Introduction
Weedy rice (also referred to as red rice) is one of the most problematic weeds of U.S. rice production and ranks as the fourth most problematic weed in Arkansas rice, behind barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], sprangletops (Leptochloa spp.), and Northern jointvetch [Aeschynomene virginica (L.) Britton, Sterns & Poggenb] (Norsworthy et al. Reference Norsworthy, Bond and Scott2013). Direct competition of weedy rice with cultivated rice during the season and seed contamination at harvest reduce grain yield and quality (Diarra et al. Reference Diarra, Smith and Talbert1985; Kwon et al. Reference Kwon, Smith and Talbert1991; Ottis et al. Reference Ottis, Smith, Scott and Talbert2005); thus, weedy rice has been classified as a noxious weed in the United States. Similar physiological and morphological features of weedy rice and cultivated rice make it difficult to differentiate between species early in the season and impossible to selectively control in rice fields (Pantone and Baker Reference Pantone and Baker1991). Before the 21st century, weedy rice was controlled using water-seeded rice production and crop rotation with alternative crops including soybean [Glycine max (L.) Merr.], corn (Zea mays L.), and grain sorghum [Sorghum bicolor (L.) Moench] (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Smith Reference Smith1981).
In 2002, a nontransgenic, imidazolinone-resistant rice (Clearfield®, BASF Corp., Research Triangle Park, NC) was commercialized, which allowed over-the-top use of imazethapyr, an acetolactate synthase (ALS)-inhibiting herbicide, in rice for control of weedy rice. The imidazolinone-resistant technology has been successful, providing 95% or greater control of weedy rice, and can be combined with other rice herbicides, including propanil or quinclorac, for excellent POST control of other grass and broadleaf weeds (Avila et al. Reference Avila, Senseman, McCauley, Chandler and O’Barr2005; Ottis et al. Reference Ottis, O’Barr, McCauley and Chandler2004; Steele et al. Reference Steele, Chandler and McCauley2002).
Despite high levels of weedy rice control, it is inevitable that escapes occur in production rice fields, regardless of herbicide, due to various environmental, biological, and application factors (Bagavathiannan and Norsworthy Reference Bagavathiannan and Norsworthy2012; Scursoni et al. Reference Scursoni, Forcella and Gunsolus2007). Weedy rice escapes are particularly problematic because flowering often occurs simultaneously in weedy rice and cultivated rice; thus, when weedy rice is not controlled, herbicide-resistance genes can be introgressed into weedy rice populations, resulting in herbicide-resistant weedy rice (Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007). Although the outcrossing rate is reported to be low (0.109% to 0.434%), the fecundity of weedy rice could easily turn a few escapes into an infestation of herbicide-resistant plants in a commercial rice field (Burgos et al. Reference Burgos, Scott and Guice2007).
In addition to outcrossing concerns, the repetitive use of imidazolinone herbicides in the early to mid 2000s placed significant selection on weed populations, increasing the frequency of resistance alleles and ultimately leading to herbicide resistance (Jasieniuk et al. Reference Jasieniuk, Brûlé-Babel and Morrison1996). To delay or prevent herbicide resistance associated with release of imidazolinone-resistant rice, stewardship recommendations include use of alternative herbicide sites of action and crop rotation or rotation away from imidazolinone-resistant rice (BASF Corp. 2010). However, on the basis of survey data from Arkansas and Mississippi, these guidelines were largely disregarded: 42% of the surveyed rice hectares use ALS-inhibiting herbicides as the only herbicide treatment and 11% of surveyed hectares are in continuous imidazolinone-resistant rice production over a 5-year span (Norsworthy et al. Reference Norsworthy, Bond and Scott2013). Today, nine weed species have evolved resistance to ALS-inhibiting herbicides in Arkansas, including four of the five most problematic species in Arkansas rice production: weedy rice, barnyardgrass, junglerice [E. colona (L.) Link], and Palmer amaranth [Amaranthus palmeri (S.) Watson] (Heap Reference Heap2018; Norsworthy et al. Reference Norsworthy, Bond and Scott2013). The evolution of herbicide resistance to ALS inhibitors and other herbicide sites of action in problematic rice weeds has limited effective chemical control options.
