Introduction
Rigid ryegrass is the most problematic grass weed species in winter grain crops in Australia. This weed currently infests more than 8 million ha of grain cropping areas, resulting in losses of AU$93 million annually for Australian grain growers (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). Uncontrolled rigid ryegrass can cause a significant reduction in the yields of wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), and canola (Brassica napus L.), for example, up to 80% in wheat (Lemerle et al. Reference Lemerle, Tang, Murray, Morris and Tang1996). It is a prolific seed producer (Chauhan et al. Reference Chauhan, Gill and Preston2007), and the lightweight seeds are easily dispersed by natural (water, wind, animals, etc.) and anthropogenic (farm implements, produce, grain, etc.) means (Beckie and Jasieniuk Reference Beckie, Jasieniuk and Chauhan2021).
A recent Australian study reported that rigid ryegrass accessions can germinate across a wide range of diurnally fluctuating temperatures, from 15/5 C to 35/25 C (day/night temperatures) (Thompson et al. Reference Thompson, Mahajan and Chauhan2021). More than 90% of seeds germinated at 30/20 C and 35/25 C, suggesting that this weed could germinate in the summer months in Australia provided that soil moisture is sufficient. Although rigid ryegrass is a well-recognized problem in winter crops and winter fallow, there are recent reports of its occurrence in summer crops (e.g., cotton) and summer fallow in the southeastern region of Australia (Thompson and Chauhan Reference Thompson and Chauhan2022; Thompson et al. Reference Thompson, Mahajan and Chauhan2021).
In Australia, herbicides have become the most common method of weed control, and overreliance on herbicides has resulted in the evolution of resistance in rigid ryegrass (Heap Reference Heap2022). This weed has developed resistance to several classes of POST herbicides, including acetyl coenzyme-A carboxylase (ACCase) inhibitors, acetolactate synthase (ALS) inhibitors, and the 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase inhibitor glyphosate. Some factors contributing to the evolution of resistance in rigid ryegrass are its high seed production and high genetic diversity within accessions (Saini et al. Reference Saini, Malone, Preston and Gill2015).
Glyphosate is the most commonly used herbicide to control weeds in glyphosate-resistant cotton and summer fallow in Australia. Owing to the continuous use of this herbicide in these situations, several summer annual weed species have evolved resistance (Heap Reference Heap2022). Rigid ryegrass has also evolved resistance to glyphosate and ACCase inhibitors, such as haloxyfop, in winter crop production systems. However, there is no information on the effectiveness of these and other selective and nonselective herbicides on summer-emerging accessions of rigid ryegrass.
Before rigid ryegrass becomes a widespread weed in summer fallow and summer crops (Thompson and Chauhan Reference Thompson and Chauhan2022), there is an urgent need to identify different herbicide options for its control. Therefore an outdoor pot study was conducted with two summer-emerging and two winter-emerging accessions of rigid ryegrass to answer the following questions: (1) what POST herbicide options exist for selective control of summer-emerging rigid ryegrass accessions and (2) if summer-emerging and winter-emerging rigid ryegrass accessions respond differently to various POST herbicide treatments.
Material and Methods
Seed Description
Four accessions of rigid ryegrass were used in this study: W3 and W8 as winter-emerging accessions and S3 and S6 as summer-emerging accessions. Seeds of W3 were collected on December 8, 2018, from the fence line of a sorghum field in Croppa Creek, New South Wales (NSW), Australia (S27°36.980; E152°12.486). W8 seeds were collected on November 12, 2018, from a wheat field in Wagga Wagga, NSW (S34°53.092; E147°04.555). Seeds of S3 were collected on March 25, 2019, from the fence line of a cotton field in Griffith, NSW (S34°30.019; E146°08.611). Seeds of S6 were collected on March 26, 2019, from a cotton field in Griffith, NSW (S34°43.417; E146°03.409). Plants were still green, suggesting that these plants were germinated in the last summer season. In Australia, winter, spring, summer, and autumn seasons are from June to August, September to November, December to February, and March to May, respectively. Seeds were stored at room temperature (25 ± 2 C) until used for experiments.
General Protocol
Pot trials were conducted during the spring and summer seasons of 2021 to 2022 at the weed science research facility of the University of Queensland, Gatton, Australia. All trials were conducted two times. Plastic pots (14 cm diameter) filled with potting mix (Centenary Landscape, Queensland, Australia) were used in this study. Seeds (10 to 12 per pot) were planted at 0.5 cm depth, and seedlings were thinned immediately after emergence to keep five plants per pot. Pots were kept on benches under natural temperature and light conditions. The average minimum and maximum temperatures during the experiment were 17.7 C and 30.1 C, respectively. Irrigation was applied two times every day using an automated sprinkler system, but no fertilizer was added to the pots.
Herbicides were applied at the 4- to 5-leaf stage of rigid ryegrass. All herbicide treatments were applied in a spray cabinet with a track sprayer fitted with TeeJet® XR110015 flat-fan nozzles (TeeJet® Technologies, Wheaton, IL, USA). The sprayer delivered a spray volume of 108 L ha−1. After treatment, pots were returned to the outdoor area. At 28 d after herbicide application, plant survival and biomass data were recorded. Plants were considered alive if they had at least 1 new leaf. Surviving plants from each pot were harvested together from the base and dried in an oven at 70 C for 72 h. Biomass of dry plants was weighed.
Experiment 1: Performance of POST Herbicides
Using the procedures described earlier, five plants per pot of four rigid ryegrass accessions were established in pots and grown outdoors to the 4- to 5-leaf stage. The performance of 11 commonly used POST herbicides was evaluated on rigid ryegrass accessions (Table 1). There were also unsprayed control treatments for each accession. The selected herbicides are commonly used in Australian farming systems to control rigid ryegrass. Each treatment was replicated four times.
