Introduction
Low-rate exposure to or spray-tank contamination with dicamba can be highly injurious to and possibly reduce yield of dicamba-sensitive soybean (Auch and Arnold Reference Auch and Arnold1978; Boerboom Reference Boerboom2004; Egan et al. Reference Egan, Barlow and Mortensen2014; Solomon and Bradley Reference Solomon and Bradley2014; Wax et al. Reference Wax, Knuth and Slife1969; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). With the advent of dicamba-resistant (DR) cotton (Gossypium hirsutum L.) and soybean and approval for use of dicamba in-crop, there will be greater opportunity for damage to susceptible crops. Neighboring fields planted in dicamba- and glyphosate-sensitive soybean may be at high risk for injury if dicamba is applied. If sprayers are not properly cleaned following a dicamba application, subsequent spray applications to dicamba-sensitive soybean are likewise expected to damage the crop (Boerboom Reference Boerboom2004). Injury symptoms from dicamba exposure to soybean have been previously described mostly as leaf cupping, stem epinasty, and swelling of the stem (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Andersen et al. Reference Andersen, Clay, Wrage and Matthees2004; Sciumbato et al. Reference Sciumbato, Chandler, Senseman, Bovey and Smith2004). In addition, pod malformation is a result of low doses of dicamba applied to soybean during reproductive stages (McCown et al. Reference McCown, Barber, Norsworthy, Palhano, Hale, Lancaster and Doherty2016a).
Historically, most dicamba applications occur in late winter or early spring for preplant removal of broadleaf vegetation before planting crops or in-crop to V3 to V5 corn (Zea mays L.), which is a time when few soybean fields have emerged or emerged plants are in an early vegetative stage. Exposure to dicamba at vegetative stages may result in severe injury, but soybean often recovers from this injury before reaching its reproductive stage (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Wax et al. Reference Wax, Knuth and Slife1969). Soybean compensates for terminal death by initiating branches from the cotyledon and unifoliate axils that reach a height comparable to that of nontreated plants (Wax et al. Reference Wax, Knuth and Slife1969). These axillary branches produce flowers and pods to offset possible yield reduction from exposure to dicamba (Andersen et al. Reference Andersen, Clay, Wrage and Matthees2004; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). Therefore, injury resulting from dicamba in vegetative stages may not always result in yield reduction (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999). Furthermore, the extent of injury may vary due to environmental conditions during and after application (Auch and Arnold Reference Auch and Arnold1978; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). Soybean exposed to dicamba when plants are drought stressed will be delayed in recovery when compared with plants experiencing adequate moisture levels (Auch and Arnold Reference Auch and Arnold1978; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). For these reasons, the extent of injury to vegetative soybean may not be a good predictor of yield loss, because soybean has the ability to recover when exposed to good environmental conditions (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Auch and Arnold Reference Auch and Arnold1978).
DR cotton and soybean have been deregulated by the U.S. Department of Agriculture (USDA) and commercially launched in 2015 and 2016, respectively. Registration of dicamba-containing products (XtendiMax® with VaporGrip®, Monsanto, St Louis, MO; Engenia®, BASF Corporation, Research Triangle Park, NC) for over-the-top use in DR soybean and cotton was recently granted for certain states (Anonymous 2016a, 2016b). Although a balanced PRE followed by POST herbicide program is recommended, dicamba applied in-crop will add an effective site of action to control problematic broadleaf weeds in cotton and soybean (Byker et al. Reference Byker, Soltani, Robinson, Tardif, Lawton and Sikkema2013; Flessner et al. Reference Flessner, McElroy, McCurdy, Toombs, Wehtje, Burmester, Price and Ducar2015; Inman et al. Reference Inman, Jordan, York, Jennings, Monks, Everman, Bollman, Fowler, Cole and Soteres2016; Spaunhorst and Bradley Reference Spaunhorst and Bradley2013). Research involving possible non-target effects of mixtures to be applied in this technology must be studied to examine any negative effects because of reports that extensive dicamba off-target movement has occurred (Barber et al. Reference Barber, Norsworthy, Scott and Hightower2017).