Very-long-chain fatty acid (VLCFA)-inhibiting herbicides are currently labeled in U.S. row-crop production for control of annual grasses and small-seeded broadleaf weeds (Monaco et al. Reference Monaco, Weller, Ashton and Monaco2002). These are soil-applied residual herbicides that are absorbed primarily by shoots and secondarily through roots of germinating seedlings, where disrupted cell division results in distorted tissue formation or plant death (Fuerst Reference Fuerst1987). Experiments by Khodayari et al. (Reference Khodayari, Smith and Black1987) in soybean production demonstrated effective weedy rice control by VLCFA-inhibiting herbicides. When applied PPI, S-metolachlor at 2.2 kg ai ha−1 or alachlor at 3.6 kg ha−1 provided greater than 90% control of weedy rice in soybean production (Khodayari et al. Reference Khodayari, Smith and Black1987). Zemolin et al. (Reference Zemolin, de Avila, Agostinetto, Cassol, Bastiani and Pestana2014) evaluated weedy rice control in soybean using PRE and early POST applications of S-metolachlor at 768, 1,152, and 1,680 g ha−1. They observed that without the addition of glyphosate, early POST applications of S-metolachlor were not effective; however, S-metolachlor applied PRE provided 74% to 84% and 53% to 64% control of weedy rice 28 d after application in the first and second year of the study, respectively. Complementary greenhouse trials were conducted to evaluate control of susceptible and imidazolinone-resistant weedy rice biotypes using S-metolachlor, and no difference in sensitivity between weedy rice biotypes was observed (Zemolin et al. Reference Zemolin, de Avila, Agostinetto, Cassol, Bastiani and Pestana2014). These results indicated populations were equally sensitive to S-metolachlor irrespective of imidazolinone resistance. Thus, VLCFA-inhibiting herbicides such as S-metolachlor, pyroxasulfone, and acetochlor provide weedy rice control in U.S. row crops; however, no VLCFA-inhibiting herbicides are currently registered for use in rice production.
In water-seeded rice production, PRE applications of alachlor (2.4 kg ha−1), dimethenamid-P (1.4 kg ha−1), S-metolachlor (2.5 kg ha−1), and acetochlor (1.5 kg ha−1) caused significant reduction in weedy rice stems and panicles, relative to the nontreated plots; however, alachlor, S-metolachlor, and acetochlor provided the greatest weedy rice control at 92%, whereas dimethenamid-P provided slightly less control at 84%. Rice was water seeded 15 d after herbicide treatments, and no phytotoxic effects were observed over the course of the experiment, indicating crop tolerance and potential for selectivity for weedy rice control using the VLCFA-inhibiting herbicides (Eleftherohorinos and Dhima Reference Eleftherohorinos and Dhima2002).
Although previous studies have demonstrated adequate crop tolerance and weedy rice control using VLCFA-inhibiting herbicides in row crops and water-seeded rice, there has been limited research in drill-seeded rice. Recent studies of VLCFA-inhibitor use in drill-seeded rice by Godwin et al. (Reference Godwin, Norsworthy and Scott2018) demonstrated that rice is adequately tolerant to acetochlor and pethoxamid; however, application timing and rate greatly influence crop injury. In addition, PRE and delayed PRE (i.e., 5 to 6 d after planting) applications of acetochlor and pethoxamid can be used to control weedy rice and annual grasses in drill-seeded rice (Norsworthy et al. Reference Norsworthy, Fogleman, Barber and Gbur2018). However, these application timings also pose significant risk to growers because severe crop injury can result when rainfall occurs soon after application.
Lawrence et al. (Reference Lawrence, Bond, Edwards, Golden, Montgomery, Eubank and Walker2018) evaluated the effect of fall-applied clomazone, pyroxasulfone, S-metolachlor, and trifluralin on rice growth and yield. At 14 d after emergence (DAE), rice seedling density and height were negatively affected by all herbicides except clomazone. Averaged across pyroxasulfone rates (170 and 340 g ha−1), rice injury and shoot density 14 DAE were the equivalents of 37% and 72%, respectively, of the nontreated control. Similarly, rice injury and relative shoot density in plots treated with S-metolachlor (1,420 and 2,840 g ha−1) were 30% and 73%, respectively. By 28 DAE, rice injury from the lower rates of pyroxasulfone and S-metolachlor had declined to 17% and 9%, respectively. Regardless of application rate, plots treated with pyroxasulfone or S-metolachlor yielded 90% or greater than the nontreated plots.