Table 1. Herbicide treatments, their sites of action, doses, and adjuvants used in Experiment 1. a, b
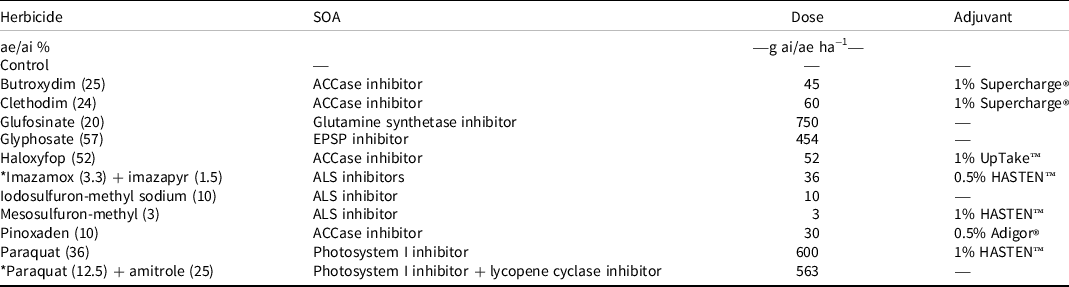
a Adigor® contains 440 g L−1 methyl esters of canola oil fatty acids; HASTEN™ contains 704 g L−1 ethyl and methyl esters of vegetable oil with 196 g L−1 nonionic surfactants; Supercharge® is 471 g L−1 paraffin oil; and UpTake™ contains 582 g L−1 paraffinic oil and 240 g L−1 alkoxylated alcohol nonionic surfactants. An asterisk indicates a commercial mixture.
b Abbreviations: ACCase, acetyl-coenzyme-A carboxylase; ae, acid equivalent; ai, active ingredient; ALS, acetolactate synthase; EPSP, 5-enolpyruvylshikimate-3-phosphate; SOA, site of action.
Experiment 2: Dose–Response Experiment
Experiment 1 identified differential responses of summer-emerging and winter-emerging accessions of rigid ryegrass to glufosinate, glyphosate, haloxyfop, and pinoxaden. To further examine these differences, these accessions were screened in dose–response pot studies using the procedures described earlier. As winter-emerging accessions (W3 and W8) were effectively controlled at the field rates of glufosinate, glyphosate, haloxyfop, and pinoxaden in Experiment 1, these accessions were screened at the seven rates 0X, 0.0625X, 0.125X, 0.25X, 0.5X, 1X, and 2X (Table 2). Summer-emerging accessions (S3 and S6) were not controlled at the field rates of glufosinate, glyphosate, haloxyfop, and pinoxaden in Experiment 1; these accessions were screened at the seven rates 0X, 0.25X, 0.5X, 1X, 2X, 4X, and 8X (Table 2). Summer- and winter-emerging accessions responded similarly to imazamox (33 g L−1) + imazapyr (15 g L−1) (a commercial mixture), iodosolfuron, and mesosulfuron in Experiment 1. Therefore rates were kept the same (0X, 0.25X, 0.5X, 1X, 2X, 4X, and 8X) for these three herbicides. Herbicides that provided complete control of the four accessions of rigid ryegrass in Experiment 1 were not included in the dose–response experiment.
Table 2. Herbicide doses used for four accessions of rigid ryegrass in Experiment 2. a
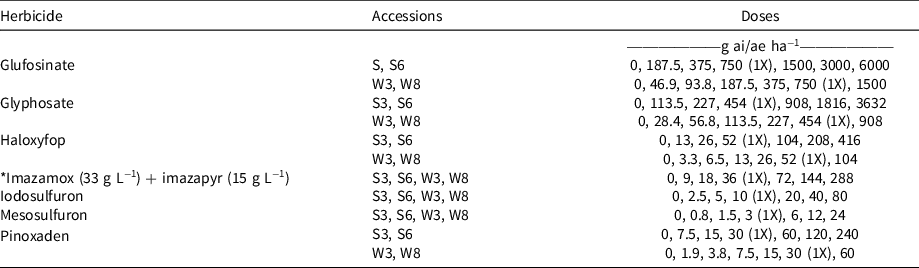
a Recommended doses are shown with 1X. An asterisk indicates a commercial mixture.
Statistical Analyses
Experiments were conducted in a randomized complete block design with four replications in Experiment 1 and three replications in Experiment 2. A pot with five plants was considered an experimental unit (one pot representing a replication). All experiments were repeated within a week after the completion of the first run. Data were subjected to analysis of variance (ANOVA) (Genstat 2021) to test for treatment by experimental run interaction. There was no interaction, and therefore data were pooled over the two experimental runs. In Experiment 1, each accession was analyzed separately, and means of survival and biomass were separated at P ≤ 0.05 using Fisher’s protected least significant difference test. In Experiment 2, seedling survival and biomass (percent of nontreated control) data were regressed against herbicide dose using a three-parameter logistic model using SigmaPlot 14.5 (Systat Software Inc., Palo Alto, CA, USA):
In this model, Y is the survival or biomass data at herbicide dose x, a is the maximum seedling survival or biomass, H 50 is the herbicide dose (g ae or ai ha−1) required for a 50% reduction in seedling survival (LD50) or biomass (GR50), and b is the slope.
Results and Discussion
Experiment 1: Performance of POST Herbicides
Summer-emerging accessions (S3 and S6) of rigid ryegrass were more poorly controlled by glufosinate, glyphosate, haloxyfop, and pinoxaden herbicide treatments than were winter-emerging accessions (W3 and W8) (Table 3). Glyphosate-, haloxyfop-, and pinoxaden-resistant rigid ryegrass accessions have been reported in Australia (Boutsalis et al. Reference Boutsalis, Gill and Preston2012; Heap Reference Heap2022; Owen and Powles Reference Owen and Powles2010), but glufosinate-resistant rigid ryegrass has not previously been reported in Australia. The first case of glufosinate-resistant rigid ryegrass was reported in Greece in 2018 (Travlos et al. Reference Travlos, Cheimona, De Prado, Jhala, Chachalis and Tani2018). Although 70% to 75% of seedlings of S3 and S6 accessions survived the field rate of glufosinate (750 g ai ha−1), the growth of these seedlings was severely affected, as indicated by the 79% to 87% reductions in biomass compared to the nontreated control plants (Table 3). In the nontreated control treatment, the biomass of S3, S6, W3, and W8 accessions was 2.2, 3.4, 2.1, and 1.8 g pot−1, respectively.
Table 3. Effect of herbicide treatments on survival and biomass of four accessions of rigid ryegrass when sprayed during summer months (Experiment 1). a, b
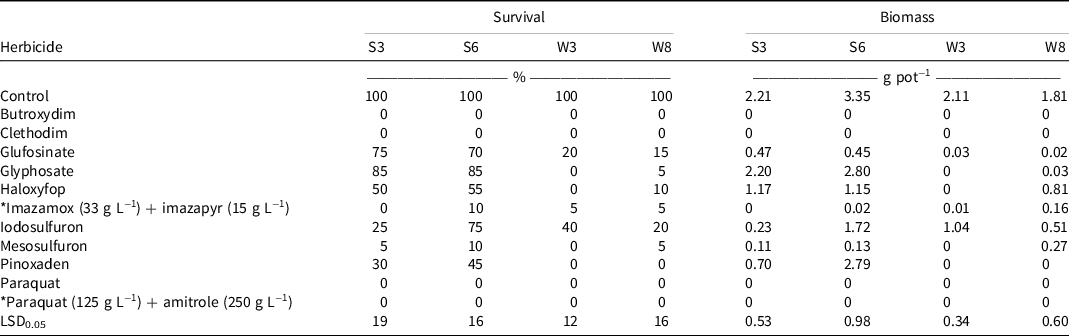
a An asterisk indicates a commercial mixture.
b Abbreviation: LSD0.05, least significant difference at the 5% level of significance.
