culture and three wash individual microdrops each 30 μl were placed in a circle in the centre of the dish’s base, separately, the day before oocyte retrieval. In detail, 10 μl of culture medium (G1-plus, Vitrolife, Sweden) were pipetted directly onto the dish, overlaid with 3.5 ml oil (Vitrolife) and then refilled with another 20 μl culture medium. Dishes were prepared once at room temperature without airflow and then equilibrated overnight in a 37°C, 6% CO2 in air incubator before use. Usually, one or two dishes were prepared for each patient, with six drops each dish.
The pronuclei were examined under a microscope at 16–18 h after insemination. Then, one, two or three zygotes with two normal pronuclei were placed randomly in a single microdrop on the culture dish unselectively for ongoing culture. One or two embryos per drop were prepared one by one. If there were still more embryos after all the drops were used, three embryos were then put together in one drop. Zygotes with three or more pronuclei and degenerated oocytes were discarded. Zygotes with one pronucleus and unfertilized oocytes were subjected to group culture in different microdrops, which were not included in this study. On day 3, embryo morphology was recorded based on the scoring system reported by Pruissant and colleagues (1987). Briefly, the number and evenness of the blastomeres were analyzed, as well as the percentage of fragments. Cleaved embryos with 7–10 equal or slightly unequal blastomeres and ≤15% fragments were considered as grade I. When the percentage of fragments was 16–29% or the number of blastomeres did not meet the grade I standard, the embryos were considered grade II. When the percentage of fragments was between 30% and 49%, the embryos were considered grade III. Finally, when there were 50% fragments or the embryo development was retarded, embryos were considered grade IV. Embryos at grade I or II were selected with priority for transferring or freezing on day 3, and the others were group cultured in blastocyst medium for another 2 or 3 days.
Study design
The present retrospective cohort study was designed to address the relationship between embryo density and cell number of day 3 embryos. The target independent variable was the embryo density, while the dependent variable was the cell number of day 3 embryos and clinical result of day 3 embryos. Embryo density was defined as follows: the microdrop volume (30 μl) divided by the number of normally fertilized zygotes that had been cleaved until day 3. In our laboratory, normally fertilized zygotes were routinely assigned into a single microdrop with three different embryo densities, i.e. 30 μl/embryo (one embryo in a microdrop), 15 μl/embryo (two embryos together in a microdrop) or 10 μl/embryo (three embryos together in a microdrop). These procedures were performed by technicians who were unaware of the patients’ characteristics and the treatment cycles. Therefore, embryo density was recorded as a categorical variable. Cell number at day 3 embryos was defined as the number of blastomeres recorded from the embryo morphology, which was considered as a continuous variable. Clinical result of day 3 embryos, which was defined as whether the transferred day 3 embryos implanted successfully, was considered as a categorical variable. Covariates included the continuous variables of maternal age, paternal age, antral follicles, level of AMH, length of stimulation, number of zygotes (two pronuclei) and number of retrieved oocytes, as well as the categorical variables of type of infertility, COS protocol and type of categorical variable insemination.
Statistical analysis
Statistical analysis was performed with the statistical software packages R (http://www.R-project.org; the R Foundation) and EmpowerStats (http://www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). Demographic and cycle characteristics of all the participants were analyzed. Continuous variables were presented as mean ± standard error or median (Q1, Q3). Categorical variables were presented as frequency and percentage. Univariate analysis was used to analyze the effect of each variable on cell number of day 3 embryos. To analyze the relationship between the embryo density and the cell number and clinical result of day 3 embryos, multivariable logistic regression analyses were performed. Each participant had several embryos, which were assigned into different embryo density groups. The generalized estimate equation (GEE) model based on the patient’s unique medical record number was used in both the univariate analysis and the multivariable logistic regression analysis to avoid the effect of repeated measurement of participants’ characteristics. In the multivariable logistic regression analyses, the following three models were used: In model 1, no covariates were adjusted; in model 2, maternal age, the number of retrieved oocytes and type of insemination were adjusted for the cell number of day 3 embryos; significantly different factors in univariate analysis were adjusted for the clinical result of day 3 embryos; and in model 3, all the covariates were adjusted. To verify the results from the multivariable logistic regression analyses based on embryo density as a categorical variable, a sensitivity analysis was performed by converting the embryo density into a continuous variable and calculating the P for trend. P-values less than 0.05 (two-sided) were considered statistically significant.