Winter flooding of rice fields is a common practice in the southern U.S. rice-producing region for habitat conservation and hunting of local and migratory waterfowl (Eadie et al. Reference Eadie, Elphick, Reinecke, Miller and Manley2008; Elphick and Oring Reference Elphick and Oring2003). Not only does flooding facilitate habitat management, it also benefits growers by reducing viability of weed seed, decreasing soil erosion, and promoting decomposition of rice straw (Anders et al. Reference Anders, van Kessel, Eadie and Manley2008; Manley et al. Reference Manley, Kaminski, Reinecke and Gerard2005). In addition, waterfowl that find refuge in flooded fields are reported to enhance straw decomposition through trampling, and they even feed on waste rice, suggesting that weed-seed populations could be diminished, although this has not been proven (Brogi et al. Reference Brogi, Pernollet, Gauthier-Clerc and Guillemain2015; Manley et al. Reference Manley, Kaminski, Reinecke and Gerard2005; Suh Reference Suh2015). Thus, winter flooding has environmental and recreational benefits and potential benefits for weed management, which justifies investigation of how this practice affects herbicide efficacy.
Limited options for controlling weedy rice in rice, fecundity of escaped plants, and longevity of weedy rice seed in the soil seedbank has led to the exploration of other means of control. Because of the prevalence of weedy rice and inability for growers to control weedy rice in non-Clearfield rice systems, growers could potentially reduce population size in the soil seedbank and limit weedy rice early-season emergence by applying residual herbicides in the fall. Thus, an experiment was conducted to evaluate the efficacy of flooded and non-flooded, fall-applied, VLCFA-inhibiting herbicides on weedy rice control the following spring.
Materials and Methods
Experimental site
Field experiments were initiated in September 2016 and September 2017 on a Calloway silt loam (fine-silty, mixed, active, thermic Aquic Fraglossudalfs) at the Pine Tree Research Station near Colt, AR (35.12°N, 90.93°W). The soil at the research station was representative of rice fields in Arkansas, with a pH of 7.5, 1.3% organic matter, 10.6% sand, 68.6% silt, and 20.8% clay.
Experimental setup and design
The experiment was conducted as split-plot design with the whole-plot factor being flooded or non-flooded winter conditions, and the split-plot factor being a factorial arrangement of herbicide and rate in a randomized complete block. A weedy nontreated plot and a weed-free nontreated plot were included in all four replications for comparison of rice injury and weedy rice control. In 2016, the experimental site was prepared by sowing a mixture of the hybrid cultivar ‘XL753’ (RiceTec Inc., Alvin, TX) and weedy rice at 33 seed m−1 of row. To ensure adequate weedy rice populations in the subsequent spring, cultivated rice and weedy rice were allowed to compete throughout the growing season without any herbicide applications. At maturity, the area was mowed to disperse seeds across the soil surface.
Fall herbicide applications were made on September 28 and October 9 in 2016 and 2017, respectively, using a CO2-pressurized backpack sprayer calibrated to deliver 140 L ha−1 through AIXR 110015 nozzles (TeeJet Technologies, Wheaton, IL). In total, 10 herbicide treatments were used, including five active ingredients applied at two rates (i.e., field rate [low] and double field rate [high]). The full list of herbicides and application rates evaluated in this experiment is provided in Table 1. Plots that received flooding treatments were flooded on November 8 and November 15 in 2016 and 2017, respectively, and remained flooded until they were drained on February 10 and February 19 in 2017 and 2018, respectively.
Table 1. Description of fall-applied herbicides evaluated in rice experiments at Colt, AR, in 2017 and 2018.

a Pethoxamid is not registered in any crop and thus does not have a trade name.
For control of winter annual weeds, flooded and non-flooded bays were treated with glyphosate (Roundup PowerMAX®II, 1.26 kg ae ha−1; Monsanto Company, St. Louis, MO) on March 29 and April 11 in 2017 and 2018, respectively. After application of glyphosate, plots were left undisturbed from herbicide application in the fall until planting in the spring. ‘CL172’ was drill seeded using a no-till drill at 72 seeds m−1 of row into 1.8-m × 6.1-m plots on April 6 and April 19 in 2017 and 2018, respectively. Immediately after planting, clomazone (Command®, 336 g ha−1, FMC Corp., Philadelphia, PA) plus glyphosate (Roundup PowerMAX®II, 1.26 kg ae ha−1) was applied to the entire experiment to simulate the beginning of a standard rice herbicide program in Arkansas.