The summer- and winter-emerging rigid ryegrass accessions were susceptible to widely used ACCase-inhibiting herbicides with apparent low-level resistance to ALS-inhibiting herbicides. Butroxydim, clethodim, paraquat, and paraquat + amitrole provided complete control of all four accessions of rigid ryegrass (Table 3). The susceptibility of all four accessions to butroxydim and clethodim is unusual given the high frequencies of resistance to these herbicides occurring in rigid ryegrass accessions in Australian cropping systems (Boutsalis et al. Reference Boutsalis, Gill and Preston2012; Broster et al. Reference Broster, Koetz and Wu2013; Owen et al. Reference Owen, Martinez and Powles2014; Saini et al. Reference Saini, Malone, Preston and Gill2015). There was low-level survival (≤10%) in three of the rigid ryegrass accessions following imazamox + imazapyr and mesosulfuron treatments (Table 3). Although there was survival, the plants were severely affected by these herbicide treatments, as indicated by the biomass reductions of at least 80%. The iodosulfuron treatment resulted in 20% to 75% survival of rigid ryegrass accessions, with the growth of these seedlings reduced by 50% to 90% (Table 3).
Experiment 2: Dose–Response Experiment
Glufosinate
The dose–response assay revealed that a glufosinate rate of 500 and 400 g ai ha−1 resulted in 50% mortality (LD50) in W3 and W8 accessions, respectively, whereas the same mortality was achieved with 1,050 and 1,130 g ai ha−1 in S3 and S6, respectively (Table 4; Figure 1). The GR50 values for the winter accessions (W3 and W8) were 270 to 320 g ai ha−1, whereas these values for the summer-emerging accessions were 680 to 1,120 g ai ha−1.
Table 4. Estimated herbicide doses required for a 50% reduction in accession survival (LD50) and plant biomass production (GR50) of rigid ryegrass accessions (Experiment 2). a
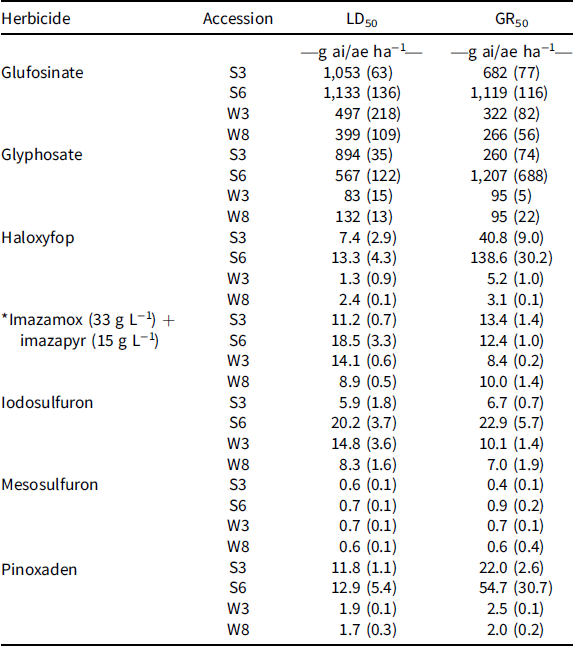
a Values in paratheses are standard errors. An asterisk indicates a commercial mixture.
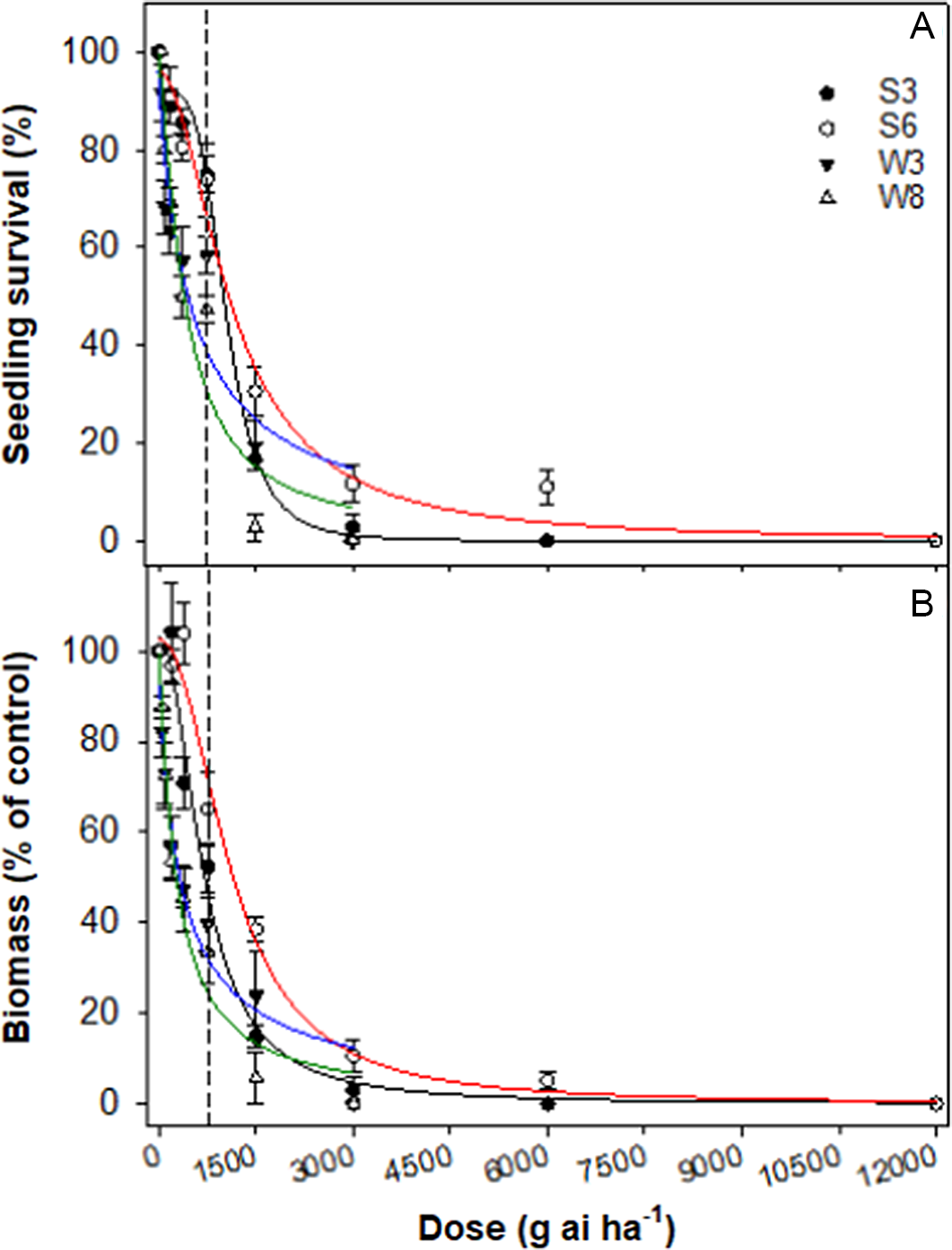
Figure 1. Effect of glufosinate dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Glufosinate is a nonselective, POST herbicide that is registered for fallow and preseeding weed control in Australian grain production systems. Although glufosinate-resistant rigid ryegrass has not been reported in Australia, there are reports that rigid ryegrass is not well controlled by glufosinate (Kumaratilake et al. Reference Kumaratilake, Lorraine-Colwill and Preston2002). Environmental factors, such as temperature and relative humidity, can greatly affect the efficacy of glufosinate (Kumaratilake and Preston Reference Kumaratilake and Preston2005; Ramsey et al. Reference Ramsey, Stephenson and Hall2002). However, these parameters were not studied in the current study. In Australia, glufosinate usage is expected to increase to manage glyphosate-resistant weed species (Chauhan et al. Reference Chauhan, Congreve and Mahajan2021). Summer-emerging accessions were collected from the cotton-growing fields and fence lines, and the poor control of these accessions with glufosinate suggests that the use of XtendFlex™ (Bayer Cropscience, Hawthorn East, Victoria, Australia) cotton (resistant to glyphosate, glufosinate, and dicamba) in the future (Werth et al. Reference Werth, Thornby, Keenan, Hereward and Chauhan2021) may not provide effective control of these accessions.
