Introduction
Wild oat is a problematic global weed species causing crop yield reductions in agronomic crops and horticultural cropping systems (Beckie et al. Reference Beckie, Francis and Hall2012; Llewellyn et al. Reference Llewellyn, Ronning, Clarke, Mayfield and Walker2016). The intensive and continual use of herbicides to control wild oat has led to the widespread evolution of herbicide resistance (Beckie et al. Reference Beckie, Hall, Meers, Laslo and Stevenson2004; Broster et al. Reference Broster, Koetz and Wu2013; Mengistu et al. Reference Mengistu, Messersmith and Christoffers2003) and associated pleiotropic effects such as altered temperature requirements for seed germination and phenology of seed production initiation (Lehnhoff et al. Reference Lehnhoff, Keith, Dyer, Peterson and Menalled2013a). In Australia, wild oat is the third most economically damaging weed species, costing grain growers US$20.8 million in annual revenue loss (Llewellyn et al. Reference Llewellyn, Ronning, Clarke, Mayfield and Walker2016). The widespread and diverse adaptations within wild oat populations highlight the need to monitor the evolution and spread of herbicide resistance and identify effective means of control. One major impediment to conducting research on wild oat is seed coat-mediated dormancy (Atwood Reference Atwood1914; Hsiao et al. Reference Hsiao, McIntyre and Hanes1983).
Wild oat seed can exhibit combinational dormancy type 1: physical (PY) plus physiological (PD; Baskin and Baskin Reference Baskin and Baskin2004; Soltani et al. Reference Soltani, Baskin and Baskin2017). The PY dormancy occurs when the embryo is constrained by its surrounding structures and a water-impermeable seed coat impedes imbibition (Baskin and Baskin Reference Baskin and Baskin2004; Bewley Reference Bewley1997; Finch-Savage and Leubner-Metzger Reference Finch-Savage and Leubner-Metzger2006). When the embryos are removed from the seed coat, they are not dormant. In contrast, PD is a more complex type of dormancy, which is classified as deep, intermediate and non-deep; moreover, these different categories are classified into different types (Baskin and Baskin Reference Baskin and Baskin2004; Finch-Savage and Leubner-Metzger Reference Finch-Savage and Leubner-Metzger2006). In deep dormancy, embryos excised from the seeds do not grow or may produce abnormal growth and several months of stratified cold or warm temperature exposure is required for germination. In non-deep dormancy, embryos excised from the seeds produce normal seedlings; giberellic acid (GA) treatment can break this dormancy as well as scarification, after-ripening in dry storage, and cold or warm stratification depending on species (Baskin and Baskin Reference Baskin and Baskin2004). In non-deep PD type 1 dormancy, seeds can germinate initially only at low temperatures, but the maximum temperature at which seeds will germinate increases as dormancy break progresses (Soltani et al. Reference Soltani, Baskin and Baskin2017).
Accordingly, the following methods have been identified as being effective techniques for overcoming dormancy in wild oat seeds. Methods that overcome PD dormancy include altering temperature and photoperiod regimes for germination (Somody et al. Reference Somody, Nalewaja and Miller1984), adjusting the osmotic potential of the seed and light conditions (Boyd and Van Acker Reference Boyd and Van Acker2004), exposing the seed to ammonia gas (Cairns and De Villiers Reference Cairns and De Villiers1986), presoaking the seed in potassium nitrate solution (Hilton Reference Hilton1985), application of sodium hypochlorite and hydrogen peroxide in combination with GA; (Hsiao and Quick Reference Hsiao and Quick1984), application of a variety of soluble sugars and GA (Foley Reference Foley1992), as well as other reagents (Beckie et al. Reference Beckie, Francis and Hall2012; Kępczyński et al. Reference Kępczyński, Wójcik and Dziurka2021; Saini et al. Reference Saini, Bassi and Spencer1986). Methods that overcome PY dormancy include exposure of the seed to high-pressure gas (Hoffmann Reference Hoffmann1961), manual removal of lemma and palea (Kommedahl et al. Reference Kommedahl, DeVay and Christensen1958), seed dipping in diluted sulfuric acid, and cold stratification treatment (Shahvand et al. Reference Shahvand, Miri and Bagheri2015). Overall, these studies described variable germination outcomes depending on the level of PD plus PY dormancy, efficacy of the treatment applied, and the wild oat population.
