Introduction
Yellow nutsedge (YNS) is one of the most widely distributed and troublesome weeds in the world (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1991). In turfgrass, the presence of YNS reduces turfgrass uniformity and visual appeal in home lawns and playability on golf courses and athletic fields. Tubers are the primary form of reproduction, forming at the apical ends of rhizomes during late summer and then sprouting in the spring (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1991). Viable tubers may remain dormant in the soil for multiple years and may sprout repeatedly (Stoller and Sweet Reference Stoller and Sweet1987). Yellow nutsedge is primarily a problem in poorly drained soils or areas that are maintained at high soil water potential (Bendixen and Nandihalli Reference Bendixen and Nandihalli1987; Webster Reference Webster2005; Ransom et al. Reference Ransom, Rice and Shock2009), but YNS can tolerate drought and can also be found in well-drained soils (Day and Russell Reference Day and Russell1955).
Control of YNS with herbicides has been investigated in a variety of settings, including turfgrass. Halosulfuron is a sulfonylurea herbicide that is labeled for postemergence control of YNS in both warm- and cool-season turfgrasses (Blum et al. Reference Blum, Isgrigg and Yelverton2000; Derr Reference Derr2012; Patton and Weisenberger Reference Patton and Weisenberger2013). Nonionic surfactant is required for improved control (Patton and Weisenberger Reference Patton and Weisenberger2012, Reference Patton and Weisenberger2013). Halosulfuron applied once at 36 or 70 g ai ha–1 with 0.25% (v/v) nonionic surfactant in late spring or early summer resulted in 75% to 90% control and is safe on all turfgrass species (Patton and Weisenberger Reference Patton and Weisenberger2013). Halosulfuron applied once on June 25 in North Carolina to YNS in bermudagrass [Cynodon dactylon (L.) Pers.] at 70 g ai ha–1 or twice at 70 g followed by (fb) 70 g ai ha–1 8 wk later resulted in >90% YNS control at 7 or 13 wk after initial treatment (WAI) (Blum et al. Reference Blum, Isgrigg and Yelverton2000). However, in the second year of the study by Blum et al. (Reference Blum, Isgrigg and Yelverton2000), halosulfuron control results were 5% at 13 WAI for a single application but improved to 82% for sequential applications. Fry et al. (Reference Fry, Dernoeden, Upham and Qian1995) reported that a single application of halosulfuron (70 g ai ha–1, application timing not specified) resulted in reductions of YNS ground cover as low as 52% in Kansas, but >97% in Maryland. Neal (Reference Neal1995) reported that a single application of halosulfuron (70 g ai ha–1) resulted in complete control of YNS throughout the growing season when applied at three- to five-leaf stage in 1993. However, YNS was only suppressed for 6 wk in 1994, and a second application 6 WAI resulted in ±70% control (Neal Reference Neal1995).
The protoporphyrinogen oxidase inhibitor sulfentrazone is used for pre- and postemergence control of YNS in select cool- and warm-season turfgrasses (Senseman Reference Senseman2007). Sulfentrazone is readily absorbed by YNS roots and translocated to the foliage. When applied postemergence (28 d after planting, or four- to six-leaf stage) at 336 g ai ha–1, sulfentrazone reduced YNS shoot numbers by 72%, shoot weight by 77%, and belowground biomass (roots, rhizomes, and tubers) by 75% rated 60 d after treatment (Gannon et al. Reference Gannon, Yelverton and Tredway2012). Postemergence applications of sulfentrazone to established YNS require herbicide–root contact for maximum efficacy (Gannon et al. Reference Gannon, Yelverton and Tredway2012; Wehtje et al. Reference Wehtje, Walker, Grey and Hancock1997). Blum et al. (Reference Blum, Isgrigg and Yelverton2000) observed a linear increase in YNS control when sulfentrazone rates were increased from 140 to 562 g ai ha–1. Sequential applications of sulfentrazone at 8-wk intervals of 281 fb 281 or 421 fb 140 g ai ha–1 resulted in >90% control 7 and 13 WAI. In the absence of bermudagrass cover, sequential applications of sulfentrazone resulted in higher control compared to halosulfuron, especially at 13 WAI (Blum et al. Reference Blum, Isgrigg and Yelverton2000). In the second year of the study by Blum et al. (Reference Blum, Isgrigg and Yelverton2000), however, control was 53% at 13 WAI for a single application of sulfentrazone (140 g ha–1) but improved to 93% with sequential applications. Hart et al. (Reference Hart, Mansue and McCullough2008) reported that single application of sulfentrazone (140 g ha–1) resulted in 70% of YNS control when applied on June 20, 2006 in New Jersey, but only 28% when applied on July 2, 2007. Sequential applications 2 WAI improved control to 85% and 75%, respectively (Hart et al. Reference Hart, Mansue and McCullough2008).