Glyphosate
The dose–response trial confirmed poor control of S3 and S6 accessions of rigid ryegrass by glyphosate. The LD50 value for S3 and S6 accessions were 890 and 570 g ai·ha−1, respectively, whereas those values for W3 and W8 accessions were 80 and 130 g ai·ha−1, respectively (Table 4; Figure 2). The GR50 values for S3 and S6 were 260 and 1,210 g ai·ha−1, respectively—greater than for the winter accessions (95 g ai ha−1).
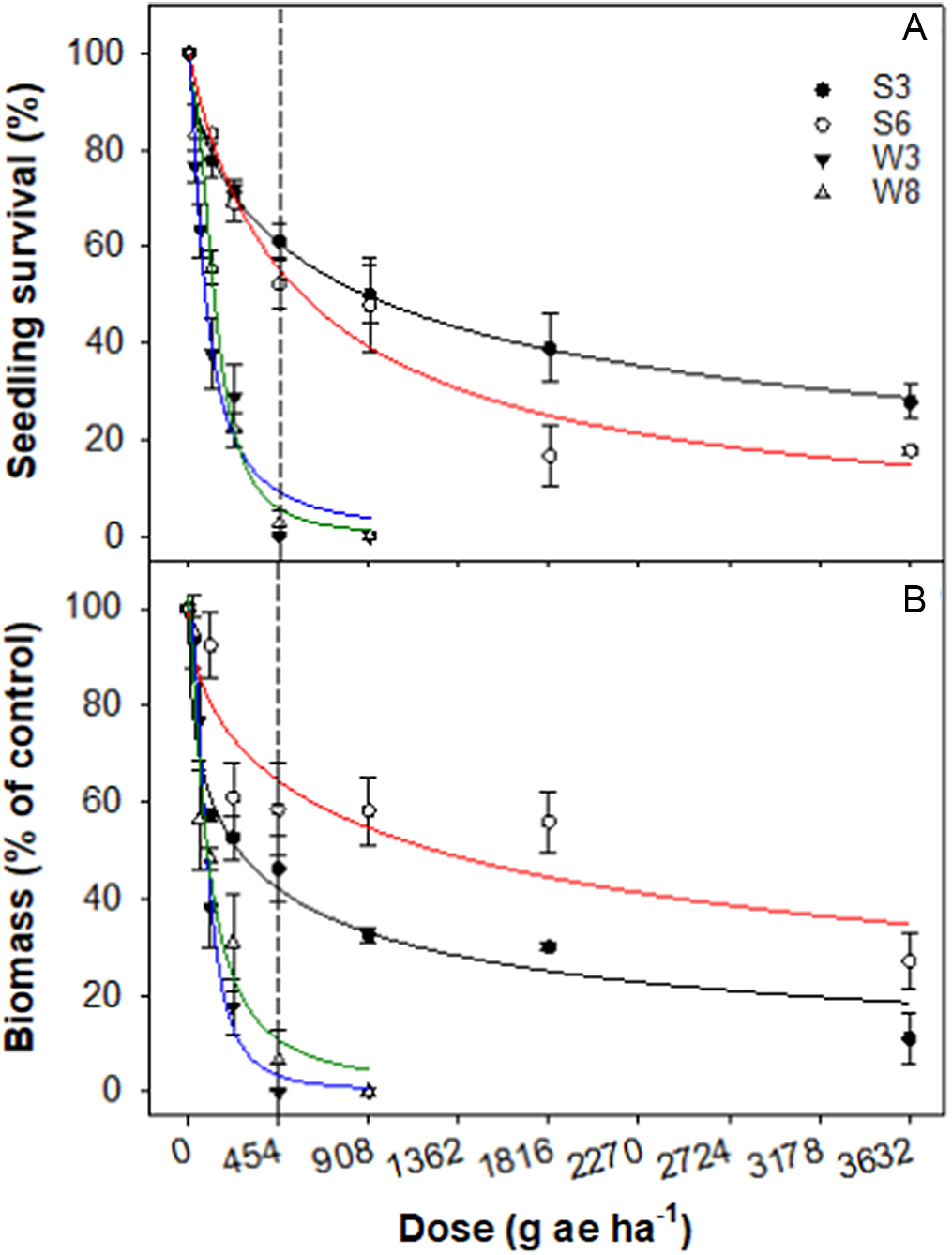
Figure 2. Effect of glyphosate dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Studies have reported glyphosate resistance in several weed species, including rigid ryegrass, in Australia and other parts of the world (Desai et al. Reference Desai, Thompson and Chauhan2020; Heap Reference Heap2022; Owen and Powles Reference Owen and Powles2010). The first case of glyphosate-resistant rigid ryegrass was reported more than 20 years ago in Australia (Powles et al. Reference Powles, Lorraine-Colwill, Dellow and Preston1998; Pratley et al. Reference Pratley, Urwin, Stanton, Baines, Broster, Cullis, Schafer, Bohn and Krueger1999), and since then, several accessions of glyphosate-resistant rigid ryegrass have been reported in several countries (Fernandez-Moreno et al. Reference Fernandez-Moreno, Rojano-Delgado, Menendez and De Prado2017). Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] and perennial ryegrass (Lolium perenne L.), closely related species of rigid ryegrass, have also evolved resistance to glyphosate (Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020; Yanniccari et al. Reference Yanniccari, Vila-Aiub, Istilart, Acciaresi and Castro2016).