Results
Demographic and cycle characteristics of study participants
In total, 206 participants were enrolled for data analysis based on the inclusion and exclusion criteria. The demographic and cycle characteristics of all these study participants are shown in Table 1. In general, the average ages of the female and male participants were 35.0 ± 5.1 and 36.6 ± 6.4 years old, respectively. The median (Q1, Q3) number of antral follicles for the female participants was 6.00 (3.00, 12.00). The median (Q1, Q3) level of AMH for the female participants was 2.18 (1.12, 3.92) ng/ml. From these female participants, 107 participants (51.94%) were diagnosed with primary infertility, and 121 participants (58.74%) were treated using the long antagonist protocol for ovary stimulation. The average length of stimulation for the female participants was 9.1 ± 2.5 days. The median (Q1, Q3) number of the retrieved oocytes for the female participants was 7.00 (3.00, 12.00). The median (Q1, Q3) number of zygotes (two pronuclei) was 5.00 (2.00, 7.50).
Table 1. Demographic and cycle characteristics of study participants
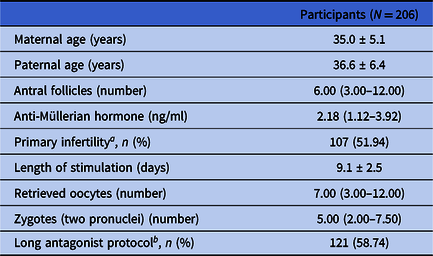
a Proportion of primary infertility.
b Proportion of long antagonist protocol.
Cycle and developmental characteristics of embryos in different embryo density groups
In total, 1196 embryos were assigned into three embryo density groups, i.e. the 30 μl/embryo group (with 434 embryos), 15 μl/embryo group (with 646 embryos) and 10 μl/embryo group (with 116 embryos). The cycle and developmental characteristics of embryos in these groups are shown in Table 2. A significantly higher percentage of oocytes was inseminated by conventional IVF in both the 15 and 10 μl/embryo groups compared with the 30 μl/embryo group (70.74% vs. 63.13%, P = 0.009; 82.76% vs. 63.13%, P < 0.001). The average cell numbers of day 3 embryos in both the 15 and 10 μl/embryo groups were higher than the 30 μl/embryo group (7.18 ± 2.12 vs. 6.96 ± 2.13; and 7.33 ± 2.58 vs. 6.96 ± 2.13), with, however, no significant difference (P = 0.263 and P = 0.253, respectively).
Table 2. Cycle and developmental characteristics of embryos among different embryo density groups (N = 1196)
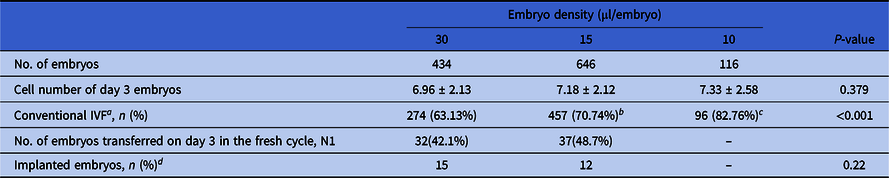
a Proportion of conventional in vitro fertilization (IVF).
b P = 0.009, compared with the 30 µl/embryo group.
c P < 0.001, compared with the 30 µl/embryo group.
d Whether the transferred embryo implanted successfully or not.
In total, 32 embryos in the 30 μl/embryo group, 37 in the 15 μl/embryo group, and 7 in the 10 μl/embryo group were transferred in the fresh COS cycle, resulting in 15, 12 and 0 embryos being successful implanted separately. As there were only seven embryos transferred in the 10 μl/embryo group, resulting no successful implantation, only the implantation rates of the 30 μl/embryo and 15 μl/embryo groups were analyzed statistically. There was no significant difference between these two groups (P = 0.22).