Recommendations commonly suggest optimum application timing for YNS as prior to summer solstice (on or around June 22) (FMC Corp. 2019; Monsanto Company 2011; Stoner Reference Stoner2012; Umeda Reference Umeda2015). This recommendation was based on the photoperiodic response of YNS reproduction, that vegetative growth peaks at long photoperiods (16 h), with tuberization initiating with diminished photoperiods (Jansen Reference Jansen1971). However, YNS control based on this recommendation produces inconsistent results and occasional failure (Blum et al. Reference Blum, Isgrigg and Yelverton2000; Hart et al. Reference Hart, Mansue and McCullough2008). In addition, control from a single application of either herbicide are often less consistent and result in lower control compared to sequential applications (Blum et al. Reference Blum, Isgrigg and Yelverton2000; Fry et al. Reference Fry, Dernoeden, Upham and Qian1995; Hart et al. Reference Hart, Mansue and McCullough2008; Neal Reference Neal1995).
Ransom et al. (Reference Ransom, Rice and Shock2009) and Patton and Weisenberger (Reference Patton and Weisenberger2013) suggest that YNS management strategies should focus on prevention with early detection and treatment to prevent exponential growth. New YNS tubers start forming at 4 to 8 wk after shoot emergence (Thullen and Keeley Reference Thullen and Keeley1987). We hypothesized that initial application of herbicides shortly after YNS emergence (late May to early June in Nebraska) could provide more effective control compared to applications near summer solstice. Furthermore, sequential applications are likely required for maximum and consistent YNS control.
Tuber production in YNS is highly prolific (Hauser Reference Hauser1968; Lapham Reference Lapham1985; Shrestha and Grantz Reference Shrestha and Grantz2005; Tumbleson and Kommedahl Reference Tumbleson and Kommedahl1961) and is the primary means of establishment, reproduction, and persistence of YNS populations (Horak et al. Reference Horak, Holt and Ellstrand1987; Mulligan and Junkins Reference Mulligan and Junkins1976; Stoller Reference Stoller1974). Long-term control of YNS is likely influenced by efficacy of applied herbicides to reduce number of new tubers and overall soil tuber bank. In our previous work, halosulfuron (70 g ai ha–1 rate) or sulfentrazone (140 g ai ha–1 rate) applied on June 3, July 15, or June 3 + July 15 in Nebraska resulted in inconsistent YNS control with a single application (Li et al. Reference Li, Sousek, Gaussoin and Reicher2019). Control was based on ground cover in that study, and we could not identify whether YNS surviving after treatment was a result of herbicide failure or of new plants germinating from tubers later in the season. Previous results indicate that efficacy of both herbicides can be highly variable. Research was undertaken to optimize strategies with halosulfuron and sulfentrazone, and to evaluate application and removal (simulated hand-pulling and mowing) strategies that improve YNS control. The hypothesis was that early initial application ahead of tuber formation, with a second application 3 wk later will improve overall YNS control in terms of both reduced ground cover and tuber development. The results of this study will provide a better understanding of herbicide application timing and removal for YNS management in turfgrass.