Wild oat seed can remain dormant in the soil seedbank for up to 4 yr, although most of the population germinates within the first 2 yr (Conn et al. Reference Conn, Beattie and Blanchard2006; Mahajan and Chauhan Reference Mahajan and Chauhan2021). Additionally, wild oat dormancy in the field can be affected by abiotic and biotic stresses such as crop competition, moisture availability, temperature, and seed pathogens (Lehnhoff et al. Reference Lehnhoff, Miller, Brelsford, White and Maxwell2013b; Mahajan and Chauhan Reference Mahajan and Chauhan2021; Maqbool et al. Reference Maqbool, Naz, Ahmad, Nisar, Mehmood, Alwahibi and Alkahtani2020; Ņečajeva et al. Reference Ņečajeva, Bleidere, Jansone, Gailīte and Ruņģis2021; Peters Reference Peters1982; Sahil et al. Reference Sahil, Loura, Raymont and Chauhan2020). Lehnhoff et al. (Reference Lehnhoff, Miller, Brelsford, White and Maxwell2013b) found that the maternal environment of wild oat had a negative effect on the viability of the seed located below the crop canopy; these effects were potentially driven by a combination of crop competition and changes in seed pathogens. Additionally, Peters (Reference Peters1982) showed that temperature and soil moisture conditions affected dormancy; the seeds from plants grown under no water-stressed environments at 15C were more likely to have more dormancy compared with seeds from plants grown under water-stressed conditions at 20 C.
Multiple studies had demonstrated that aeration and moisture need to be available to the seed embryo for wild oat germination, with puncturing of the seed coat effectively overcoming PY dormancy in wild oat (Foley Reference Foley1987; Hay Reference Hay1962; Hsiao et al. Reference Hsiao, McIntyre and Hanes1983; Raju et al. Reference Raju, Hsiao and McIntyre1988). As a result, the puncturing of the mid-dorsal side of the seed is a highly effective and most common method used for breaking seed coat dormancy in wild oat studies (Bourgeois et al. Reference Bourgeois, Kenkel and Morrison1997; Cruz-Hipolito et al. Reference Cruz-Hipolito, Osuna, Dominguez-Valenzuela, Espinoza and De Prado2011; Mansooji et al. Reference Mansooji, Holtum, Boutsalis, Matthews and Powles1992; Ņečajeva et al. Reference Ņečajeva, Bleidere, Jansone, Gailīte and Ruņģis2021; Owen and Powles Reference Owen and Powles2009). However, puncturing individual seeds is time-consuming, making wild oat research less efficient. For example, wild oat resistance testing using classical pot assays demand consistent germinable seed populations. Accordingly, this study aims to develop an efficient and effective mechanical scarification technique to break seed dormancy and induce wild oat germination using a commercially available seed thresher.
Materials and Methods
Seed Collection and Multiplication
At maturity (November 2017), eight wild oat populations were collected from commercial dryland agricultural farms in the Western Australian grain belt (Table 1). To eliminate maternal effects that can affect seed dormancy, 20 seeds from each population were germinated in May 2018 in plastic trays containing 1% (wt/vol) agar dissolved in deionized water. Each seed was hand-punctured to aid germination (Ian and Leonard Reference Ian and Leonard1982). Trays were transferred to a growth cabinet at optimal germination conditions of 11 C (Naylor and Fedec Reference Naylor and Fedec1978) with no light. At the four-leaf stage, 20 seedlings were transplanted at the University Western Australia Shenton Park Field Station, Perth, WA, Australia (31.95030556°S, 115.79375000°E) at 1-m intervals in the center of 1.5-m-wide plastic-lined rows to eliminate weed competition. Seedlings were fertilized with 6 g of NPK Blue Special™ (N 12% [NH2 8%, NH4 1.9%, NO3 2.1%], P 5.2%, K 14.1%, S 6%, Mg 1.2%, Ca 4.3%, B 0.02%, and Fe 0.08%; CSBP Ltd, Kwinana, WA, Australia) and 2 g Yates Flowtrace™ (Fe 24%, Cu 0.75%, Mn 0.5%, Zn 0.2%, Mo 0.04%, and B 0.033%; Yates Australia, Padstow, NSW, Australia). Six weeks after emergence, all plants were fertilized with 4 g of ammonium sulphate (N 21%, S 24%) to maintain growth. In November 2018, the mature seed was hand-collected and stored in a laboratory at room temperature (21 ± 3 C) until the start of the first experiment.
Table 1. Location of wild oat populations used in this study collected from the Western Australian grain belt in 2017.