Data collection
Visible estimates of rice injury and weedy rice control were taken at 3 and 5 wk after planting (WAP). Rice injury and weedy rice control were rated on the basis of reductions in plant height, stand, or tillering, and were recorded on a scale of 0 to 100, with 0 being no injury or control and 100 being complete crop death or control. Physical counts of rice shoot density per meter of row and weedy rice shoot reduction per square meter were evaluated at 5 WAP. Rice shoot densities were determined by counting two 1-m row sections and then calculating the mean. Rice shoot densities were then converted to proportions of the average density in weed-free nontreated plots within each replication and presented as a percentage of the nontreated plots. Weedy rice densities were determined by counting the number of plants in two 1-m2 quadrats in each plot and then calculating the mean. Weedy rice densities were converted to proportions of the average density in weedy nontreated plots within each replication and presented as percent reduction from the nontreated plots. Because no POST treatments were evaluated in this experiment, plots were not harvested at maturity, due to excessive weediness.
Statistical analysis
All data were subjected to ANOVA as a split-plot design using the GLIMMIX procedure in SAS, version 9.4 (SAS Institute Inc., Cary, NC). Main effects of herbicide, rate, year, and winter condition and all interactions were treated as fixed effects, and block nested within year was treated as a random effect. Data were checked for normality by reviewing residual plots from SAS output, and means were separated using Fisher protected LSD (α = 0.05). Data for rice injury, weedy rice control, rice shoot density, and weedy rice density were square-root transformed for ANOVA, and means separation was then back-transformed for presentation in tables.
Results and Discussion
Rice injury and shoot density
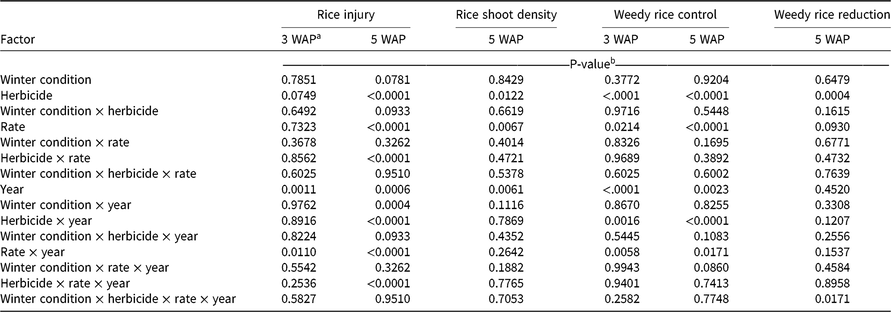
At 3 WAP, the only factors exhibiting a significant effect on rice injury were the main effect of year and the interaction of application rate and year (Table 2). In 2017, when averaged across all herbicides, the low application rates caused less visible injury to rice than did high rates; however, rice injury was 7% at the low rates and 10% at the high rates (Table 3). In 2018, no significant injury was observed at 3 WAP in response to any treatment. The highest level of rice injury was observed in response to the low rate of dimethenamid-P; however, rice injury was only 3%, which was not statistically different from treatments that caused no injury (Table 3).
Table 2. ANOVA output for rice injury, rice shoot density, weedy rice control, and weedy rice reduction from field trials in Colt, AR, in 2017 and 2018.
a Abbreviation: WAP, wk after planting.
b Before generating P-values, rice injury and weedy rice control ratings were square-root transformed to address non-normal variance of the data.
Table 3. Rice injury and rice shoot density at Colt, AR, in 2017 and 2018.
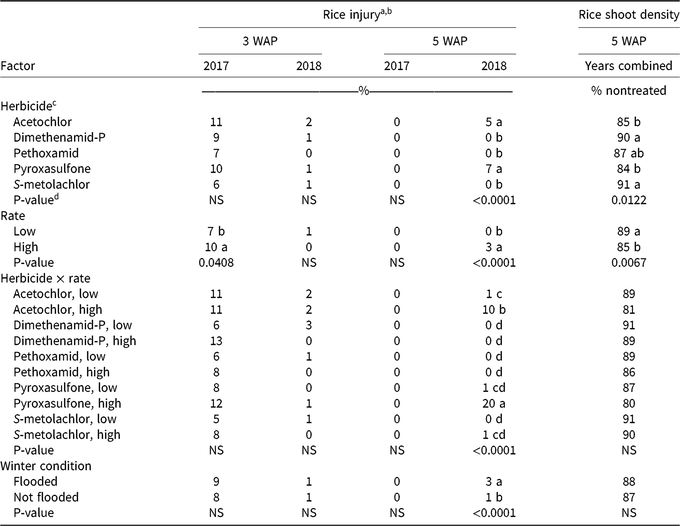
a Means within a column followed by the same lowercase letter are not different according to Fisher protected LSD at α = 0.05. Lack of letters indicates the F statistic was not significant at α = 0.05. Injury data were square-root transformed for ANOVA and means separation, then back-transformed for presentation in this table.