Glyphosate is relied on for broad-spectrum preplanting and fallow weed control in conservation agriculture systems as well as for selective weed control in glyphosate-resistant crops grown in Australia (Duke and Powles Reference Duke and Powles2008). Therefore the increasing frequency of glyphosate-resistant rigid ryegrass accessions is of concern to Australian growers. These summer-emerging accessions were collected from glyphosate-resistant cotton fields, suggesting that the use of this herbicide in cotton increases the selection pressure for resistance evolution. With the expected increasing problem of summer-emerging rigid ryegrass, it will be very important to monitor the evolution of glyphosate resistance in this weed species as it occurs in summer fallows and glyphosate-resistant cotton crops.
Haloxyfop
Although 30% of the seedlings of S6 survived the 8X rate of haloxyfop, the LD50 value was only 13 g ai·ha−1 (Table 4; Figure 3). The GR50 value for this accession was 140 g ai·ha−1. The GR50 value for S3 (another summer-emerging accession) was 40 g ai·ha−1, whereas the GR50 values for the winter-emerging accessions were 3 to 5 g ai·ha−1.
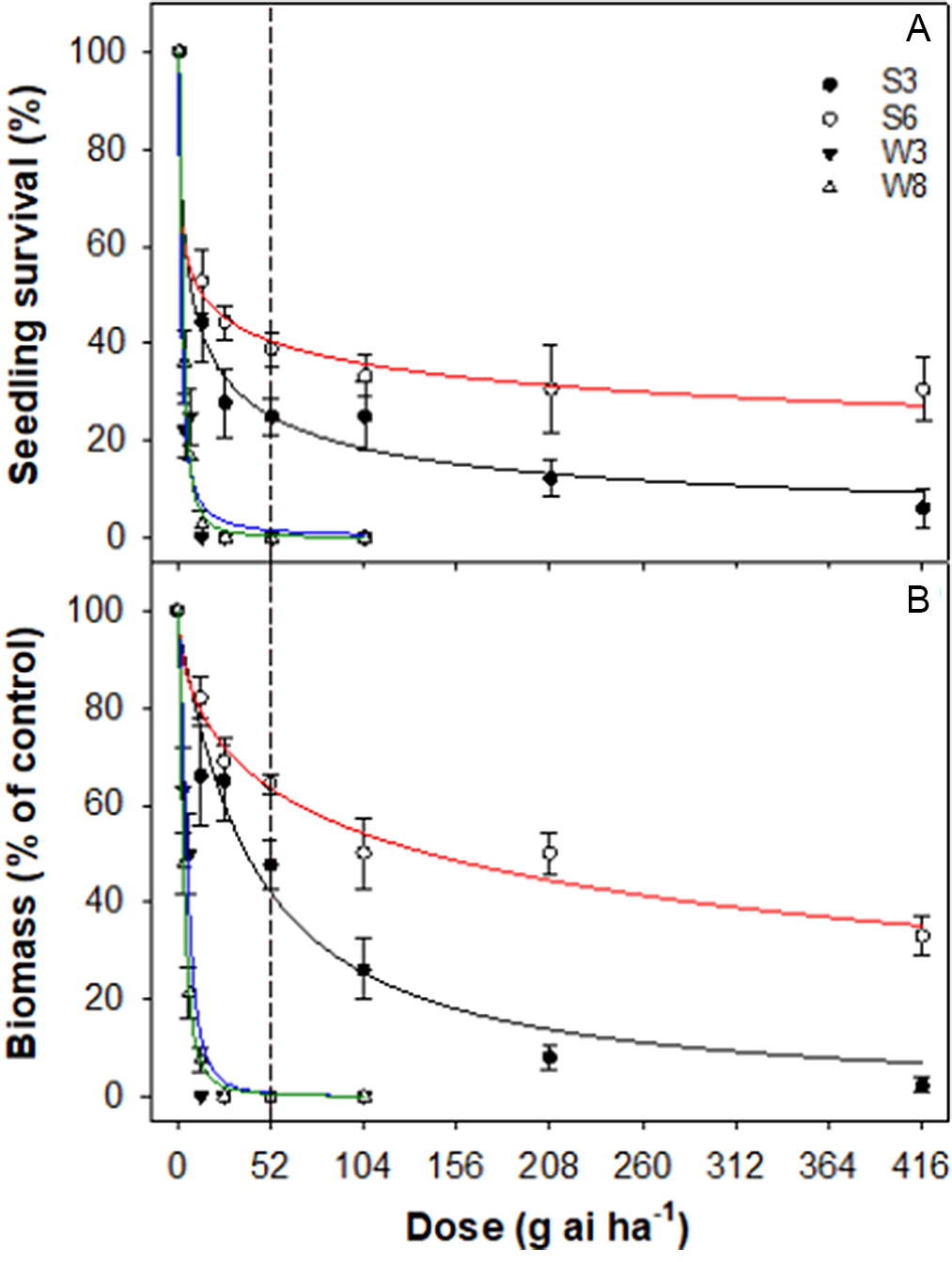
Figure 3. Effect of haloxyfop dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Haloxyfop is used to control grass weeds in fallow and broadleaf crops (Chauhan et al. Reference Chauhan, Congreve and Mahajan2021). Although there are several cases of winter-emerging rigid ryegrass accessions resistant to haloxyfop, this is the first study showing poor control of summer-emerging accessions. The summer-emerging accessions were collected from cotton fields and edges, and growers use haloxyfop to control grass weeds in cotton (from the crop’s second leaf to before the onset of flowering). Therefore a possible reason for the poor control could be the selection pressure for this herbicide, as herbicides from this mode of action group are prone to the risk of developing herbicide resistance. It is possible that these accessions evolved resistance to ACCase-inhibiting herbicides prior to evolving to be summer-emerging biotypes. Resistance to some ACCase-inhibiting herbicides can occur because of a decreased rate of herbicide activation, an increased rate of herbicide detoxification, sequestration of the herbicide, or overproduction of ACCase activity (Saini et al. Reference Saini, Malone, Preston and Gill2015). In such situations, growers need to use other effective ACCase-inhibiting herbicides (e.g., clethodim, butroxydim) in rotation or mixture.