Materials and Methods
Field Study
Research was conducted at the University of Nebraska–Lincoln’s John Seaton Anderson Turfgrass Research Facility near Mead, NE (41.17° N, 96.47° W), starting in June of 2013, 2014, 2015, and 2016. The location of the plots was adjacent to the study of Li et al. (Reference Li, Sousek, Gaussoin and Reicher2019) with the same source of YNS tubers and identical management. A local YNS population was found in a large shallow waterway near the research facility and was the site of the field study initiated in 2013 and 2014. The YNS in the waterway was distributed in an 8- by 18-m area with approximately 40 to 50 plants m–2 and intermixed with perennial ryegrass. The propagules of YNS from the original site were transplanted to an area adjacent to the waterway in Fall 2014 to expand the trial size and reduce the risk of flooding. Perennial ryegrass (5-Iron Blend; United Seed Inc. Ralston, NE 68127) was seeded concurrently at 330 kg ha–1. Soil type was a Tomek silt loam (fine, smectitic, mesic Pachic Argiudolls) with pH 6.9, 31 g 100 kg–1 soil organic matter, and 240 mg phosphorus 100 g–1 of soil. Each year, 49 kg N ha–1 of polymer-sulfur–coated urea (43-0-0 N-P-K; Van Diest Supply Company, Webster City, IA) was applied in May each year. The study area was mowed weekly at 7.6 cm and was mowed 3 d before and after the application date.
Treatments were arranged as a 2 × 2 × 4 factorial with two herbicides (halosulfuron or sulfentrazone), two application strategies (single or sequential applications), and four application dates (June 3, June 23, July 15, or August 5). The GDDs accumulated based on 10 C base at the time of each application were, 200 to 250, 400 to 550, 650 to 750, and 900 to 1,050 GDD, respectively. Experimental design was a randomized complete block with three replications and 0.9- by 0.9-m plots. Study was repeated on an adjacent previously untreated YNS-infested site in 2014, 2015, and 2016.
Herbicides were applied using a CO2-powered backpack sprayer at 206 kPa in 813 L water ha–2 through a spray boom with an 8002VS flat-fan nozzle (TeeJet Spraying Systems, Glendale Heights, IL). A single application of halosulfuron at 70 g ai ha–1 with a crop oil at 0.25% (v/v) or sulfentrazone at 140 g ai ha–1 were made on June 3, June 23, July 15, or August 5 (± 1 d). Sequential applications were made 3 WAI. Percent YNS cover was visually rated from June 3 through September 17 (± 2 d) in 2013, 2014, 2015, and 2016, and rated June 3 ± 1 d the following year. Percent YNS control was calculated as [(mean percent YNS cover of control plots – mean percent YNS cover of rated plots)/ mean percent YNS cover of control plots] × 100%. For brevity YNS control is reported for the growing season (September 17) and the following spring (June 3).
Data from the field study were analyzed as a generalized linear mixed model using PROC GLIMMIX in SAS (Version 9.4; SAS Institute Inc., Cary, NC). Mean separation was performed using Fisher’s protected LSD at P < 0.05. To better understand inconsistent results among years, orthogonal contrasts were used to compare means of ground cover among years (Gomez and Gomez Reference Gomez and Gomez1984; Nogueira Reference Nogueira2004). Three orthogonal contrasts were performed based on the maximum number statistically allowed (Nogueira Reference Nogueira2004).
Greenhouse Study
Untreated tubers harvested from the same population as the field study were hand-separated, and tubers with fresh biomass of 56.2 to 65.5 mg tuber–1 were used. One YNS tuber (“mother tuber”) per treatment was planted at a depth of 2.5 cm in a 7-L pot with a mixture of 85% sand (Lyman-Richey Corp., Omaha, NE) and 15% reed sedge peat with pH 5.6, 87 g 100 g–1 organic matter, and 4 mg phosphorus 100 g–1 (Dakota Peat & Equipment, East Grand Forks, MN). Greenhouse temperatures were 34 C/24 C day/night with a 16-h light photoperiod with supplemental lighting at the beginning and end of the photoperiod with a mean peak-light-irradiance of 1,000 µmol m–2 s–1. Soil moisture in pots was maintained by hand irrigation twice daily during germination and once daily post-germination during the duration of the study. Pots received the same amount of polymer-sulfur–coated urea as plots in the field study (49 kg N ha–1). All experimental units except the unmowed control were clipped with scissors at 7.6 cm weekly. Mowing during the week of herbicide application was conducted 3 d before and after the application date.