Applications of dicamba to DR soybean are allowed up to the R1 growth stage; therefore, nearby dicamba-sensitive soybean planted at similar dates will also be in the reproductive stages (Anonymous 2016a, 2016b). Previous research has examined the effect of dicamba applied at low rates during reproductive development. Yield reduction of 20% required only 4 g ae ha−1 when applied at bloom, whereas 35 g ha−1 was required for the same yield reduction in vegetative stages (Wax et al. Reference Wax, Knuth and Slife1969). Furthermore, the dicamba applied at 11 g ha−1 at early bloom reduced yield 9% to 42% while not affecting yield at any other growth stage (Auch and Arnold Reference Auch and Arnold1978). More recent research also supports the previous claims of Wax et al. (Reference Wax, Knuth and Slife1969) and Auch and Arnold (Reference Auch and Arnold1978), who documented greater yield reduction from dicamba at R2 compared with V3 applications when applied at the same rate (Robinson et al. Reference Robinson, Simpson and Johnson2013; Solomon and Bradley Reference Solomon and Bradley2014). In other research, soybean yield loss was 2.5 times greater at the R1 growth stage than at V3/V4 when exposed to dicamba at 4.4 and 17.5 g ha−1 (Griffin et al. Reference Griffin, Bauerle, Stephenson, Miller and Boudreaux2013). Previous research may warrant the concern some have over dicamba applications near reproductive dicamba-sensitive soybean, as studies reveal that yield loss is of more concern once soybean reaches the flowering stages.
In most instances, glyphosate will be combined with dicamba to achieve broad-spectrum control of both grass and broadleaf weed species. Interactions have been documented concerning the addition of glyphosate to other herbicides in terms of soybean phytotoxicity and weed control. For instance, the addition of glyphosate at 1,270 g ha−1 to dicamba at 5.6 g ha−1 applied at the V7 growth stage to glyphosate-resistant/dicamba-sensitive soybean caused 30% to 35% injury compared with 27% to 28% injury when dicamba was applied alone at 2 wk after application (Kelley et al. Reference Kelley, Wax, Hager and Riechers2005). Control of glyphosate-resistant tall waterhemp (Amaranthus tuberculatus (Moq.) J. D. Sauer) increased when glyphosate was mixed with dicamba (Spaunhorst and Bradley Reference Spaunhorst and Bradley2013). It was assumed that the effect seen in glyphosate-resistant soybean was because glyphosate slowed the metabolism of dicamba, increasing the intensity and duration of injury over dicamba alone (Kelley et al. Reference Kelley, Wax, Hager and Riechers2005); however, no explanation was included in regard to waterhemp control by the tank mixture (Spaunhorst and Bradley Reference Spaunhorst and Bradley2013).
Dicamba-sensitive soybean exposed to low doses of dicamba at the reproductive stages results in offspring that display dicamba-like injury symptoms soon after emergence (Barber et al. Reference Barber, Norsworthy, Bond, Steckel and Reynolds2015; Thompson and Egli Reference Thompson and Egli1973). Conversely, for glyphosate, there is no effect on glyphosate-sensitive offspring when low doses of the herbicide are applied to parent plants during reproductive development (Norsworthy Reference Norsworthy2004). Again, the addition of glyphosate to dicamba increases leaf injury to glyphosate-resistant soybean over dicamba alone (Kelley et al. Reference Kelley, Wax, Hager and Riechers2005); however, the effect of low doses of the mixture on offspring needs to be examined.