Experiment 1: Mechanical Scarification
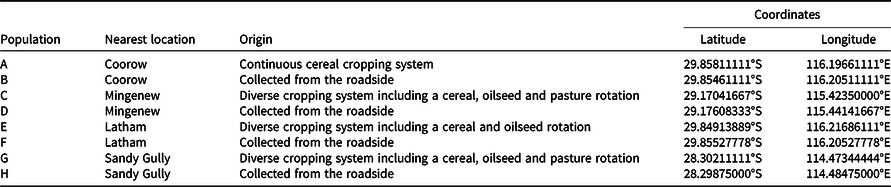
The aim of this experiment was to identify the most effective mechanical settings to break physical dormancy in a randomly selected population of wild oat (population ‘e’, Table 1). Seed germination was assessed after three manual treatments and four threshing treatments (varying in revolutions per minute [rpm]) and duration [s]). The treatments applied are outlined in Table 2. The thresher used in this study was the Haldrup LT-15 laboratory benchtop thresher (Haldrup, Germany). As per the manual specifications, the LT-15 thresher has a drum diameter of 200 mm containing three blades of 70-mm width. The drum rotational speed can be regulated from 150 rpm to 1,500 rpm. The rotational speed was verified and set with a laser tachometer (Nidec-SHIMPO DT-2100). Most laboratories have a similar mechanical thresher that can be used for the same purpose. There were three replicates of 50 seeds each per treatment. The experiment was initially conducted in January 2020 and repeated in July 2020. The condition of the seeds as affected by the treatments is shown in Figure 1.
Table 2. Treatments used for determining the most appropriate settings for the mechanical scarification of wild oat.

a Abbreviation: rpm, revolutions per minute.

Figure 1. Wild oat seed after scarification. T1 is intact seed with husk (lemma and palea), T2 is punctured seed with husk, T3 is manual extraction of caryopsis plus needle puncture, and T4 is manual extraction of caryopsis. Mechanical scarification treatments are T5: 1,350 rpm for 5 s, T6: 1,500 rpm for 5 s, T7: 1,350 rpm for 10 s, T8: 1,500 rpm for 10 s.
Following mechanical scarification treatment (Table 2), all wild oat seeds were surface-sterilized by individually immersing each seed in a 1:16 ratio of 12.5% NaOCl solution for 2 s, followed by rinsing with deionized water. Immediately after sterilization, the seeds were placed in rectangular plastic trays containing 1% (wt/vol) agar dissolved in deionized water (three replicates of 50 seeds each) for germination. The trays were placed in a growth cabinet at optimal germination conditions of 11 C (Naylor and Fedec Reference Naylor and Fedec1978) with no light for 15 d. Seeds were then assessed for germination, with the criterion being visible protrusion of the radicle from the seed. To ensure the nongerminated seed was viable, all nongerminated seeds were incubated in 1% (wt/vol) solution 2,3,5-triphenyl tetrazolium chloride for 48 h at 30 C. Evidence of pink staining indicated that the seed was viable (Verma and Majee Reference Verma and Majee2013); nonviable seeds were omitted from the calculations.
Experiment 2: Replication of Mechanical Scarification Results on Multiple Wild Oat Populations
To verify the value of using mechanical scarification to break physical dormancy, the most effective treatment from Experiment 1 (T6) was repeated using eight wild oat populations collected from the Western Australian grain belt (Table 1). These populations came from fields with different cropping rotations and management practices. Each population was scarified in the LT-15 Haldrup thresher as previously described at 1,500 rpm for 5 s (i.e., T6, Table 2). This treatment was replicated three times per population (50 seeds each). To serve as a comparative control, each population (three replicates) was also manually punctured as previously described (50 seeds each; T2). Immediately after applying the mechanical scarification treatment, the frequency of embryo damage was assessed for each population by visual inspection of a fragmented or missing embryo (Figure 2). Treated seeds were placed on agar to assess germination responses of each treated population and assessed 15 d after imbibition as previously described. This experiment was conducted immediately after Experiment 1 in January 2020 and repeated in July 2020. The seeds that did not germinate were evaluated using 2,3,5-triphenyl tetrazolium chloride, with nonviable seed omitted from the calculations as previously indicated.

Figure 2. Caryopsis at different levels of seed coat and embryo damage can be observed: (A) intact caryopsis; (B) caryopsis with ideal damage to the seed coat located on the distal part to the embryo where the endosperm is slightly exposed, allowing for water movement to the embryo to trigger germination; (C) the embryo has been destroyed; (D) successful germination after the application of treatment T6 (1,500 rpm for 5 s); (E) imbibed caryopsis which failed to germinate after the application of treatment T8 (1,500 rpm for 10 s).