Treatments were arranged in a 2 × 2 × 3 factorial with two herbicides (halosulfuron or sulfentrazone), two application strategies (single or sequential applications applied 3 WAI) and three initial application dates [2, 4, or 6 wk after emergence (WAE)]. Herbicide treatments were applied using a single-tip chamber sprayer (DeVries Manufacturing Corp., Hollandale, MN) fitted with an 8001E flat-fan nozzle (TeeJet Spraying Systems, Glendale Heights, IL) calibrated to deliver 813 L ha–1 carrier volume at 207 kPa.
In addition to the herbicide treatments, we reserved 20 pots that were not treated with herbicides. Among these pots, three sets (four replications each) underwent apical meristem removal (AMR) using scissors to cut directly below the basal bulb where the apical meristem is located. The AMR treatment was designed to simulate hand-pulling while minimizing treatment variability by ensuring all plants were cut at the same location. The timing of AMR treatment coincided with the herbicide application timing at 2, 4, or 6 WAE. The remaining eight pots were untreated check pots under weekly mowing at 7.6 cm (mowed control) or no mowing (unmowed control).
Experimental design was a completely randomized design with four replications. Experimental units were randomly rotated within the greenhouse bench on a weekly basis during the experimental period to minimize variation and to reduce edge effects in the greenhouse. Two trials were conducted, with the first trial initiated in May 2017 and concluded in September 2017, and the second trial initiated in May 2018 and concluded in September 2018. In this setting, YNS plants matured to the 3- to 5-leaf stage 2 WAE, 5- to 8-leaf stage 4 WAE, and 11- to 12-leaf stage 6 WAE.
All data were recorded 4 wk after the last application. Yellow nutsedge plants were hand-washed to separate tubers, roots, and rhizomes. Roots and rhizomes were placed in a forced-air dryer at 55 C for 72 h before total root and rhizome dry mass per pot was recorded. Average fresh tuber weight per pot was measured after tubers were submerged in water for 24 h and then air-dried for 2 h to remove surface water. Re-emergence was recorded, defined as any new shoot observed between when the last application was made and when experimental unit was destructively sampled. All YNS plants were alive when receiving treatment or when the experimental unit was destructively sampled. The mother tuber in each pot was retrieved at the time of sampling. The mother tubers were then planted in pots under identical greenhouse growing condition to test re-sprouting.
Data from the greenhouse study were analyzed as a general linear model, which assumes homogeneous variance. However, variance for experimental trials tends to be heterogeneous, and thus variance for each trial was modeled separately using PROC GLLIMIX in SAS to account for heterogeneity, and data for the two trials were combined in one analysis (Littell et al. Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger2006). Mean separation was performed using Fisher’s protected LSD at P < 0.05.
The three AMR treatments, and the mowed and unmowed controls were not a part of the factorial design that included herbicide treatment, timing, and strategy. In AMR treatments, no regrowth occurred upon cutting. No herbicide or AMR treatment were applied to the mowed and unmowed controls, and the controls were destructively sampled 13 WAE. Means from these five treatments were calculated, and data were excluded from the model used to analyze and compare results from herbicide treatment. Means between AMR treatments and the controls can be statistically compared among each other, or with means of herbicide treatments using linear contrasts (Gomez and Gomez Reference Gomez and Gomez1984).
Results and Discussion
Field Study
Herbicide main effect was significant on the September 17 rating date. Control of YNS with halosulfuron resulted in 91% control, which was higher than control with sulfentrazone (82%), regardless of timing or strategy. Admittedly, single application of sulfentrazone was at the low recommended rate of 140 g ai ha–1, whereas halosulfuron was applied at the high recommended rate of 70 g ai ha–1. A direct comparison should not be made between herbicides, as the low rate likely reduces the efficacy of a single sulfentrazone application.
Averaged across years, herbicides, and timings, sequential applications resulted in 91% YNS control on September 17 compared to 82% with a single application. When rated on June 3 the following year, sequential applications resulted in 78% YNS control compared to 63% for a single application. Our results agree with previous studies (Blum Reference Blum, Isgrigg and Yelverton2000; Neal Reference Neal1995; Patton and Weisenberger Reference Patton and Weisenberger2013), who reported two postemergence applications to be more effective and consistent than a single application.