Impact of each variable on the cell number and clinical results of day embryos from univariate analysis based on GEE model
As shown in Table 3, the cell numbers of day 3 embryos in the 15 and 10 μl/embryo groups increased by 0.22 and 0.37 compared with the 30 μl/embryo group (95%CI: −0.14, 0.58; and −0.51, 1.25). The cell number of day 3 embryos in the ICSI fertilization group was decreased by 0.05 compared with the conventional IVF fertilization group (95%CI: −0.42, 0.32). The cell number of day 3 embryos in the female participants diagnosed with secondary infertility was decreased by 0.10 compared with those diagnosed with primary infertility (95%CI: −0.51, 0.30). The cell number of day 3 embryos in the female participants treated with the other COS protocol was increased by 0.11 compared with those treated with the long antagonist protocol (95%CI: −0.39, 0.61). Along with increased age of male participants, stimulation length of female participants, the number of retrieved oocytes from female participants and number of zygotes (two pronuclei), and cell numbers of day 3 embryos were decreased by 0.01, 0.04, 0.0001 and 0.00, respectively (95%CI: −0.05, 0.02; −0.14, 0.05; −0.0186, 0.0182 and −0.03, 0.03). In contrast, along with the increased age of female participants, antral follicle number of female participants and AMH level of female participants, the cell numbers of day 3 embryos were increased by 0.01, 0.02, and 0.05, respectively (95%CI: −0.04, 0.05; −0.01, 0.05; and −0.03, 0.13). However, based on P-value, none of these above variables including embryo density had a significant effect on day 3 embryo cell number.
Table 3. Univariate analysis for cell number and implanted embryos of day 3 embryos
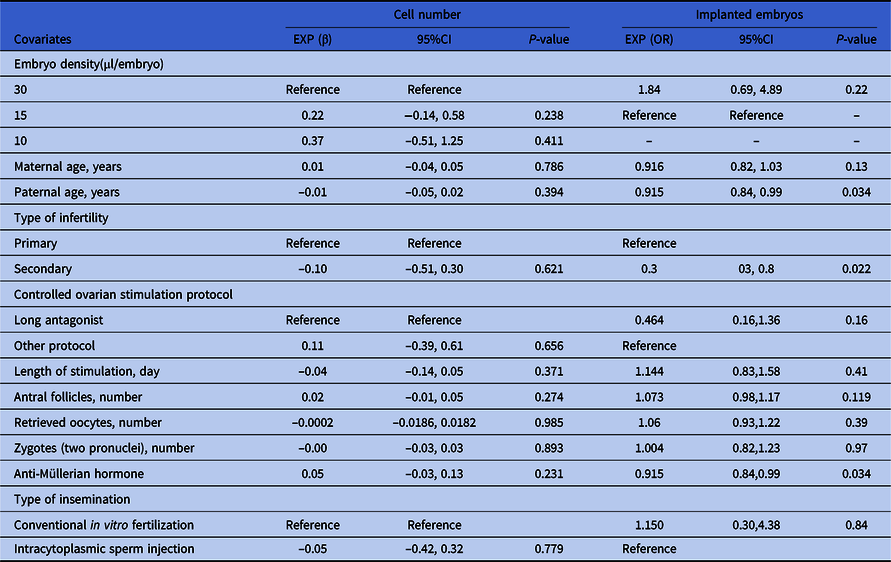
For the outcome of clinical results (recorded as ‘whether the transferred embryo implanted successfully or not’, a binary variable), paternal age, AMH and type of infertility had significant power. Specifically, along with increased age of male participants, less embryos implanted successfully (OR = 0.92, 95% CI: 0.84–0.99, P = 0.03) (OR = 0.94, 95% CI: 0.91–0.97, P < 0.01). In contrast, along with increased AMH level of female participants, more embryos implanted successfully (OR = 1.27, 95% CI: 1.01–1.60, P = 0.045). The implantation rate of day 3 transferred embryos in female participants diagnosed with secondary infertility was increased by 0.3 compared with those diagnosed with primary infertility (95%CI: 0.3, 0.8, P = 0.022).