Experiment 3: Effect of Mechanical Scarification on Wild Oat Growth
To quantify the effect of mechanical scarification on wild oat seedling growth, the mechanical scarification technique (T6) was compared with the manual puncturing technique (T2; Table 2). Seeds of similar size in each population (Table 1) were germinated in agar medium as previously described. Six germinated seedlings for each population, harvest date and treatment were transplanted into seedling trays that contained potting mix (50% fine composted pine-bark, 20% coco peat, 30% brown washed river sand). Seedlings were maintained in a controlled-environment cabinet (Conviron A-1000) set at a 12-h photoperiod of cool white fluorescent light (30 to 60 μmol m−2 s−1), at temperatures of 20 C day and 12 C night with 70% relative humidity. At 3 and 24 d after emergence, all six plants per treatment were cut at the soil surface and dried for 72 h at 65 C to assess dry biomass. The relative growth rate (RGR) was calculated as follows (Hunt 1982):
where M1 and M2 are the dry biomass of the sample at times 1 and 2, respectively, and T1 and T2 are the harvest times. The experiment was initially conducted in February 2020 and repeated in August 2020.
Experiment 4: Comparing Mechanical Threshing and Known Methods of Increasing Wild Oat Germination
The mechanical scarification of wild oat was compared with other known seed germination methods to quantify its effectiveness, including chemical scarification with H2SO4, partial endosperm removal, sandpaper scarification, immersion of the seed in sodium nitroprusside (NO donor SNP) solution, and untreated control (Table 3). These seed germination techniques were applied to population ‘e’ (Table 1) using the methodology outlined in Table 3. There were three replicates for each treatment (50 seeds each). The experiment was conducted in parallel to Experiment 3 in February 2020 and repeated in August 2020. To validate the germination results, all ungerminated seeds were treated with 2,3,5-triphenyl tetrazolium chloride to assess viability as previously described.
Table 3. Methods used to compare the mechanical scarification technique and other commonly used methods to break the dormancy of wild oat. a

a Abbreviation: SNP, sodium nitroprusside.
b All treated seeds were surface-sterilized by individually immersing each seed in a 1:16 ratio of 12.5% sodium hypochlorite (NaOCl) solution for 2 s, followed by rinsing with deionized water. Immediately after sterilization, the seeds were placed in rectangular plastic trays containing 1% (wt/vol) agar dissolved in deionized water (three replicates of 50 seeds each) for germination, then the trays were placed in a growth cabinet at optimal germination conditions of 11 C (Naylor and Fedec Reference Naylor and Fedec1978) with no light for 15 d.
Data Analysis
All experiments were arranged in a completely randomized design. To determine the most suitable thresher settings for germination and to compare the germination using the mechanical thresher versus other commonly used treatments, germination percentages between treatments were analyzed through a one-way ANOVA followed by Tukey’s test. ANOVA tests were also carried out between the first and second repetition of the experiments; there were no significant differences (P > 0.05) and therefore the results were pooled across repetitions. Additionally, an ANOVA test was conducted between the three times of exposure tested for the H2SO4 treatment. There was no significant difference (P > 0.05), and therefore pooled results are presented. To examine the effect of the mechanical thresher (T6) in comparison to the manual puncturing technique (T2) on the germination of each individual population, each population was compared for total germination against a control treatment via a two-tailed t-test. The RGR was analyzed by comparing the averages between treatments for each population (six seedlings for each treatment) at 3 and 24 d after germination according to Equation 1 described above. Normality was tested through a Shapiro-Wilk test and homogeneity of variance was tested through the Brown-Forsythe test. The analyses were performed at the 0.05 significance level with SigmaPlot v13.0 (Systat Software, San Jose, CA, USA).
Results and Discussion
Experiments 1 and 2: Mechanical Scarification and Replication of Results on Multiple Wild Oat Populations
Using the Haldrup LT-15 thresher, it was determined that the most efficient setting for breaking seed coat-mediated dormancy of wild oat seed (population e; Figure 3) was 1,500 rpm for 5 s (T6), which resulted in a mean germination rate of 65%. The untreated control treatment did not germinate (0%). These results support previous studies suggesting that overcoming physical barriers to facilitate water movement to the embryo is essential in germinating wild oat seed (Foley Reference Foley1987; Hsiao et al. Reference Hsiao, McIntyre and Hanes1983; Raju et al. Reference Raju, Hsiao and McIntyre1988).