Averaged over strategy, application of herbicides on June 3 and July 15 resulted in the highest control in 3 of the 4 yr of the study (Table 1). GDDs at 10 C base calculated for the June 3 application date indicated the initial application was made between 200 and 250 GDD. The traditionally recommended summer solstice timing (approximately June 22, 400 to 550 GDD) resulted in lower control in 2013 and 2015 compared to the early application (June 3), but not in 2014 and 2016. The consistent control results from the August 5 application were likely due to the short interval between application date and September 17 rating date, given that vegetative growth of YNS was slowing with shortening day length (Bell et al. Reference Bell, Lachman, Rahn and Sweet1962; Jansen Reference Jansen1971; Williams Reference Williams1982). Herbicide timing was not significant when YNS cover was rated in June the year following the application.
Table 1. Percent yellow nutsedge control in the field study rated on September 17 in 2013 through 2016. Single or sequential (3 wk after initial) applications of halosulfuron or sulfentrazone were made on June 3, June 23, July15, or August 5. Cumulative growing degree days (GDD) were based on 10 C. Means were calculated from three replications, two application strategies, and two herbicides.
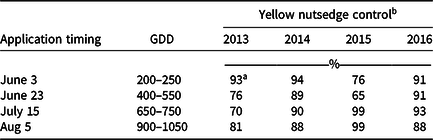
a Fisher’s protected LSD (P < 0.05) = 11 for means across columns at the same application timing. LSD (P < 0.05) = 8 for means within columns in the same year.
b Percent control calculated as [(mean percent yellow nutsedge cover of control plots – mean percent yellow nutsedge cover of rated plots)/ mean percent yellow nutsedge cover of control plots] × 100%. Percent yellow nutsedge covers were 35%, 50%, 55%, and 45% in 2013, 2014, 2015, 2016, respectively.
Later applications were effective in 2015 when rated in September (Table 1). This is likely due to unseasonably cool temperatures in late spring of 2015 delaying YNS development. Growth stage at the June 3 application was the one- to two-leaf stage, and many plants did not emerge until late June. Limited leaf surface area, a vegetative barrier created by surrounding perennial ryegrass that thrived under the cooler temperatures, and late-season emergence after herbicides were applied might have reduced control from both herbicides. Therefore, optimum application timing for YNS should be based on development stage of the plants instead of a fixed date or time window. Optimal application timing was further examined in the greenhouse study to determine whether application made on July 15 can also be an effective application timing, as it provided highest control in 3 of 4 yr in the field study.
Control in 2014 and 2016 ranged between 88% and 94%, whereas control in 2013 and 2015 ranged from 65% to 99% regardless of application timing (Table 1). Three orthogonal contrasts were performed to further investigate the inconsistencies among years. When herbicides, application timing and strategy were combined, no differences were observed comparing September 17 and June 3 results between 2013 and 2015, and between 2014 and 2016. However, YNS control results 2014/2016 combined were greater than 2013/2015 combined, both in September (91% vs. 83%) and June (72% vs. 62%). This study was started in 2013 and used YNS from the original site that was transplanted to a research plot adjacent to the previous study site in Fall 2014, while the study remained in progress that year at the previous site. The first year the study was conducted on the new plots was 2015. Therefore, 2013 and 2015 were years when YNS started to undergo routine mowing at 7.6 cm weekly, whereas 2014 and 2016 were the second year of routine mowing at 7.6 cm. Routine mowing at 3.8 cm once a week reduce YNS shoot numbers and lateral spread beginning 3 to 8 wk after initial mowing (Summerlin et al. Reference Summerlin, Coble and Yelverton2000), and this may have affected our results. Higher overall YNS control was also observed the following June from applications made 2014 (82%) and 2016 (76%) compared to 2013 (65%) and 2015 (58%). The effects of mowing on YNS growth were further examined in the greenhouse study.