Imazamox (33 g L−1) + imazapyr (15 g L−1)
The response of the summer- and winter-emerging accessions of rigid ryegrass was similar to the application of imazamox + imazapyr (Table 4; Figure 4). The LD50 values for the commercial mixture ranged from 9 (imazamox at 6.2 g ai·ha−1 and imazapyr at 2.8 g ai·ha−1) to 18.5 g ai·ha−1 (imazamox at 12.7 g ai·ha−1 and imazapyr at 5.8 g ai·ha−1), whereas the GR50 values for different accessions were 8.4 (imazamox at 5.8 g ai·ha−1 and imazapyr at 2.6 g ai·ha−1) to 13.4 g ai·ha−1 (imazamox at 9.2 g ai·ha−1 and imazapyr at 4.2 g ai·ha−1).

Figure 4. Effect of the commercial mixture of imazamox (33 g L−1) + imazapyr (15 g L−1) dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate (36 g ai ha−1 of the commercial mixture; 24.75 g ai ha−1 of imazamox + 11.25 g ai ha−1 of imazapyr) of the herbicide.
Imazamox + imazapyr, a commercial mixture of ALS-inhibiting imidazolinone herbicides, is used for the control of certain annual grass and broadleaf weeds in Clearfield® (BASF Australia, Southbank, Victoria, Australia) crops and igrowth® (Pacific Seeds, Toowoomba, Queensland, Australia) herbicide-resistant grain sorghum. Although the LD50 and GR50 values for different accessions of rigid ryegrass were well below half the field rate of this herbicide, up to 6% of seedlings survived the 4X rate of this herbicide. These results suggest monitoring the escaped seedlings and the evolution of resistance to this herbicide mixture as the area under Clearfield® and igrowth® herbicide-resistant sorghum increases. After applications of these imidazolinone herbicides, there is a requirement for significant intervals for planting crops, such as chickpea (Cicer arietinum L.; 10 mo) and cotton (22 mo).
Iodosulfuron
Accessions responded differently to different doses of iodosulfuron. S6 and W3 accessions had greater LD50 and GR50 values than the field rate (10 g ai ha−1) of iodosulfuron. The LD50 value for S6 and W3 accessions were 20 and 15 g ai ha−1, respectively, whereas these values for S3 and W8 were 6 and 8 g ai ha−1, respectively (Table 4; Figure 5). Similarly, the GR50 values for S6 and W3 were 23 and 10 g ai ha−1, respectively, whereas the GR50 value for S3 and W8 was 7 g ai ha−1.
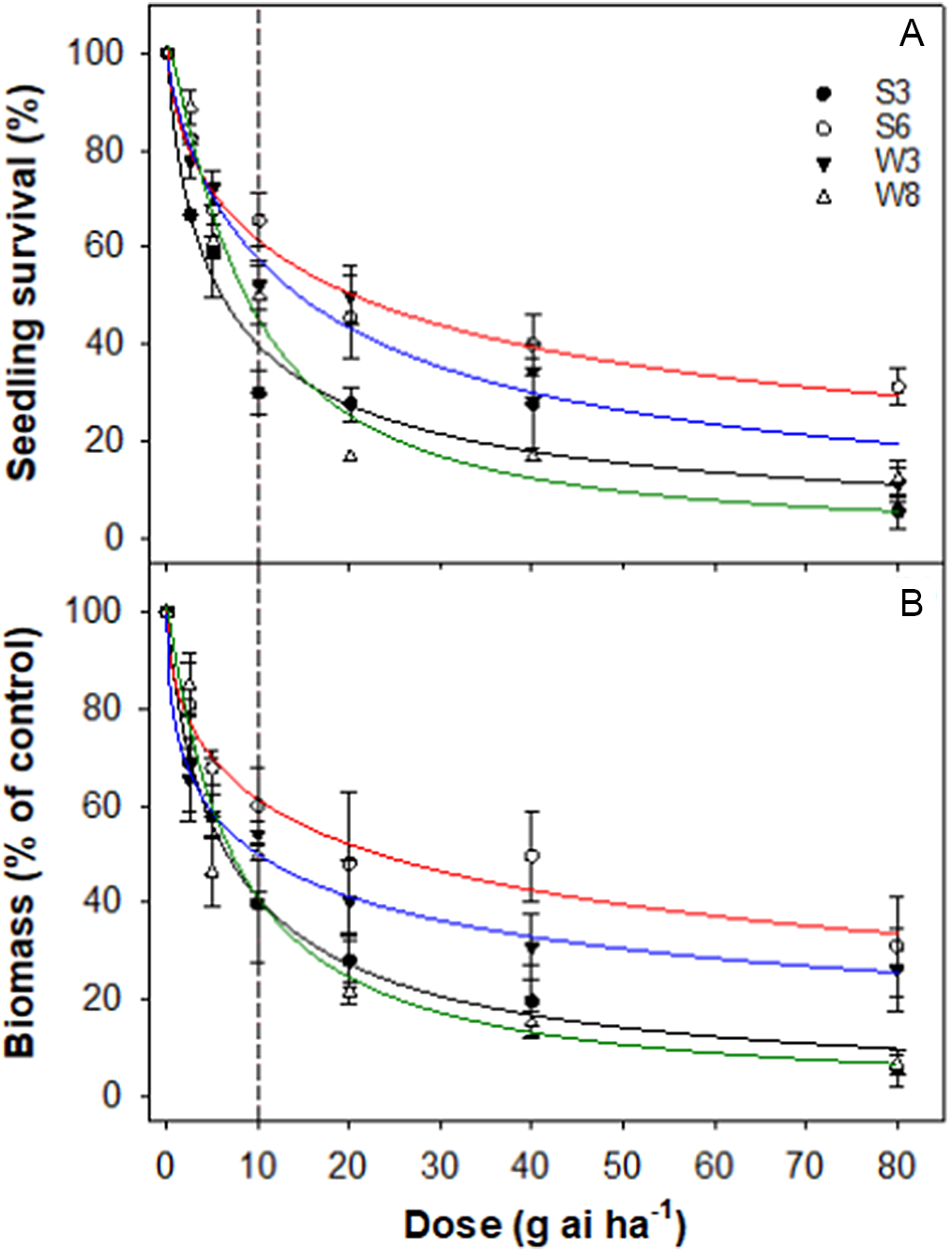
Figure 5. Effect of iodosulfuron dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Iodosulfuron, an ALS-inhibiting herbicide, is recommended for the control of rigid ryegrass in wheat. In Australia, only one case of iodosulfuron-resistant rigid ryegrass has been reported, from South Australia (Heap Reference Heap2022). The current study found two accessions (S6 and W3) resistant to iodosulfuron. Recently, iodosulfuron-resistant rigid ryegrass was also reported in Spain (Torra et al. Reference Torra, Montull, Taberner, Onkokesung, Boonham and Edwards2021). The study reported the evolution of multiple and cross-resistance to ALS- and ACCase-inhibiting herbicides by mechanisms consistent with the presence of both target-site and non-target-site resistance mechanisms.