b Abbreviations: WAP, wk after planting; NS, not significant.
c Low (high) application rates (g ha−1) were as follows: acetochlor, 1,050 (2,100); dimethenamid-P, 525 (1,050); pethoxamid, 420 (840); pyroxasulfone, 205 (410); S-metolachlor, 1,070 (2,140).
d P-values were generated separately for year using the SLICE statement on any significant (P < 0.05) interaction effects involving the main effect of year, using the GLIMMIX procedure in SAS, version 9.4.
At 5 WAP, rice injury response differed between 2017 and 2018 and is reflected in the interaction terms involving the main effect of year (Table 2). Total precipitation and timing of rainfall events differed between 2017 and 2018, which may have played a role in the activity of fall-applied residual herbicides. From planting until 3 WAP, a total of 8.3 cm and 4.5 cm of rainfall occurred in 2017 and 2018, respectively. From planting until 5 WAP, a total of 20.5 cm and 14.2 cm occurred in 2017 and 2018, respectively. In 2017, no injury symptoms were observed in rice plots at 5 WAP; however, in 2018, the main effects of herbicide, rate, winter condition, and the interaction of herbicide and application rate each had a significant effect on rice injury (Table 3). The lack of symptoms at 5 WAP in 2017 may be associated with an 11.4-cm rainfall event that occurred in a 4-d span preceding 5 WAP rice injury ratings. Averaged across application rates at 5 WAP, acetochlor and pyroxasulfone caused 5% and 7% rice injury, respectively.
As expected, high application rates caused increased levels of rice injury, but the observed level of injury was only 3%, due to the number of herbicide treatments that caused little or no injury. The most significant rice injury was observed in response to the high rates of acetochlor and pyroxasulfone, which caused 10% and 20% injury, respectively (Table 3). Lawrence et al. (Reference Lawrence, Bond, Edwards, Golden, Montgomery, Eubank and Walker2018) also observed rice injury after fall applications of pyroxasulfone, although to a greater extent. Reduced pyroxasulfone injury in the current study was likely because herbicide applications were made on September 28 and October 9 in 2016 and 2017, respectively, in comparison to an early November application in the Lawrence et al. (Reference Lawrence, Bond, Edwards, Golden, Montgomery, Eubank and Walker2018) study, which may have allowed more herbicide degradation before planting rice.
Rice shoot density at 5 WAP was affected by herbicide and by application rate; no significant interactions were observed (Table 2). Shoot densities in plots treated with acetochlor, dimethenamid-P, or S-metolachlor did not differ from shoot densities in the nontreated plots, which averaged 52 plants per meter of row (data not shown). Plots treated with acetochlor and pyroxasulfone had the lowest rice shoot densities, at 85% and 84% that of the nontreated plot, respectively (Table 3). High herbicide rates resulted in lower rice shoot densities than did low rates, though at both rates, rice shoot density was 85% or greater of the density in the nontreated plots.. All herbicide treatments showed some degree of reduction in rice shoot density relative to the nontreated plots; however, rice shoot densities ranged from 80% to 91% of the density in the nontreated plots, so rice shoot densities were not drastically reduced in response to herbicide treatments. When comparing visible injury and rice shoot density, it is worth noting that treatments causing greatest visible injury (i.e., high rates of acetochlor and pyroxasulfone) were also observed to have the lowest rice shoot densities (Table 3). This alignment of qualitative and quantitative assessments offers two lines of evidence that, of the selected herbicides, acetochlor and pyroxasulfone pose the greatest risk to cause injury to rice, particularly at high application rates.