Tubers could not be counted in these field plots, so it was not possible to differentiate YNS surviving herbicide application from that resulting from late-season emergence. It is possible that our inconsistent results were caused by later YNS emergence from tubers after applications. Furthermore, root and rhizome formation, as well as the number of new tubers post application could not be evaluated in the field study, where the initial tuber population and developmental stage were not known. These questions were examined in the subsequent greenhouse study, which allowed us to better evaluate the effect of herbicide, timing, and strategy on YNS root growth, tuber development, and re-emergence.
Greenhouse Study
Herbicide control
Applications made at 2 WAE produced the lowest root dry mass, almost regardless of herbicide or strategy (Table 2). Sequential applications of either herbicide also resulted in lower root dry mass than a single application at all application timings. Timing × herbicide and herbicide × strategy were the highest-order interactions for rhizome dry mass. Rhizome dry mass was generally lower when either herbicide was applied 2 WAE compared to applications made at 4 or 6 WAE (Table 3). When application timings were combined, a single application of halosulfuron resulted in the highest amount of rhizome dry mass among all herbicide treatments (Table 3). Our results suggest that early application of herbicide can effectively reduce or terminate rhizome formation. Such an effect is important in controlling the reproduction of YNS, as rhizomes constitute one means of YNS reproduction, as well as the location at which tubers are formed at the apical ends (Bell et al. Reference Bell, Lachman, Rahn and Sweet1962; Wills Reference Wills1977; Wills et al. Reference Wills, Hoagland and Paul1980). Similar to these results, the number of new tubers formed was generally lower when herbicide application was made at 2 WAE, compared to application made at 6 WAE (Table 2). Application timing affected tuber weight. When herbicide and strategy were combined, tuber weights were 6, 14, and 23 mg when herbicides were applied at 2, 4, and 6 WAE, respectively (data not shown). Tuber weights were also lower in herbicide-treated YNS than in the mowed controls (42 mg). Increased tuber weight, while having little known effect on germination rate, increased plant weights of YNS (Stoller et al. Reference Stoller, Nema and Bhan1972). Therefore, any treatment reducing tuber weights could help with long-term YNS control by reducing total biomass the following season. The 2-WAE timing in the greenhouse is similar to the June 3 application timing in terms of YNS growth stage (three- to five-leaf stage), whereas the 6-WAE timing was similar to the July 15 application timing. Results from the greenhouse study further confirmed that June 3 is the optimum application date in the field when taking into consideration of root, rhizome, and tuber development.
Table 2. Root dry mass of yellow nutsedge and the number of new tubers formed when single or sequential (3 wk after initial) applications of halosulfuron or sulfentrazone were applied in the greenhouse at 2, 4, or 6 wk after emergence (WAE). All samples were collected 4 wk after the last application was made. Means were calculated from four replications and two trials.
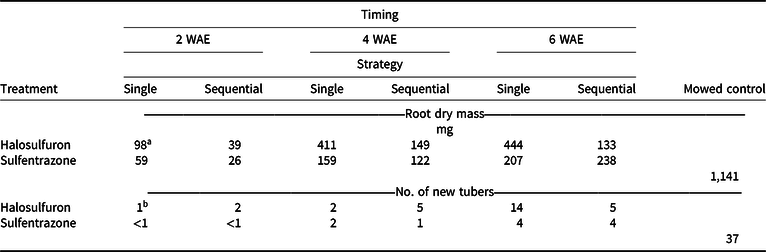
a LSD (P < 0.05) = 176 for means across columns. LSD (P < 0.05) = 82 for means within columns. Mowed control is presented for reference only.
b LSD (P < 0.05) = 3 for means across columns with the same herbicide treatment. LSD (P < 0.05) = 4 for means within columns at the same application timing with the same strategy. Mowed control is presented for reference only.
Table 3. Rhizome dry mass of yellow nutsedge when single or sequential (3 wk after initial) applications of halosulfuron or sulfentrazone were applied in the greenhouse at 2, 4, or 6 wk after emergence (WAE). All samples were collected 4 wk after the last application was made. Means were calculated from four replications, two application strategies, and two trials.