Previous research has documented that glyphosate is accumulated in bolls of cotton plants exposed during reproductive growth (Pline et al. Reference Pline, Price, Wilcut, Edmisten and Wells2001); however, research pertaining to growth, maturity, and yield effects of low doses of dicamba plus glyphosate on glyphosate- and dicamba-sensitive soybean is limited and needs to be expanded to further to understand potential risks associated with using both herbicides as a mixture or premix in DR crops. Greater soybean yield loss and transmission of dicamba-like symptoms to offspring have been associated with applications of low doses of dicamba during reproductive development (Auch and Arnold Reference Auch and Arnold1978; Barber et al. Reference Barber, Norsworthy, Bond, Steckel and Reynolds2015; Solomon and Bradley Reference Solomon and Bradley2014; Thompson and Egli Reference Thompson and Egli1973; Wax et al. Reference Wax, Knuth and Slife1969). Therefore, an experiment was conducted to examine the effects of low doses of dicamba and glyphosate alone and in combination on soybean sensitive to dicamba and glyphosate during reproductive development. Subsequently, seeds collected from parent plants exposed to dicamba and glyphosate were evaluated to assess the impact of both herbicides alone and in combination on offspring.
Materials and Methods
Field Experiment
Experiments were planted to glufosinate-resistant (glyphosate- and dicamba-sensitive) soybean on April 30, 2015, and May 4, 2016, at the Arkansas Agriculture Research and Extension Center (AAREC) in Fayetteville, AR (2015: 36.0941 N, 94.1744 W; 2016: 36.0952 N, 94.1732 W), and on May 14, 2016, at the Pine Tree Research Station (PTRS) near Colt, AR (2016: 35.1249 N, 90.9633 W). Previous research has identified indeterminate soybean to be more sensitive than determinate soybean varieties to height and yield reduction from low rates of dicamba applied in reproductive stages (McCown et al. Reference McCown, Barber, Norsworthy, Rose, Ross and Collie2016b). Therefore, to limit variability in results, all varieties used in these experiments were of indeterminate growth habit (Table 1). The soil series at PTRS was a Calhoun silt loam (fine-silty, mixed, active, thermic Typic Glossaqualfs) with a pH of 7.8 and 2.23% organic matter. Fields at AAREC were classified as Leaf silt loam (fine, mixed, active, thermic Typic Albaquults) with a pH of 6.1 and 1.75% organic matter. Trials were seeded at 345,800 seeds ha−1 with the intention of obtaining a population of 275,000 plants ha−1 given 80% germination. Trials were conventionally tilled and beds were pulled to a 76-cm (PTRS) or 91-cm (AAREC) row spacing. At PTRS, soybean was furrow irrigated; at AAREC, plots were irrigated with overhead lateral irrigation. Experiments were irrigated once weekly at 2.5 cm if less than 2.5 cm of rainfall occurred over a 7-d period. Other agronomic information pertaining to each location is provided in Table 1.
Table 1 Soybean cultivars, plot sizes, planting dates, and application dates for experiments conducted in Fayetteville and Pine Tree, AR.
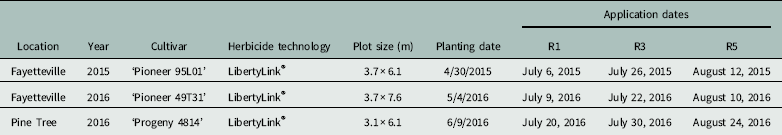
Weeds were controlled at the experimental sites with a PRE application of flumioxazin at 70 g ai ha−1 at planting followed by two POST applications of glufosinate at 530 g ai ha−1 (Liberty®, Bayer CropScience, Research Triangle Park, NC 27709) plus S-metolachlor (Dual Magnum®, Syngenta, Greensboro, NC 27408) at 1,064 g ai ha−1 added to the first POST application. Treatments were arranged in a randomized complete block (RCB) design with four replications. Dicamba (Clarity®, BASF), glyphosate (Roundup PowerMax®, Monsanto), or a mixture of the two herbicides was applied at 1/64X (dicamba at 8.75 g ae ha−1, glyphosate at 13.44 g ae ha−1) or 1/256X (dicamba at 2.19 g ha−1, glyphosate at 3.36 g ha−1) of the recommended rate (dicamba at 560 g ha−1, glyphosate at 870 g ha−1) for DR cotton and soybean. Nonionic surfactant was added at 1/64X or 1/256X the full rate of 0.25% v/v (Induce®, Helena Chemical, Collierville, TN) to dicamba-alone treatments, but not dicamba plus glyphosate treatments, because the glyphosate product already contained an adjuvant. Treatments were mixed using serial dilution from a stock 1X rate, and applications were made on each variety at R1 (initial flower), R3 (initial pod set), and R5 (initial seed formation) soybean growth stages. Treatments were applied using a handheld boom and CO2-pressurized backpack sprayer with an output of 140 L ha−1 at 270 kPa tipped with AIXR110015 nozzles (TeeJet® Technologies, Springfield, IL 62703). Only the center 2 rows of each 4-row plot were treated. Plot sizes are given in Table 1.