Increased rice injury was expected in plots that were not flooded in the fall, because chloroacetamide herbicides are known to break down more rapidly in anaerobic than aerobic soils (Loor-Vela et al. Reference Loor-Vela, Simmons, Simmons and Raskin2003). However, although flooded and non-flooded conditions were a major component of the experimental design of this study, herbicide and application rate had a much greater effect on rice injury (Table 2). Where differences were observed, rice injury was slightly lower in the flooded than in the non-flooded conditions (Table 3). Because the herbicides in this experiment were subject to the same environmental conditions and no herbicide winter condition was significant, we can infer that the differences observed in rice injury were mainly a function of herbicidal properties and application rates. Thus, there is no crop safety benefit or increased risk from herbicide use associated with winter flood condition, and growers should choose to flood or not flood on the basis other benefits and expenses associated with the practice (Eadie et al. Reference Eadie, Elphick, Reinecke, Miller and Manley2008; Elphick and Oring Reference Elphick and Oring2003).
Weedy rice control and density
Analysis of weedy rice control ratings at 3 and 5 WAP revealed a significant effect of herbicide, application rate, and year, as well significant interactions of herbicide and year and of application rate and year (Table 2); therefore, we report weedy rice control for herbicide and application rate separately for 2017 and 2018 (Table 4). The interactions with year may be associated with differences in rainfall in 2017 and 2018. At 3 WAP, weedy rice control ratings ranged from 39% to 59% and from 80% to 85% in 2017 and 2018, respectively. Larger amounts of rainfall were observed in the initial 3 WAP in 2017 (8.3 cm) than in 2018 (4.5 cm), which may have diluted the residual herbicides or caused herbicides to leach below the depth of weedy rice germination. In 2017, acetochlor and pyroxasulfone provided 59% and 53% weedy rice control, respectively—the highest weedy rice control of the selected herbicides. All herbicides offered greater weedy rice control in 2018 than in 2017, but no differences were observed among herbicides or application rates.
Table 4. Weedy rice control and weedy rice shoot reduction at Colt, AR, in 2017 and 2018.

a Means within a column followed by the same lowercase letter are not different according to Fisher protected LSD at α = 0.05. Lack of letters indicates the F statistic was not significant at α = 0.05. Weedy rice control data were square-root transformed for ANOVA and means separation, then back-transformed for presentation in this table.
b Abbreviations: WAP, wk after planting; NS, not significant.
c Low (high) application rates (g ha−1) were as follows: acetochlor, 1,050 (2,100); dimethenamid-P, 525 (1,050); pethoxamid, 420 (840); pyroxasulfone, 205 (410); S-metolachlor, 1,070 (2,140).
d P-values generated separately for year using the SLICE statement on any significant (P < 0.05) interaction effects involving the main effect of year, using the GLIMMIX procedure in SAS, version 9.4.
At 5 WAP, herbicide residual activity had declined and weedy rice control ratings decreased substantially, ranging from 22% to 42% and from 3% to 43% in 2017 and 2018, respectively (Table 4). Despite a significant herbicide and year interaction, acetochlor and pyroxasulfone performed consistently across years, providing the highest weedy rice control in 2017 and 2018. Weedy rice control at 5 WAP was 42% and 43% for acetochlor in 2017 and 2018, respectively, and 33% and 34% for pyroxasulfone in 2017 and 2018, respectively. Similar to 3 WAP, pethoxamid provided the lowest levels of weedy rice control at 5 WAP: 22% and 3% in 2017 and 2018, respectively (Table 4).
Weedy rice densities were assessed relative to a weedy nontreated plot at 5 WAP. A comparison among weedy rice reductions revealed a significant main effect of herbicide and a four-way interaction of winter condition and herbicide and application rate and year (Table 2). Unfortunately, no meaningful pattern was observed when data were analyzed according to this interaction, and 36 of 40 treatment combinations fell within the same statistical groupings after means separation (data not shown). Instead, we report the main effect of herbicide (Table 4). Among herbicides, acetochlor and pyroxasulfone caused the greatest reduction in weedy rice densities, reducing weedy rice–shoot density by 56% and 48%, respectively. Weedy rice reduction was lower in the other three treatments, but all herbicides caused at least 31% weedy rice reduction by 5 WAP.
It is worth comparing the visible ratings of weedy rice control with the quantitative counts of weedy rice–shoot reduction. The herbicide treatments with the highest weedy rice control also produced the fewest weedy rice shoots per square meter. Though in all cases, in 2017 and 2018, weedy rice control according to visible assessment was lower than the weedy rice reduction based on weedy rice shoot counts (Table 4). This can be explained by differences between the assessments. Visible control ratings assess the weed density as well as the weed size, architecture, and symptomology, whereas shoot counts assess only the number of plants in an area. Thus, late-emerging weedy rice plants may be undersized and register visually as greater weedy rice control. However, a late-emerging weedy rice plant and one that emerged alongside the crop would both count for a single weedy rice shoot, despite differences in size. Regardless, the alignment of these data are strong indicators of a significant effect of herbicide on weedy rice control and weedy rice reduction and that all herbicides retained some activity up to 5 WAP.