Mesosulfuron
All four accessions were found to be highly susceptible to the field rate of mesosulfuron. The LD50 values for different accessions ranged from 0.6 to 0.7 g ai ha−1, whereas the GR50 values ranged from 0.4 to 0.9 g ai ha−1 (Table 4; Figure 6).
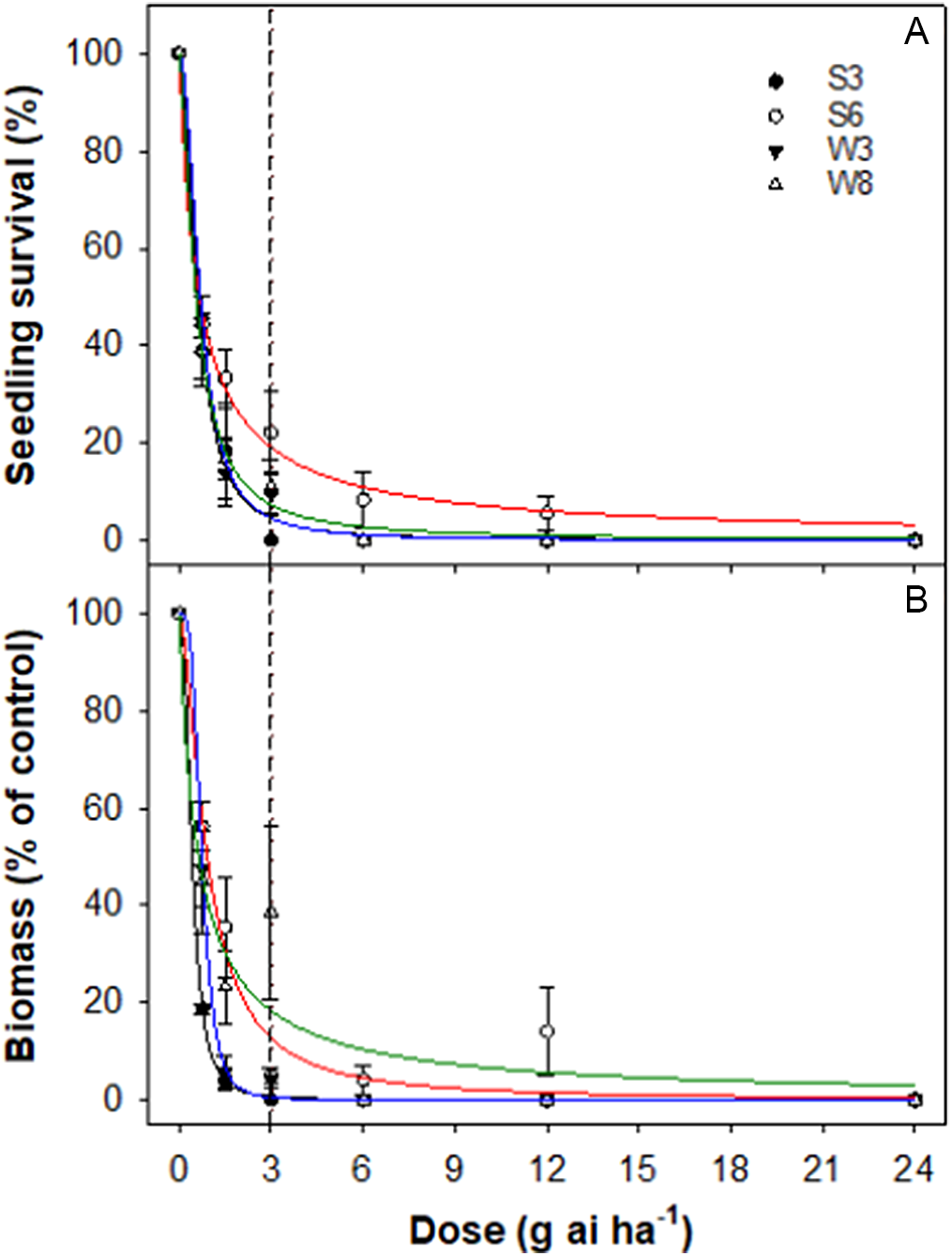
Figure 6. Effect of mesosulfuron dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Mesosulfuron, an ALS-inhibiting herbicide, is used to control rigid ryegrass in wheat. Mesosulfuron-resistant rigid ryegrass has been reported in France, Israel, and Spain (Heap Reference Heap2022; Torra et al. Reference Torra, Montull, Taberner, Onkokesung, Boonham and Edwards2021). In the United States, a dose–response assay revealed 37-fold resistance in Italian ryegrass to mesosulfuron compared with a susceptible standard (Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020). The widespread use of sulfonylurea herbicides in Australian crop production systems may result in the evolution of mesosulfuron-resistant rigid ryegrass; therefore the use of this herbicide needs to be managed using a diversified weed management system. After an application of mesosulfuron, there is a need to wait for 9 to 12 mo if growers want to plant barley, chickpea, or cotton.
Pinoxaden
Summer- and winter-emerging accessions of rigid ryegrass responded differently to different rates of pinoxaden. The LD50 values for summer- and winter-emerging accessions were 12 to 13 and 2 g ai ha−1, respectively (Table 4; Figure 7). The GR50 values were much greater than the LD50 values. The GR50 values for S3 and S6 accessions were 22 and 55 g ai ha−1, respectively, whereas the GR50 values for W3 and W8 accessions were 3 and 2 g ai ha−1, respectively.