At 2 and 4 wk after application, visual assessments of percent leaf malformation were recorded on a scale of 0% to 100%, with 100% being severe malformation throughout the plant. Pod malformation was rated similarly at harvest. Soybean height (cm) was recorded at 4 wk after application (canopy height) and again at soybean maturity by measuring from the soil surface to the terminal shoot of three representative plants. Plots were harvested using a small-plot combine, and soybean grain yield was adjusted to 13% moisture. Soybean heights and yield were later converted to a percentage relative to the nontreated control. The day each plot reached R8 was recorded for examining any delay in maturity that may have occurred. In addition, a sample of approximately 500 seeds from each plot was stored at −10 C after harvest.
Greenhouse Experiment
Seed samples from the previous field experiments were evaluated in a greenhouse at the University of Arkansas Altheimer Laboratory in Fayetteville, AR. One experiment for each site-year was completed using offspring from both years at AAREC and from 2016 t PTRS. Twenty-five seeds from each sample were planted at a 2-cm depth into 33 by 18 by 13 cm trays that were filled with potting mix (Sun Gro Horticulture, Seba Beach, AB, Canada). Trays from each of the four replications were arranged in an RCB design in the greenhouse. The greenhouse was maintained at 32 C daytime and 22 C nighttime temperatures (±3 C). Natural lighting was supplemented by a metal-halide lighting system and set to a 16-h photoperiod. Plants were watered daily to maintain adequate moisture levels. At 21 d after planting (DAP), emergence (%), injury (0% to 100% with 0% being no injury and 100% being plant death relative the nontreated control), and number of plants injured (number of plants injured/total number of emerged plants) were recorded for each tray. Plants were considered injured if they exhibited leaf cupping, leaf strapping, stem epinasty, or stunting, which are common symptoms of soybean exposed to dicamba (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Andersen et al. Reference Andersen, Clay, Wrage and Matthees2004; Sciumbato et al. Reference Sciumbato, Chandler, Senseman, Bovey and Smith2004). Additionally, plant vigor was rated on a 1 to 5 scale for each tray, where 1 was extremely low vigor (delayed and/or reduced emergence) and 5 was extremely high vigor (seedlings quickly emerged and exhibited normal growth). A standardized rating for vigor has yet to be realized, but the concept of vigor and its importance in crop development are well accepted (Pollock and Roos Reference Pollock and Roos1972). Aboveground biomass was collected at 21 DAP, dried at 66 C for 7 d, and weighed. Percent reduction in biomass was calculated relative to the nontreated control.
Statistical Analysis
Data from field and greenhouse trials were subjected to an ANOVA procedure using JMP 12 Pro (SAS Institute, Cary, NC 27511). Site-year and replication nested within site-year were considered random effects. Soybean growth stage (timing), herbicide treatment, and rate were considered fixed effects. Previous research has documented little to no response by soybean to low rates of glyphosate applied during reproductive development (Norsworthy Reference Norsworthy2004). In the current experiment, glyphosate treatments caused no response and were excluded from the analysis, thereby reducing the herbicide treatment factor level to two. Remaining data met the assumptions necessary for ANOVA. Main effects and interactions for dependent variables were assessed. Means were separated using Fisher’s protected LSD test (α = 0.05).