Relationship between embryo density and the cell number and clinical results of day embryos in different multivariable logistic regression GEE models
Here, three models were used to analyze the independent effects of embryo density on cell number of day 3 embryos. The effect sizes (β) and 95%CI are listed in Table 4. In the unadjusted model (model 1), the cell numbers of day 3 embryos in the 15 and 10 μl/embryo groups were increased separately by 0.22 (95%CI: −0.14, 0.58) and 0.37 (95%CI: −0.51, 1.25) compared with the 30 μl/embryo group. In the minimum-adjusted model (model 2) (adjusted for the maternal age, number of retrieved oocytes and type of insemination), the cell numbers of day 3 embryos in the 15 and 10 μl/embryo groups were increased separately by 0.21 (95%CI: −0.18, 0.59) and 0.45 (95%CI: −0.43, 1.33) compared with the 30 μl/embryo group. There were no significant differences in both these two models. However, in the fully adjusted model (model 3) (adjusted for all covariates), the cell numbers of day 3 embryos in the 15 and 10 μl/embryo groups were significantly increased separately by 0.40 (95% CI 0.00, 0.79; P = 0.048) and 0.78 (95% CI 0.02, 1.54; P = 0.044) compared with the 30 μl/embryo group. For sensitivity analysis, embryo density was converted from a categorical variable to a continuous variable, and the P-value for trend of embryo density was used as a continuous variable in the fully adjusted model. The results (P = 0.018) were consistent with the results when embryo density was classified as a categorical variable.
Table 4. Relationship between embryo density and cell number of day 3 embryos in different models
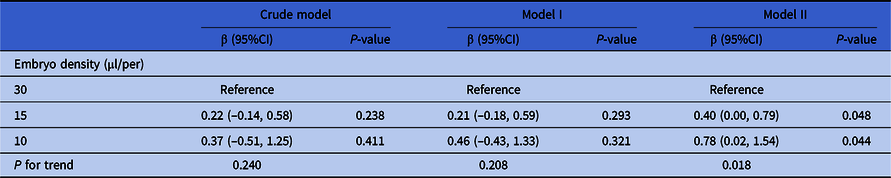
Note: Crude model adjusted for: None. Model I adjusted for maternal age, the number of retrieved oocytes and type of insemination. Model II adjusted for maternal age, paternal age, primary infertility, type of insemination, COS protocol, AMH, the number of retrieved oocytes, length of stimulation and the number of antral follicles. Generalized estimate equation was used. Subject ID = patient unique medical record number (independence).
As shown in Table 5, the crude model was not adjusted for covariates and embryo density did not have significant effect on the clinical results (recorded as ‘whether the transferred embryo implanted successfully or not’, a binary variable) (group 30 µl/per embryo: P = 0.22, group 15 µl/per embryo: reference). Model I was adjusted for paternal age, AMH and type of infertility (significantly risk factors in univariate analysis), and embryo density still did not have a significant effect (group 30 µl/per embryo: P = 0.54, group 15 µl/per embryo: reference). Model II was adjusted for all the confounders and embryo density still did not have an effect on clinical results (group 30 µl/per embryo: P = 0.19, group 15 µl/per embryo: reference).
Table 5. Relationship between embryo density and implanted embryos of day 3 embryos in different models
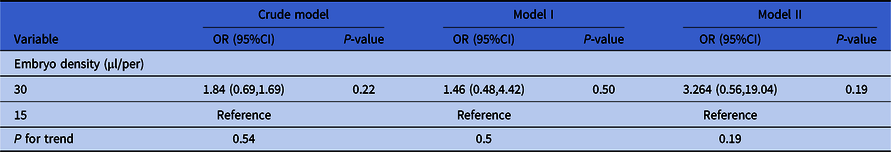
Note: Crude model was not adjusted for covariates. Model I was adjusted for paternal age, type of infertility and AMH. Model II was adjusted for maternal age, paternal age, primary infertility, type of insemination, COS protocol, AMH, number of retrieved oocytes, length of stimulation and the number of antral follicles.
Discussion
In this study, the relationship between embryo density and cell number and clinical result of day 3 embryos was investigated using multivariable logistic regression with adjusted covariates combined with GEE. Covariates were first adjusted in this study to explore the relationship between embryo density and the developmental outcome. Our results showed that in the 30 μl microdrop cleavage medium culture, after hypoxia culture for 48 h, the cell numbers of day 3 embryos in group cultures of two or three embryos together were higher compared with the individual culture of one embryo. After adjusting for covariates of maternal/paternal age, level of AMH, number of antral follicles, type of infertility, COS protocol, length of stimulation, number of retrieved oocytes, number of zygotes (two pronuclei) and type of insemination, the effects became more significant.