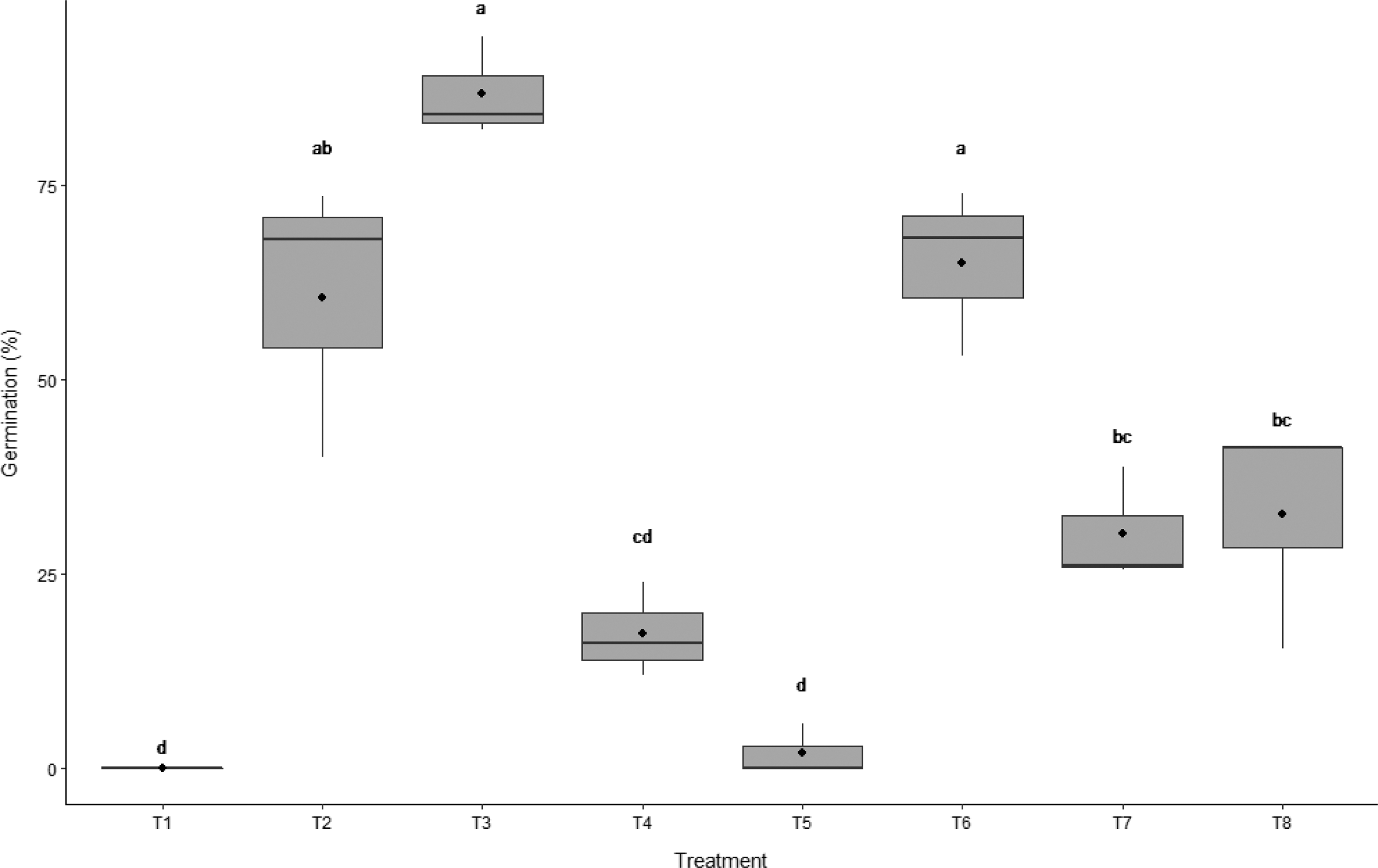
Figure 3. Boxplot for the comparison of germination between multiple treatments. T1 is intact seed with husk (lemma and palea), T2 is punctured seed with husk, T3 is manual extraction of caryopsis plus needle puncture, and T4 is manual extraction of caryopsis. Mechanical scarification treatments are T5: 1,350 rpm for 5 s, T6: 1,500 rpm for 5 s, T7: 1,350 rpm for 10 s, and T8: 1,500 rpm for 10 s (3 replicates, 50 seeds each). The significance of results as assessed by Tukey’s multiple comparison test are denoted by letters. In the boxplot, the ends of the box represent the 25th and 75th percentiles, the bars inside the box represent the 50th percentile or median, and the ends of the whiskers represent minimum and maximum values. Means are represented by solid circles.
The highest germination percentage observed in this experiment occurred following the extraction of the caryopsis from the seed coat plus the manual puncturing of caryopsis (87% germination, T3). Needle puncturing of the seed husk (T2) resulted in 60% germination (Figure 3), which is the most common technique for alleviating seed coat-mediated dormancy in weed management studies (Bourgeois et al. Reference Bourgeois, Kenkel and Morrison1997; Cruz-Hipolito et al. Reference Cruz-Hipolito, Osuna, Dominguez-Valenzuela, Espinoza and De Prado2011; Mansooji et al. Reference Mansooji, Holtum, Boutsalis, Matthews and Powles1992; Ņečajeva et al. Reference Ņečajeva, Bleidere, Jansone, Gailīte and Ruņģis2021; Owen and Powles Reference Owen and Powles2009). When the caryopsis was manually extracted from the husk but not manually punctured (T4), germination was reduced to 17%.