Results and Discussion
Soybean Response to Dicamba during Reproductive Development
At 14 d after application (DAA), leaf malformation was significant for the rate by timing interaction (P = 0.012) and the main effects of timing (P < 0.0001) and herbicide (P = 0.0292). Averaged across rate and timing, leaf malformation at 14 DAA was greater when glyphosate was added to dicamba (8%) compared with dicamba alone (6%) (unpublished data). Applications occurring at the R1 growth stage caused more leaf malformation than those at later timings.
At 28 DAA, an interaction between herbicide and timing was observed (P = 0.0425), along with a rate by timing interaction (P < 0.0001). When applications were made at the R3 and R5 growth stages, leaf malformation at 28 DAA was similar for dicamba alone and dicamba plus glyphosate. However, at 28 DAA, addition of glyphosate to dicamba in the R1 growth-stage treatments produced a significant 6 percentage point increase in leaf malformation compared with dicamba alone. Similarly, greater leaf malformation was observed at the 1/64X rate than the 1/256X rate at the R1 growth stage when averaged over herbicides, but no difference between rates occurred at later timings (Table 2). These results are explained by examining indeterminate soybean plants at each respective stage. During early reproductive stages (R1), vegetative growth is still occurring at a rapid pace under ideal conditions (Heatherly and Elmore Reference Heatherly and Elmore2004). Once pod formation initiates (R3), vegetative growth slows significantly and nearly ceases once seed formation begins (R5). Therefore, it is not surprising that soybean exposure to glyphosate and dicamba resulted in greater leaf malformation when plants were still undergoing vegetative growth.
Table 2 Leaf malformation, pod malformation, height, maturity delay, and yield of soybean when exposed to dicamba and glyphosate applied at two rates during the R1, R3, and R5 growth stages.a

a Means followed by the same letter within a column are not significantly different using Fisher’s protected LSD (α = 0.05).
b The 1X rates of dicamba and glyphosate were 560 and 870 g ae ha−1, respectively.
c Leaf malformation and pod malformation averaged for glyphosate-containing treatments were not included due to lack of soybean response.
Visible leaf malformation (injury) at 28 DAA resulting from dicamba at 1/64X applied at the R1 growth stage was 37% (Table 2), which was somewhat similar to that documented by Kelley et al. (Reference Kelley, Wax, Hager and Riechers2005), who reported that 38% injury resulted to indeterminate soybean from dicamba at 1/100X at 28 DAA during flowering. Solomon and Bradley (Reference Solomon and Bradley2014) observed 15% injury to indeterminate soybean at 28 DAA of dicamba at 1/200X, whereas the current study documented 27% injury at a comparable rate and timing. The extent of injury to soybean from dicamba is known to be greater for indeterminate varieties when exposure occurs in reproductive development and varies with environmental conditions, irrigation practices, and rainfall before, during, and after application (Auch and Arnold Reference Auch and Arnold1978; McCown et al. Reference McCown, Barber, Norsworthy, Rose, Ross and Collie2016b; Wax et al. Reference Wax, Knuth and Slife1969; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989).
At soybean maturity, pod malformation involved interactions of herbicide by timing (P = 0.0033) and rate by timing (P = <0.0001). Pod malformation at soybean maturity was not increased with the addition of glyphosate to dicamba, averaged over rates, at the R1 and R5 growth stages (Table 2). The addition of glyphosate to dicamba increased pod malformation by 10 percentage points when applied at the R3 growth stage (Table 2). When averaged across herbicide, pod malformation was greatest after application of the high rate at the R3 growth stage (47%). This timing by rate combination was significantly greater than the low rate at this timing (23%) and in all other combinations.