Moreover, as embryos cultured in the 30 μl/embryo and 10 μl/embryo groups were also present in the individual and group culture, the benefits were analyzed and compared between group culture and individual culture. However, any benefit was not evident without adjusting any covariates, whereas it became more evident after adjusting for all covariates. All these results indicated that group culture was superior to individual culture concerning the cell numbers of human day 3 embryos. These results are consistent with the findings from Ebner and co-workers (2010) who showed that compaction and blastulation were significantly higher and the blastocyst quality was better when embryos were cultured in groups rather than being cultured individually. Similar results have also been reported in the studies with large culture volumes (>500 μl). For example, Almagor and colleagues. (1996) indicated that communal growth of embryos resulted in significantly improved pregnancy rates compared with individual culture in a drop size of 700 μl. Moessner and co-workers (1995) found that group culture significantly enhanced cleavage rates, embryo scores and mean cell number of day 2 embryos, compared with individual culture, in a drop size of 1000 μl. However, Spyropoμlou and colleagues (1999) reported that group culture produced comparable clinical pregnancies as individual culture.
As the cell number of day 3 embryos in the 15 μl/embryo group was also significantly higher in than the 30 μl/embryo group, embryo density may affect the benefits of group culture. Lehner and co-workers (Reference Lehner, Kaszas, Murber, Rigo, Urbancsek and Fancsovits2017) studied the optimal embryo density for group culture and found that culturing five or six embryos together in a culture volume of 25 μl produced better effects on embryo density than culturing 2–4 or 7–9 embryos together. The optimal embryo density would be different for different species. Gopichandran and Leese (Reference Gopichandran and Leese2006) compared two embryo densities (i.e. 31 μl/embryo vs. 10 μl/embryo) for bovine embryos cultured in a fixed microdrop size of 500 μl, and their results showed that the 10 μl/embryo represents the optimal embryo density because embryos cultured in the 31 μl/embryo group reached the blastocyst stage at a significantly lower rate than zygotes cultured in a 10 μl/embryo group (22.2% vs. 30.3%). Vutyavanich and colleagues (1997) compared four embryo densities (i.e. 10, 2, 1 and 0.67 μl/embryo) for mouse embryos cultured in a fixed microdrop size of 10 μl, and their results showed that the 0.67 μl/embryo size represented the optimal embryo density based on the endpoint of the mean number of inner cell mass (ICM) cells. Hardy and Spanos (Reference Hardy and Spanos2002) or 8–10 feline embryos were cultured in varied microdrop sizes of 20, 50 and 100 μl, and their results showed that the 2.5–2 μl/embryo represented no optimal embryo density based on the endpoint of the blastocyst formation rate. The optimal embryo density for the same species varied according to different culture conditions, which meant that the optimal embryo density would be affected by culture conditions (such as the composition of culture medium, oxygen concentration, and Petri dish type). Kawamura et al. (Reference Kawamura, Chen, Shu, Cheng, Qiao, Behr, Pera and Hsueh2012) concluded that under 20% oxygen, compared with low embryo density, high embryo density would decrease the proportion of mouse embryos that developed to the blastocyst stage on day 4. In contrast, under 5% oxygen, compared with low embryo density, high embryo density increased the total number of blastocyst cells and trophoblast cells. Gopichandran and Leese (Reference Gopichandran and Leese2006) found that under the same embryo density (31 μl/embryo), the well dish significantly improved bovine embryo developmental potential to the blastocyst stage compared with group culture in a conventional dish. Our results indicated that cell numbers of day 3 embryos may affect the benefits of group culture. However, the embryo density did not benefit embryo implantation potential. Many studies using animal models have demonstrated that embryo development and survival is regulated by embryonically and maternally derived growth factors (Hardy and Spanos, Reference Hardy and Spanos2002; Gopichandran and Leese, Reference Gopichandran and Leese2006; Kawamura et al., Reference Kawamura, Chen, Shu, Cheng, Qiao, Behr, Pera and Hsueh2012). These growth factors include epidermal growth factor (EGF), brain-derived growth factors (BDNF), artemin, colony stimulating factor 1(CSF1), fibroblast growth factor (FGF), insulin-like growth factor-I (IGF-I), platelet derived growth factor (PDGF) and glial cell-line derived neurotrophic factor (GDNF) (Kawamura et al., Reference Kawamura, Chen, Shu, Cheng, Qiao, Behr, Pera and Hsueh2012). Growth factors secreted within the embryo and between the embryo could therefore promote optimal early embryonic development and implantation rate in IVF-ET procedures. Further in-depth studies are still needed to investigate the optimal embryo density for group culture under different culture conditions in this study.