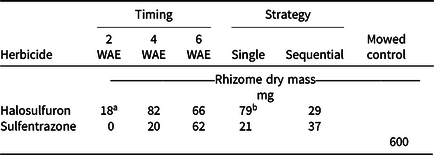
a LSD (P < 0.05) = 41 for means across columns with the same herbicide treatment. LSD (P < 0.05) = 25 for means within columns at the same application timing. Mowed control is presented for reference only.
b LSD (P < 0.05) = 17 for means across columns with the same herbicide treatment. LSD (P < 0.05) = 25 for means within columns using the same strategy. Mowed control is presented for reference only.
All main effects and interactions were significant for re-emergence. No shoot re-emergence was found when making sequential applications (Table 4). All herbicide treatments in the greenhouse study were able to inhibit new shoot growth, within 24 h for sulfentrazone and 10 to 14 d for halosulfuron. Based on these results, regrowth of YNS observed in the field study was likely a result of late-season YNS emergence from tubers, especially in plots treated with sequential applications. This helps to explain the inconsistent results turfgrass managers often report regarding herbicide control of YNS in the field. Yellow nutsedge tubers are reported to sprout at least three separate times when germinated at 25 C in darkness between vertical sheets of absorbent paper moistened with tap water (Stoller et al. Reference Stoller, Nema and Bhan1972). However, unlike results reported by Stoller et al. (Reference Stoller, Nema and Bhan1972), mother tubers collected at all application timings in our study were hollow and rotten, and failed to re-sprout when planted in greenhouse pots. The first sprout expended more than 60% of tuber dry weight, carbohydrate, oil, starch, and protein under growing conditions used by Stoller et al. (Reference Stoller, Nema and Bhan1972). It is not clear if the soil media likely increased microbial activity, or other growing conditions in our study accelerated the reserve drawdown after initial sprouting compared to Stoller et al. (Reference Stoller, Nema and Bhan1972). The hollow and rotten mother tubers found as early as the 4 wk after 2 WAE treatment suggest that most reserves were expended.
Table 4. Re-emergence, defined as the number of newly emerged shoots, when single or sequential (3 wk after initial) applications of halosulfuron or sulfentrazone were applied to yellow nutsedge in the greenhouse at 2, 4, or 6 wk after emergence (WAE). All samples were collected 4 wk after the last application was made. Means were calculated from four replications and two trials.
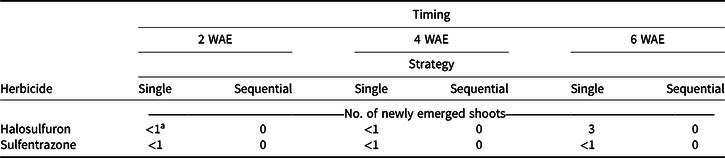
a LSD (P < 0.05) = 2 for means across columns with the same herbicide treatment. LSD (P < 0.05) = 1 for means within columns at the same application timing with the same strategy.
Physical control
The lowest root dry mass and tuber weight were observed when AMR was performed at 2 WAE causing rhizome, new tuber formation, and re-emergence to essentially cease (Table 5). Linear contrasts between means of AMR and herbicide treatments showed that both methods were equally effective at 2 WAE. In our study, AMR simulated physical removal (i.e., pulling), and caused significant reduction of root and rhizome dry weight, number of new tubers, and tuber weight. Similar results were reported by Stoller et al. (Reference Stoller, Nema and Bhan1972), who observed a significant reduction in root and rhizome weight at 43 and 91 d after planting when YNS was severed at the apical meristem. In that study, newly formed tuber weight was also lower in plants severed below the apical meristem compared to whole plants at 91 d after planting. The results combined suggest that hand-pulling can be an appropriate physical control method when YNS populations are young.
Table 5. Root and rhizome dry mass, and tuber weight (mg), number of new tubers formed, and re-emergence of yellow nutsedge. a Apical meristem removal (AMR) was performed at 2, 4, or 6 wk after emergence (WAE). All samples were collected 4 wk after the last application was made, or at 13 WAE in mowed or unmowed control. Means were calculated from four replications and two trials.