Pod malformation following dicamba exposure has been documented in previous research, but not quantified (Auch and Arnold Reference Auch and Arnold1978; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). In the present study, the greatest percentage of pod malformation followed applications to R3 soybean. The focus of soybean at the R3 growth stage is to initiate pod formation; therefore, exposure to dicamba will have the greatest possibility of generating severe pod malformation. Dicamba exposure to soybean at the R1 growth stage caused severe leaf malformation; however, pod formation has not yet begun at this timing. Hence, soybean plants have time to recover from dicamba exposure, which may lead to a lower dicamba concentration in the plant before pod formation begins and consequently result in a lower percentage of malformed pods. By the time seed formation stages (R5 to R6) are reached, pod formation has been completed in all but the top nodes of soybean plants. In the current study, pod malformation after a low dose of dicamba at R5 was minimal (2% to 5%) and only documented in the upper 2 to 4 nodes.
When averaged across rates, glyphosate alone did not reduce 28 DAA canopy or mature terminal height of soybean at any timing relative to the nontreated check at 28 DAA or maturity (Table 2). Canopy height at 28 DAA was reduced most by dicamba (24%) and dicamba plus glyphosate (26%) when applied at the R1 growth stage averaged over rate (Table 2). The application of dicamba and dicamba plus glyphosate to soybean at the R3 growth stage resulted in canopy height reductions of 14% and 10%, respectively, at 28 DAA. The application of herbicides at the R5 growth stage did not reduce soybean canopy height compared with the nontreated check whether assessments were taken at 28 DAA or at maturity. In general, height reductions decreased as dicamba applications were delayed. These findings lead to the conclusion that dicamba exposure to soybean in early flowering stages has the greatest risk for height reduction among applications during reproductive development, as has been reported in other research (Auch and Arnold Reference Auch and Arnold1978; Solomon and Bradley Reference Solomon and Bradley2014; Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989). The lack of height reduction at later stages is likely because soybean plants shift to pod and seed production and plants are already near maximum height.
The delay in maturity was minimal in the present study, with no treatment resulting in more than a 4-d delay in maturity (Table 2). The herbicide dose range in the present and past research using indeterminate soybean induced comparable delays in soybean maturity (Solomon and Bradley Reference Solomon and Bradley2014). In other research, delays in soybean maturity increased with dicamba rate (Auch and Arnold Reference Auch and Arnold1978; Kelley et al. Reference Kelley, Wax, Hager and Riechers2005; Wax et al. Reference Wax, Knuth and Slife1969). Auch and Arnold (Reference Auch and Arnold1978) reported delays in soybean maturity to range from 3 to 19 d when dicamba at 11 to 56 g ha−1 was applied at the reproductive stages. Comparable delays (4 to 24 d) were reported when 2 to 64 g ha−1 was applied in bloom stages (Wax et al. Reference Wax, Knuth and Slife1969).
Soybean grain yield reduction involved both herbicide by timing (P < 0.0001) and rate by timing (P = 0.0087) interactions. Glyphosate applications did not reduce yield at any timing compared with the nontreated control (Table 2), which agrees with previous research by Norsworthy (Reference Norsworthy2004), who found that glyphosate at 8 g ha−1 applied at the R2 or R5 stages did not reduce yield. The greatest yield reductions were from dicamba alone or with glyphosate applied at the R1 growth stage, which has been reported previously (Auch and Arnold Reference Auch and Arnold1978; Solomon and Bradley Reference Solomon and Bradley2014; Wax et al. Reference Wax, Knuth and Slife1969). Yield reductions from R3 applications of dicamba (7%) and dicamba plus glyphosate (6%), averaged over rates, were small but significant. Applications during seed fill (R5) did not reduce yield compared with the nontreated check. Yield reduction was present only in treatments in which height reduction at maturity occurred. Soybean yield reduction following mature height reduction has been documented previously (Weidenhamer et al. Reference Weidenhamer, Triplett and Sobotka1989).