So far, both theories that group culture is superior to individual culture, and that there is an optimal embryo density for group culture, have theoretical basis in animal experiments. That is, embryos can secrete autocrine and paracrine factors that promote embryo growth (Hardy and Spanos, Reference Hardy and Spanos2002; Gopichandran and Leese, Reference Gopichandran and Leese2006; Kawamura et al., Reference Kawamura, Chen, Shu, Cheng, Qiao, Behr, Pera and Hsueh2012). In addition, the nutrients in medium are also absorbed and utilized by the embryos, and toxic metabolites (such as ammonium) are also secreted and accumulate in the microdrop. If nutrients are not sufficient or if concentrations of toxic metabolites are too high, embryo development would be impaired (Virant-Klun et al., Reference Virant-Klun, Tomazevic, Vrtacnik-Bokal, Vogler, Krsnik and Meden-Vrtovec2006; Gardner et al., Reference Gardner, Hamilton, McCallie, Schoolcraft and Katz-Jaffe2013). Embryo density is determined by the volume of culture microdrop and the number of cultured embryos (Reed, Reference Reed2012). These two factors also determine the distance between embryos in the microdrop, aggregation of growth-promoting factors and dilution effect with toxic metabolites. Therefore, there would be an optimal embryo density that can effectively aggregate various growth-promoting factors secreted by embryos and reduce the effects of negative factors (Lehner et al., Reference Lehner, Kaszas, Murber, Rigo, Urbancsek and Fancsovits2017).
Embryo density indeed affects the embryo based on in vitro developmental outcome. Each IVF laboratory should explore their own optimal embryo density to obtain the optimal culture method. When exploring optimal embryo density, covariates should be considered for clinical trials. Other culture conditions (such as the oxygen concentration and culture dish type) should be selected carefully in the experimental design. Our results in this study may provide ideas for future research design. However, there were also limitations to this study. First, there was only one endpoint concerned here. As cell number of day 3 embryos could not indicate embryo quality completely, more endpoints (such as pregnancy rate after embryo transfer) should be included in future studies. Second, the sample size was relatively limited. More participants should be included to further verify the results obtained here. Finally, although several covariates had been adjusted, there were still other factors that should be adjusted such as the duration of infertility and number of treatment cycles.
In summary, our results showed that, for the 30 μl microdrop size, culture of embryos both with an embryo density of 15 or 10 μl/embryo increased the cell numbers of day 3 embryos, which may benefit embryo quality compared with individual culture. Alternatively, the cell number of day 3 embryos did not benefit embryo implantation potential compared with an individual culture of 30 μl/embryo.
Acknowledgements
This work was supported by the Chinese Medical Association Special funding Project for Clinical Medical Scientific Research (17020530722), and the Application of Clinical Features of Capital Special Subject (Z171100001017130). The authors thank the medical, paramedical and technical staff of the Department of Reproductive Medicine Center, Peking University People’s Hospital. We thank Xinglin Chen for the advice on the statistics.
Authors contribution
CS, TCS, and XC conceived and designed the experiments. CS, TCS, PW, and HJH performed the experiments. CS, TCS, and XC analyzed the data. CS and TCS prepared the manuscript. All authors contributed to the article and approved the submitted version.
Ethical approval
This study was approved by the Institutional Review Board (IRB) of Peking University Peoples’ Hospital and had been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors declare no financial competing interests.