When comparing the duration of mechanical scarification at 1,500 rpm, it was found that increasing the time of seed exposure from 5 s (T6) to 10 s (T8) reduced wild oat germination by 28% (Figure 3). When these seeds were visually assessed, treatment T8 had a greater number of wild oat seeds with damaged embryos, rendering the seed nonviable (Figure 2). The threshing intensity was also found to affect wild oat germination. Threshing the wild oat seeds at 1,350 rpm for 5 s (T5) was found to be ineffective, resulting in only 2% germination (Figure 3). However, when the duration was increased to 10 s (1,350 rpm, T7), wild oat germination was increased to 30%. Thus, a longer period of threshing is required to alleviate dormancy at a lower speed.
The most effective mechanical scarification method T6 (1,500 rpm for 5 s) was compared against the widely used technique of manually puncturing individual seeds through the husk (T2; Mansooji et al. Reference Mansooji, Holtum, Boutsalis, Matthews and Powles1992; Ņečajeva et al. Reference Ņečajeva, Bleidere, Jansone, Gailīte and Ruņģis2021; Owen and Powles Reference Owen and Powles2009) using eight populations of wild oat collected from the Western Australian grainbelt. There was no difference (P > 0.05) in germination between the mechanical scarification (T6) and the manual puncturing of the seed coat (T2) for the eight populations tested (Table 4). The mechanical scarification (T6) technique caused mean embryo damage of 1% (range between 0% to 3%) of the total seeds scarified (Table 4). Overall, mechanical scarification can achieve the same level of seed germination as the most commonly used manual method in a fraction of the time for diverse wild oat populations from different habitats and exposed to different cropping systems.
Table 4. Comparison of the effect of mechanical scarification on embryo damage and the germination of eight wild oat populations from the grain belt of Western Australia. a
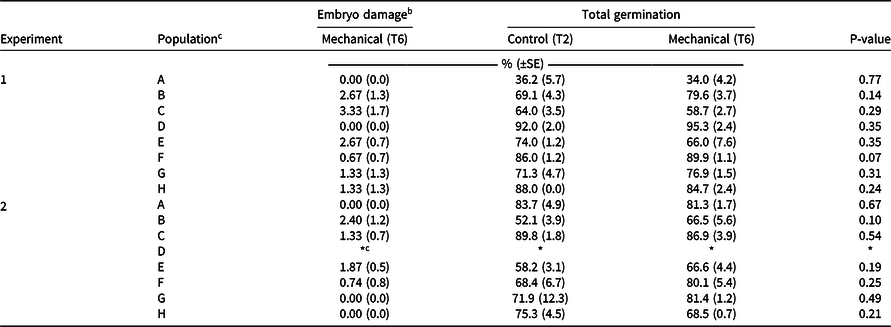
a Note: T2 indicates manually punctured seed with husk (control); T6 indicates mechanical thresher Haldrup LT-15 at 1,500 rpm for 5 s. The experiment was repeated twice (January and July 2020); data are averages with SE in parentheses (three replicates per population of 50 seeds each).
b Assessed visually and not assessed for T2 as no embryo damage from manual puncturing is expected.
c Due to insufficient seed, population ‘d’ was omitted from this experiment.
Experiment 3: Effect of Mechanical Scarification on Wild Oat Growth
This experiment found that the mechanical scarification of wild oat seeds at 1,500 rpm for 5 s was effective at alleviating seed coat–mediated dormancy without affecting seedling growth. The RGR of seedlings was assessed following mechanical scarification (T6: 1,500 rpm for 5 s) and manual puncturing (T2). There was no effect of these seed treatments (P > 0.05) on subsequent seedling growth rates (Table 5).
Table 5. Assessment of relative growth rate of seven wild oat populations comparing two treatments. a

a Note: T2 indicates manually punctured seed with husk (control); T6 indicates mechanical thresher Haldrup LT-15 at 1,500 rpm for 5 s. The experiment was repeated twice (February and August 2020).
b Abbreviation: RGR, relative growth rate.
c Due to insufficient seed, population ‘d’ was omitted from this experiment.