Effect of Soybean Exposure to Dicamba on Offspring
Emergence of soybean offspring was significant for the main effects of herbicide (P = 0.003) and rate (P = 0.0481). Glyphosate added to dicamba had no effect on offspring emergence relative to dicamba alone; however, dicamba-containing treatments lowered emergence by as much as 3 percentage points compared with the nontreated check (unpublished data). Soybean emergence from plants treated with the lowest rate was 100%, and high rates decreased emergence 2% (unpublished data), which is likely not of biological importance and would not be noticed at a commercial production scale. Ideal growing conditions in the greenhouse may have expedited soybean emergence over less than ideal field environments. Previous research using higher rates of dicamba applied during reproductive development showed reductions in germination and emergence (Thompson and Egli Reference Thompson and Egli1973; Wax et al. Reference Wax, Knuth and Slife1969). Germination was not affected by rates similar to those used in this study; yet Wax et al. (Reference Wax, Knuth and Slife1969) reported that germination was reduced to 79% and 19% when 1/32 (17.5 g ha−1) and 1/16X (35 g ha−1) rates were applied. Emergence was only 50% when dicamba at 30 g ha−1 was applied, and soybean offspring failed to emerge when dicamba at 220 g ha−1 was applied during flowering stages (Thompson and Egli Reference Thompson and Egli1973).
The occurrence of offspring plants having dicamba-like injury was dependent on the interaction of rate and timing (P = 0.0026) (Table 2). Soybean plants exposed to a low dose of dicamba at the R5 growth stage were more likely to experience a high percentage of injured offspring; however, adding glyphosate to dicamba did not increase injury to the offspring (Table 3). The highest percentage of injured plants (96%) resulted when parent plants were treated with the high rate of dicamba alone and including glyphosate applied at the R5 growth stage. The low rates applied at R5 reduced incidence of emerged soybean offspring with dicamba-like symptoms to 81%. Applications of high and low rates at R3 resulted in 59% and 34% of offspring being malformed, respectively. No difference was observed in the percentage of plants malformed between high and low rates applied at the R1 growth stage, and symptoms were less than those for other combinations of rate and timing.
Table 3 Percentage of plants injured and intensity of leaf malformation documented in offspring whose parents were exposed to low rates of glyphosate and dicamba during reproductive development.a
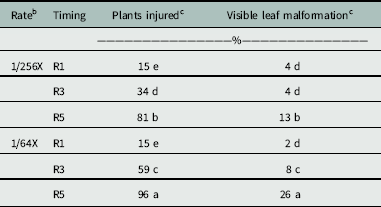
a Means followed by the same letter within a column are not statistically different using Fisher’s protected LSD (α = 0.05).
b The 1X rates of dicamba and glyphosate were 560 and 870 g ae ha−1, respectively.
c Percentage of plants injured and injury ratings for glyphosate-only treatments were not included, because no response was observed.
Overall, percentage of plants malformed and the degree of injury increased as application to soybean was delayed (Table 3), likely because application at late reproductive stages allowed for more dicamba storage in the seed. Dicamba exposure during reproductive development may allow offspring emergence, but with many of the emerged plants having malformed leaves. If auxin-like symptomology arises in newly planted soybean fields, growers may have cause for concern. In severe cases, the auxin-like symptomology could be mistaken as drift of auxin herbicides, causing growers to file false herbicide-misuse complaints to state agencies.
Reductions in vigor generally increased with later applications for all treatments, except for glyphosate alone, which maintained vigor at all applications and rates (Table 4). The addition of glyphosate to dicamba did not reduce soybean offspring vigor at any growth stage compared with dicamba alone. Vigor reduction in offspring caused by dicamba-containing solutions applied at the R1 growth stage ranged from 11% to 12%, regardless of rate. Treatment with dicamba-containing solutions at R3 resulted in reduced vigor in offspring ranging from 15% to 20% but did not differ between rates. Application of dicamba at seed fill (R5) had the greatest impact on offspring vigor. Dicamba and dicamba plus glyphosate applications at R5 at the low rate caused 22% and 30% reductions in vigor. Vigor was reduced more from the high rate of dicamba-containing solutions applied at R5 than from any other treatment.