Overall, the greatest control of weedy rice was observed soon after planting and generally declined with time. Although herbicide degradation processes generally decrease under cooler air and soil temperatures (Curran Reference Curran1999), complete weedy rice control was not expected, because degradation is likely to occur to some extent in the 211 to 213 d between application and the first evaluation of weedy rice. Nonetheless, it should be noted that all treatments were still providing some level of weedy rice suppression at 225 to 227 d after herbicide applications were made in the fall. The extended residual control of weedy rice from acetochlor and pyroxasulfone was likely due to herbicidal properties (Shaner Reference Shaner2014). In particular, acetochlor was applied as Warrant®, a microencapsulated formulation. In the microencapsulated formulation, herbicide molecules are protected from degradation processes by a polymer shell, which slowly releases herbicide after absorbing water and thus offers longer residual control (Rao Reference Rao2000).
The reason that herbicides in this study provided control of weedy rice but did not injure cultivated rice to the same extent is not clear. One explanation could be that weedy rice seeds absorbed high concentrations of herbicide over the winter months, and growth became inhibited in the spring when temperatures were conducive for germination. The process of drill seeding rice disturbs the soil surface and creates a disturbed microenvironment, which may have affected herbicide uptake or emergence timing relative to weedy rice. Furthermore, drill seeding creates a more uniform distribution of seed and may have provided a better chance for crop compensation by production of tillers where neighboring rice seed did not emerge. Future studies should identify the survival of weedy rice populations after being exposed to VLCFA-inhibiting herbicides under temperatures that promote dormancy followed by temperatures conducive for germination to further understand observations of this study.
Practical applications
The results of this experiment indicate that acetochlor, dimethenamid-P, pethoxamid, pyroxasulfone, and S-metolachlor can be applied in the fall after harvest with minimal injury to cultivated rice the following spring. However, none of these herbicides currently are labeled for use in rice production, and all have recommended plant-back intervals that exceed the approximately 6-month period evaluated in this experiment (Scott et al. Reference Scott, Barber, Boyd, Seldon, Norsworthy and Burgos2018). With the exception of the high rate of pyroxasulfone, which caused as much as 20% injury, less than 13% rice injury was observed in response to all other treatments (Table 3). Thus, there is reason to believe these herbicides could be safely implemented in rice production. The flooded or non-flooded conditions played a smaller role; but in 2018, reduced injury was observed in the non-flooded treatments (Table 3). The effect was small, but it provides evidence that environmental conditions can be managed in concert with herbicide selection to minimize carryover issues.
Treatments that caused the greatest amount of rice injury generally also provided the greatest levels of weedy rice control throughout the season (Tables 3 and 4). Overall, acetochlor and pyroxasulfone were the most effective herbicides evaluated in this experiment, although dimethenamid-P, pethoxamid, and S-metolachlor also provided a degree of weedy rice control. Although all selected VLCFA-inhibiting herbicides offered some degree of weedy rice control, none was sufficient as a stand-alone treatment. However, given the current state of herbicide-resistant weedy rice in the southern United States, any PRE control or suppression of weedy rice would be considered advantageous.
It should be noted that the results of this experiment are based on a single site, representing one soil type; therefore, it is imperative that this experiment be repeated under different conditions to confirm or refute the observations made thus far. Furthermore, weedy rice populations in this study prevented any opportunity to collect harvest data; thus, it would be beneficial to conduct similar studies with VLFCA-inhibiting herbicides to evaluate yield response of cultivated rice. Data presented here indicate the potential for fall-applied VLCFA inhibitors to provide rice growers an alternative means of managing weedy rice in cultivated rice. Should VLCFA-inhibiting herbicides be labeled for use in U.S. rice, fall applications to fields with severe weedy rice infestations or where populations of imidazolinone-resistant weedy rice are known to exist should improve in-season control.
Author ORCID
Matthew B. Bertucci https://orcid.org/0000-0002-0661-8990.
Acknowledgments
This research was funded by the Arkansas Rice Promotion Board. No conflicts of interest have been declared.