Experiment 4: Comparing Mechanical Threshing with Preexisting Methods to Break Seed Coat-mediated Dormancy of Wild Oat Seed
The mechanical scarification of the seed provided greater germination (P < 0.001) than scarification with H2SO4, partial endosperm removal, sandpaper scarification, and exposure to NO donor SNP (Figure 4). The germination percentage following mechanical scarification was significantly greater than the treatment with SNP solution (66% and 34%, respectively; Figure 4). The application of concentrated H2SO4 resulted in no germination, which contradicted the findings of Shahvand et al. (Reference Shahvand, Miri and Bagheri2015) who reported 42% germination after 15 min of exposure to diluted H2SO4 (15%). However, in this experiment we tested H2SO4 at 50% concentration for 1, 2, and 5 min, which likely accounts for the discrepancy. Previous studies found that the effectiveness of this treatment depends on duration and concentration (Brito et al. Reference Brito, Guevara and Gelabert2010; Martin and De la Cuadra Reference Martin and De la Cuadra2004; Patanè and Gresta Reference Patanè and Gresta2006; Shahvand et al. Reference Shahvand, Miri and Bagheri2015). The sandpaper method provided variable germination results (median of 25%). Other studies have demonstrated that this method is very effective on Medicago, Trifolium, and Acacia species (Kimua and Islam 2012; Martin and De la Cuadra Reference Martin and De la Cuadra2004; Shoobridge Reference Shoobridge1970). Exposure to KNO3 and NH3 have also been found to provide a similar germination percentage as the manual puncturing technique (Cairns and De Villiers Reference Cairns and De Villiers1986; Hilton Reference Hilton1985).
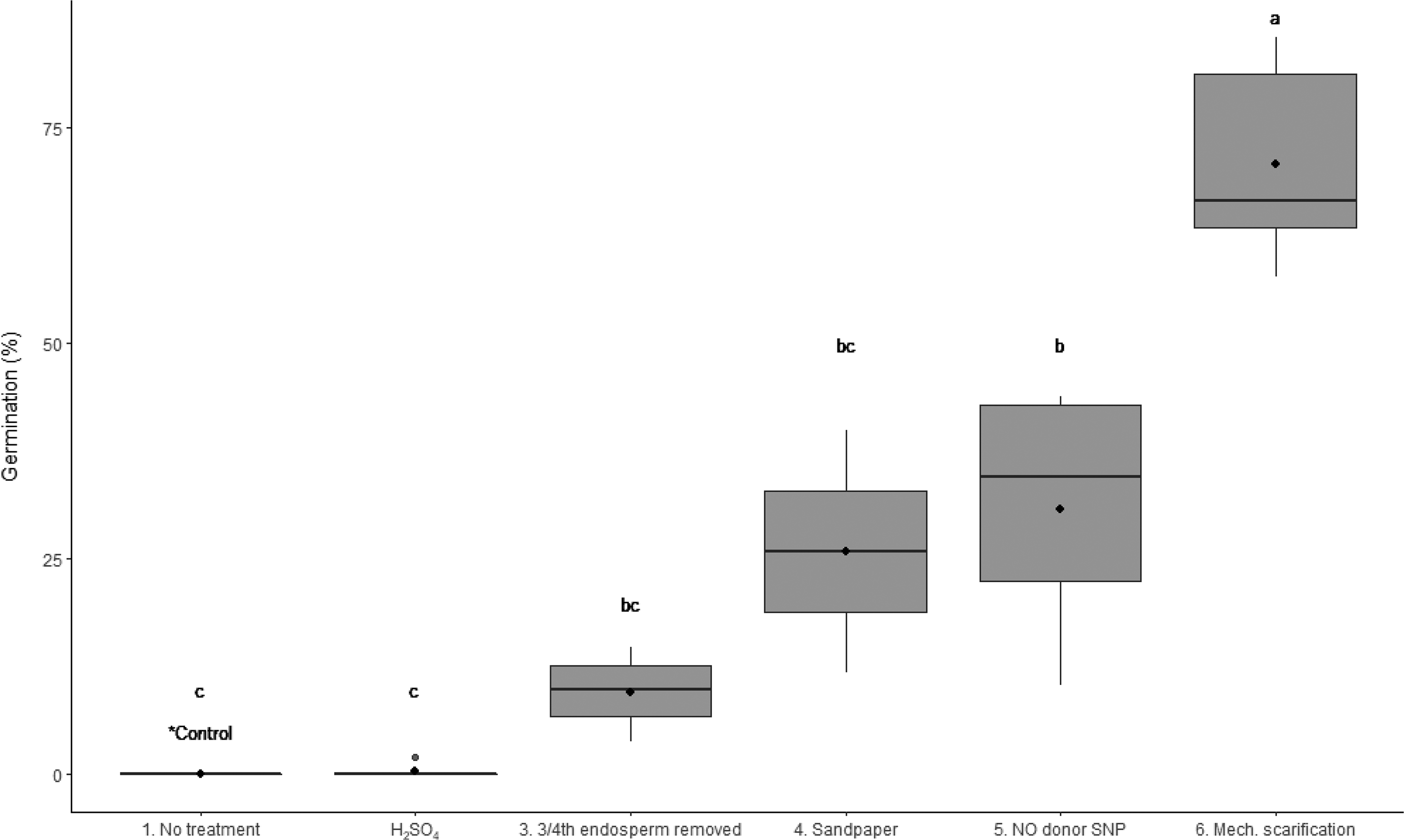
Figure 4. Boxplot for the comparison of germination between common treatments used to break the dormancy of wild oat. Tukey’s multiple comparison test found a statistically significant difference between the mechanical scarification of the seed with the thresher and all other treatments (P < 0.001). The significance of results as assessed by Tukey’s multiple comparison test are denoted by letters. In the boxplot, the ends of the box represent the 25th and 75th percentiles, the bars inside the box represent the 50th percentile or median, and the ends of the whiskers represent minimum and maximum values. Means are represented by solid circles.