Table 4 Relative vigor and biomass reduction documented in offspring whose parents were exposed to low rates of glyphosate and dicamba during reproductive development.a
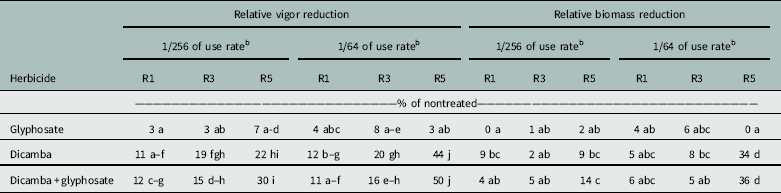
a Means followed by the same letter within relative vigor reduction and relative biomass reduction are not statistically different using Fisher’s protected LSD (α = 0.05).
b Fraction of full labeled rate (560 g ae ha−1 of dicamba and 870 g ae ha−1 of glyphosate).
Reduction in soybean offspring biomass for glyphosate-alone treatments was minimal (0% to 6%) (Table 4). The addition of glyphosate to dicamba did not further decrease biomass. Dicamba and dicamba plus glyphosate treatments caused similar biomass reduction when applied at the R1 and R3 growth stages, with values ranging from 4% to 8%. Trends for this parameter generally followed vigor reduction, as the greatest offspring biomass reduction occurred from the R5 application. At this timing, the lowest rate of dicamba alone and dicamba plus glyphosate resulted in 9% and 14% reduction in offspring biomass, respectively. At the higher rate, application of dicamba alone led to a 34% reduction, and the addition of glyphosate reduced biomass to 36% of that of the nontreated check.
These results document that dicamba exposure to soybean at the R5 growth stage can decrease vigor of offspring by as much as half and biomass up to a third. Injury observed to parents from soybean exposure to low doses of dicamba at seed fill was minimal. Therefore, it may be possible that dicamba exposure to soybean could go unnoticed in seed production fields and continue through the harvest, cleaning, and bagging processes. Furthermore, standard germination tests may not identify poor quality seed, as dicamba-containing solutions only slightly reduced emergence (2% to 3%) in this study. Identification of seed contaminated by dicamba may be difficult, as testing of seed for presence of dicamba through laboratory analysis could prove costly. Therefore, contaminated seed may not be identified and may subsequently be distributed to growers.
Practical Implications
The addition of an alternative herbicide site of action will increase diversity in soybean and cotton weed control programs. Including a grass-controlling herbicide such as glyphosate is a necessity in dicamba/glyphosate-resistant cropping systems for broad-spectrum weed control. Precautions must be taken to reduce the chance of off-target movement to susceptible crops. Increased leaf or pod malformation caused by glyphosate addition to dicamba will not further reduce yields over a comparable dose of dicamba alone. Further research must be completed to determine whether glyphosate is aiding in the translocation of dicamba to cause the observed effect in parent plants.
This research does conclude that seed fill exposure of soybean to dicamba will lead to greater offspring reductions in vigor and biomass; therefore, further research completed during seed fill using additional rates of glyphosate and dicamba may detect differences. The addition of glyphosate to dicamba had no effect on soybean offspring, as emergence, malformation, and biomass are similar to those for soybean exposed to dicamba alone. However, the appearance of auxin symptomology on soybean offspring may be troubling, in that it could lead to suspected tank contamination or drift of dicamba by applicators. Additional training may be helpful for commercial applicators involved in DR cropping systems, as not all are aware of the care that needs to be taken when applying dicamba (Bish and Bradley Reference Bish and Bradley2017). Dicamba application training is crucial to inform applicators of the stringent guidelines that must be followed to ensure dicamba initially reaches the intended target.
Acknowledgments
This research was funded by the Arkansas Soybean Promotion Board. No conflicts of interest have been declared.