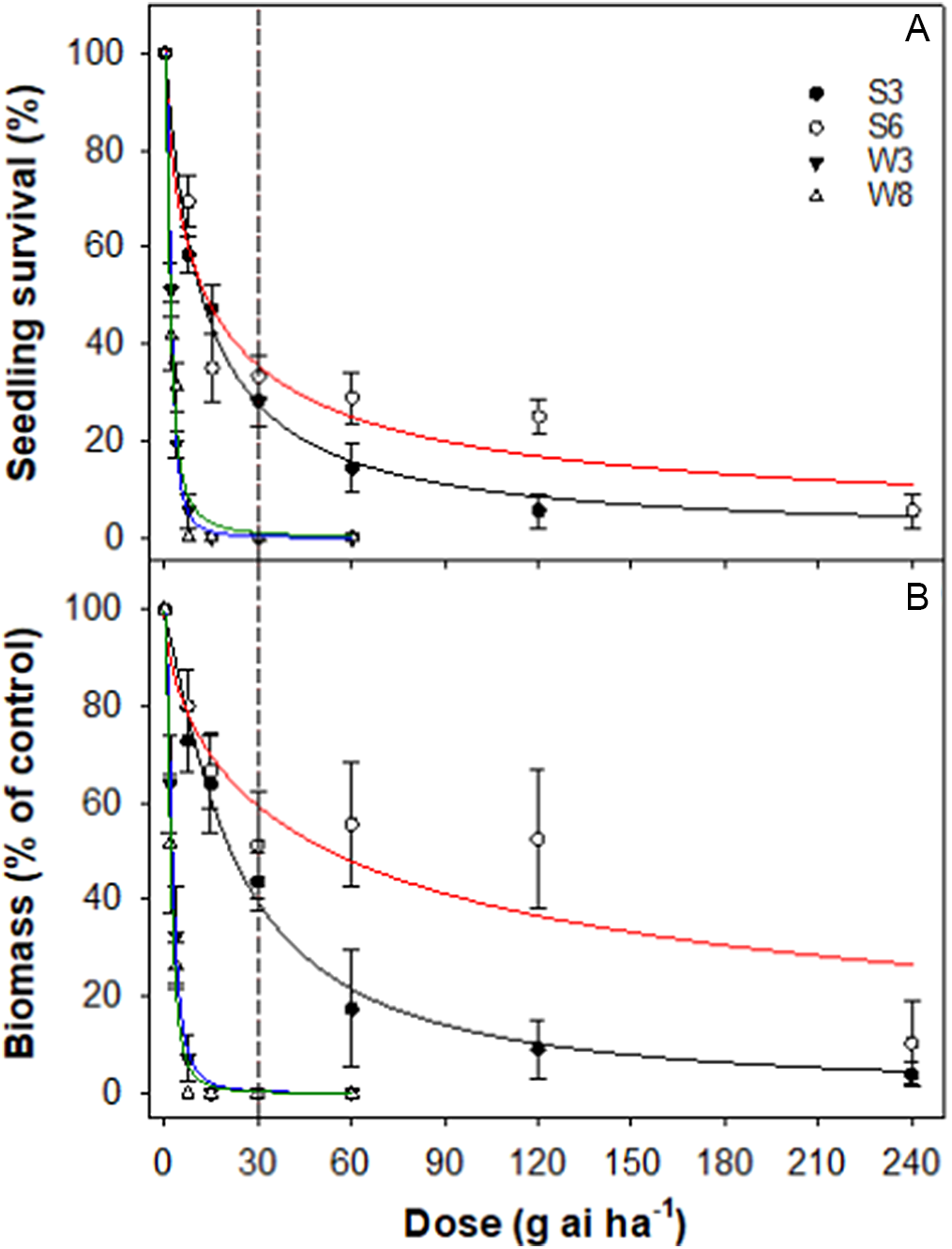
Figure 7. Effect of pinoxaden dose on survival (A) and biomass (percent of nontreated control) (B) of four accessions (S3, S6, W3, and W8) of rigid ryegrass. Plants were sprayed at the 4- to 5-leaf stage of each accession. The vertical dashed line represents the 1X rate of the herbicide.
Pinoxaden, an ACCase-inhibiting herbicide, is used to control rigid ryegrass and Avena species in wheat and barley crops in Australia. Although pinoxaden was first registered in Australia in 2006, 30% to 40% of the fields surveyed in 2003 and 2005 had pinoxaden-resistant rigid ryegrass accessions (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). These observations suggest cross-resistance in rigid ryegrass to herbicides that were not previously used. As the summer-emerging accessions of rigid ryegrass were collected from cotton fields and edges, it is possible that these accessions never received an application of pinoxaden but were exposed to haloxyfop, another ACCase-inhibiting herbicide. Italian ryegrass has also been found to have a 23-fold resistance to pinoxaden in the Texas Blacklands of the United States (Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020).
The two winter-emerging accessions were better controlled by POST herbicides compared to the two summer-emerging accessions. The summer-emerging accessions could be resistant to the herbicides; however, this cannot be concluded in this study, as there was no susceptible control of summer-emerging rigid ryegrass. Future studies should include herbicide-susceptible accessions of summer-emerging rigid ryegrass. This study was conducted outdoors during the spring and summer seasons. Although the weather conditions were optimum for summer-emerging accessions, they may not have been representative for winter-emerging accessions. Therefore future research should include only summer-emerging accessions in the spring and summer months.
This study revealed poor control of summer-emerging accessions (S3 and S6) of rigid ryegrass by multiple herbicides (glyphosate, glufosinate, haloxyfop, and pinoxaden). The S6 accession had the highest GR50 value for these herbicides, and it was also poorly controlled by iodosulfuron. Both summer-emerging accessions were collected from cotton fields and edges, suggesting that glyphosate-resistant cotton or the future availability of glyphosate- and glufosinate-resistant cotton in Australia (e.g., XtendFlex™) may not provide effective control of these summer-emerging accessions. Haloxyfop is commonly used to control dominant summer annual grass weed species, such as feather fingergrass (Chloris virgata Sw.) and jungle rice [Echinochloa colona (L.) Link], in cotton. While currently there are no reported cases of haloxyfop-resistant feather fingergrass and jungle rice in Australia, the results presented here suggest that summer-emerging rigid ryegrass accessions with evolved resistance may become more problematic in these production systems. At present, summer-emerging accessions of rigid ryegrass are mainly present along crop edges or fence lines (Thompson and Chauhan Reference Thompson and Chauhan2022); therefore preventive measures, such as field hygiene, clean water channels, or clean field boundaries, could prove very effective in delaying the spread of summer-emerging rigid ryegrass.
In Australia, annual sowthistle (Sonchus oleraceus L.) is another example that was once considered to be only a winter weed but is now present in winter as well as summer cropping systems (Manalil et al. Reference Manalil, Werth, Jackson, Chauhan and Preston2017). As rigid ryegrass is an obligate outcrossing species with high genetic diversity (Charmet et al. Reference Charmet, Balfourier and Chatard1996), stopping the spread of multiple-herbicide-resistant summer-emerging rigid ryegrass is critical. The occurrence of rigid ryegrass as a summer-emerging weed in summer cropping systems is problematic; however, the evolution of resistance in these accessions to herbicides frequently relied on for summer annual weed control is a significant threat to summer cropping systems. Further research should investigate the management of herbicide resistance in summer-emerging annual ryegrass accessions.
Acknowledgments
The authors thank the Grains Research and Development Corporation (GRDC) for investing in this research. The authors declare no conflicts of interest.