The mechanical scarification technique was found to be effective on all eight populations of wild oat evaluated in this study (Tables 1 and 4). Previous studies have identified effective techniques at alleviating wild oat seed coat-imposed dormancy, however, they are all laborious and time-consuming to perform. These highly effective germination techniques include the removal of lemma and palea (90% germination following 10 d) (Kommedahl et al. Reference Kommedahl, DeVay and Christensen1958); the removal of lemma and palea plus needle puncture as demonstrated in this study (Figure 3, T3: 87% germination); and a combination of soluble sugars and GA (100% germination after 7 d with 0.1 µM GA, 88 mM sucrose, and surgical separation of the embryo; Foley Reference Foley1992).
The results of this study confirm that mechanical threshing of wild oat seed is an effective and more efficient method of breaking seed coat-induced dormancy than the current common technique of manually puncturing individual seeds to facilitate germination and plant growth (Mansooji et al. Reference Mansooji, Holtum, Boutsalis, Matthews and Powles1992; Ņečajeva et al. Reference Ņečajeva, Bleidere, Jansone, Gailīte and Ruņģis2021; Owen and Powles Reference Owen and Powles2009). The results further support previous research indicating that a physical pathway for water movement from the seed husk to the seed embryo is required for imbibition and germination of wild oat (Foley Reference Foley1987; Hay Reference Hay1962; Hsiao et al. Reference Hsiao, McIntyre and Hanes1983; Morrison and Dushnicky Reference Morrison and Dushnicky1982; Raju et al. Reference Raju, Hsiao and McIntyre1988). The time of exposure to mechanical threshing is a critical factor in this technique’s effectiveness, with a longer time of exposure required for germination of wild oat seed at 1,350 rpm compared with 1,500 rpm. Studies have previously reported using purpose-built mechanical scarifiers to break seed dormancy on legumes and native seeds with variable degrees of success (Kimura and Islam Reference Kimura and Islam2012). Carleton et al. (Reference Carleton, Austin, Stroh, Wiesner and Scheetz1971) achieved 78% germination of Astragalus cicer L. seeds when exposed to 1,400 rpm using a Model 2 huller (Foresbergs Inc., Thief River Falls, MN, USA); Shoobridge (Reference Shoobridge1970) reported successful germination of multiple species (Fabaceae and Acacia species) when seeds were scarified in a compressed air-powered rotating drum lined with sandpaper.
While an economic analysis was not performed in this study, the mechanical scarification treatment we propose took 5 s to break the dormancy of 50 seeds compared with more than 6 min to manually puncture the same number of seeds. The results obtained for the thresher investigated in this study can be used as indicators of the rotational speed (rpm) and time of exposure required to break the dormancy of wild oat in other threshers.
In summary, the mechanical scarification of wild oat seed is a novel technique for researchers to germinate seeds efficiently and effectively to expedite research. For example, the use of a mechanical thresher can greatly enhance the ability to conduct pot studies in which uniform and sufficiently high germination and emergence of wild oat seedlings are needed. This requirement is desirable for studies ranging from screening weed accessions for herbicide resistance to assessing ecological growth, development, and fitness of different populations.
Acknowledgments
This research was supported by the Grains Research and Development Corporation through the base funding of the Australian Herbicide Resistance Initiative. We thank Mr. Shane Baxter and Dr. Shaghayegh Mehravi for their technical assistance in conducting the experiments. No conflicts of interest have been declared.