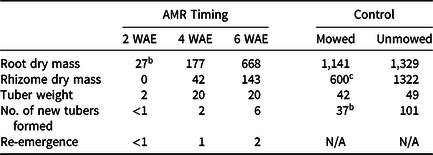
a Re-emergence was defined as any new shoot observed between when the last application was made and when experimental unit was destructively sampled.
b Excluding controls, LSD (P < 0.05) = 150, 42, and 18 for root dry mass, rhizome dry mass, and tuber weight, respectively. LSD (P < 0.05) = 3 and 1 for the number of new tubers formed and the number of newly emerged shoots, respectively.
c Results were significantly different at P < 0.05 when means of mowed and un-mowed controls were compared using linear contrast.
In control pots, mowing reduced both rhizome dry mass and the number of new tubers formed. Root dry mass and tuber weight were not affected by mowing. Summerlin et al. (Reference Summerlin, Coble and Yelverton2000) reported a complete inhibition of tuber formation, and a reduction in shoot number and lateral spread when mowed at 3.8 cm weekly or 1.3 cm three times a week. In our study, the 7.6-cm weekly mowing height caused a 55% reduction in rhizome dry mass and a 63% reduction in the number of new tubers formed compared to the unmowed control (Table 5). As a result of the more favorable growing conditions in the greenhouse, YNS developed more rapidly and reached each leaf stage approximately 1 wk earlier than in reported field trials. Faster greenhouse development may reduce the effect of routine mowing on rhizome dry mass and new tuber production. We expect that a lower mowing height and a higher mowing frequency, such as the 1.3 cm three times a week reported by Summerlin et al. (Reference Summerlin, Coble and Yelverton2000), would further reduce YNS growth. Our results suggest that physical control such as routine mowing can reduce rhizome development and the number of new tubers formed, and therefore can be an effective method to reduce YNS growth. The inhibiting effects of routine mowing on rhizome and tuber development helped explain the difference in herbicide efficacy due to maintenance, which we speculated in the field study.
Thullen and Keeley (1975) found that separating YNS plants at 2-wk intervals from the mother tuber allowed sprouting of all buds. However, in our study re-emergence after AMR was only found in one out of the four replications at 2 WAE. Similar to those retrieved from herbicide-treated pots, mother tubers were found to be hollow and rotten, and failed to re-sprout. In our study, YNS plants were detached from below the apical meristem, not at the tuber buds as in the Thullen and Keeley (1975) study. Pulling in the field is more likely to cause detachment of YNS plants right below the crown rather than at the tuber buds. Therefore, pulling may not cause additional sprouting and can be a helpful physical control technique when YNS populations are limited.
Results from field and greenhouse studies indicate that halosulfuron and sulfentrazone reduce YNS ground cover, root and rhizome dry mass, the number of new tubers, tuber weight, and re-emergence. Either herbicide should be applied at 2 WAE when YNS is in three- to five-leaf stage or 200 to 250 GDD for maximum control. Early application likely resulted in herbicide activity before tuber formation, which starts as day length begins to reduce (Jansen Reference Jansen1971). GDDs can be used to better predict the initial application timing. However, additional research is needed in different geographical regions to validate the GDD range reported here. Sequential applications of either herbicide at 3-wk interval can help improve control consistency. Yellow nutsedge plants visible after herbicide treatments are likely not a result of re-emergence from the same tuber but late germination from previously ungerminated tubers. Therefore, sequential applications of either herbicide within a 3- or 6-wk (Li et al. Reference Li, Sousek, Gaussoin and Reicher2019) interval is recommended for optimal YNS control. Lawn care professionals are often requested to attempt control late in the summer when clients start to see larger plants. Our findings suggest that top growth of YNS can be controlled, but long-term control is probably unaffected by late-summer application, as more tubers will form when treatments are made late in the season.
Mowing can be an effective method to reduce YNS growth. Mowing at 7.6 cm weekly reduced YNS rhizome dry mass by 55% and number of new tubers formed by 63%. Physical removal of YNS plants such as pulling can be an effective method when controlling young YNS in low populations. End-users can maximize YNS control by integrating early herbicide treatments and cultural practices such as mowing and hand-pulling.
Acknowledgments
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors. No conflicts of interest have been declared.








