Introduction
Protoporphyrinogen oxidase (PPO)-inhibiting herbicides have been used for more than 50 years to control weeds in agronomic crops. Although these herbicides were first used in the 1960s, resistance took a relatively long time to evolve, with the first case of resistance (in waterhemp) not observed until 2001 (Heap Reference Heap2019). The mechanism of this first case of resistance involves the deletion of a glycine residue at the 210th position (ΔGly-210) of the PPO enzyme (PPO2) (Patzoldt et al. Reference Patzoldt, Hager, McCormick and Tranel2006). The region of the sequence spanning position 210 contains two overlapping trinucleotide repeats, and the loss of one of these repeats via a proposed DNA polymerase slippage-like mechanism results in the loss of a glycine codon (Gressel and Levy Reference Gressel and Levy2006). Riggins and Tranel (Reference Riggins and Tranel2012) found that Palmer amaranth has the same repeat motif in PPX2 as waterhemp, suggesting that Palmer amaranth could also evolve resistance to PPO-inhibiting herbicides via the ΔGly-210 mutation.
Since waterhemp first evolved resistance to PPO inhibitors, 12 additional weed species have been found with resistance to PPO-inhibiting herbicides, including Palmer amaranth (Heap Reference Heap2019). PPO-inhibitor resistance in Palmer amaranth was first observed in 2011, 10 years after resistance to PPO inhibitors in waterhemp was first observed. This Palmer amaranth population was also resistant via the ΔGly-210 mutation (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). After identification of the ΔGly-210 mutation in waterhemp and Palmer amaranth, additional mechanisms conferring PPO inhibitor resistance have been identified in these species. These additional mechanisms include mutations conferring other PPO2 amino acid changes (Arg128Gly, Arg128Met, Arg128Ile, and Gly399Ala) as well as enhanced herbicide detoxification (Giacomini et al. Reference Giacomini, Umphres, Nie, Mueller, Steckel, Young, Scott and Tranel2017; Nie et al. Reference Nie, Mansfield, Harre, Young, Steppig and Young2019; Rangani et al. Reference Rangani, Salas-Perez, Aponte, Knapp, Craig, Mietzner, Langaro, Noguera, Porri and Roma-Burgos2019; Varanasi et al. Reference Varanasi, Brabham and Norsworthy2018).
Given that Palmer amaranth and waterhemp are two very closely related species, it is notable that Palmer amaranth took a decade longer to evolve resistance compared to waterhemp. Anecdotal evidence from growers indicates that Palmer amaranth is the more difficult species to control with PPO-inhibiting herbicides, and grower reports state that Palmer amaranth is unable to be controlled with PPO inhibitors after it reaches a height of 10 cm (Riar et al. Reference Riar, Norsworthy, Steckel, Stephenson, Eubank and Scott2013). These pieces of evidence suggest that Palmer amaranth is more tolerant to PPO inhibitors than is waterhemp, which may be why it evolved resistance 10 years later.
The objectives of this study were to characterize the relative levels of resistance to PPO inhibitors in Palmer amaranth and waterhemp conferred specifically by the ΔGly-210 mutation, and to determine the selective advantage of resistance to PPO inhibitors in Palmer amaranth relative to waterhemp.
Materials and Methods
Plant Materials
Palmer amaranth populations used in this research included one PPO-inhibitor–resistant (KLPA2-3) and one PPO inhibitor–sensitive (KLPAS) population from Kentucky, and waterhemp populations included one PPO-inhibitor–resistant (CHR14-7) and one PPO inhibitor–sensitive (WHWT) population from Illinois. KLPA2-3 resulted from crossing plants that were homozygous for the ΔGly-210 mutation from a Kentucky population (Lillie et al. Reference Lillie, Giacomini, Green and Tranel2019), whereas CHR14-7 resulted from crossing plants from the CHR population (Evans et al. Reference Evans, Strom, Riechers, Davis, Tranel and Hager2019) that were homozygous for the ΔGly-210 mutation. Homozygosity for the ΔGly-210 mutation was determined by performing a TaqMan quantitative polymerase chain reaction (qPCR) assay using fluorescent probes designed by Wuerffel et al. (Reference Wuerffel, Young, Matthews and Young2015) to detect the ΔGly-210 mutation. DNA was extracted from new-leaf tissue using the hexadecyltrimethyl-ammonium bromide method (Doyle and Doyle Reference Doyle and Doyle1990). The same qPCR assay was performed on a subset of the progeny from the crosses to confirm homozygosity. KLPAS was collected in 2013 from a PPO-inhibitor-sensitive Palmer amaranth plant in Fulton County, Kentucky, and WHWT was made up of multiple wild-type waterhemp accessions collected from various counties in Illinois in 2003.
Postemergence Dose Responses
Approximately 100 seeds from each population were broadcast into 12 cm by 12 cm plastic greenhouse flat inserts containing a mixture of Sunshine LC1 (Sun Gro Horticulture, Agawam, MA, USA), soil, peat, and torpedo sand (3:1:1:1 by weight) plus 13-13-13 Osmocote fertilizer (The Scotts Company, Marysville, OH, USA). The same soil mixture was used to cover the seeds at a thickness of approximately 2 mm. When they reached the 1- to 2-leaf stage, seedlings were transplanted into 8 cm by 8 cm square plastic pots containing the same mixture of Sunshine LC1, soil, peat, and torpedo sand plus fertilizer. Each pot contained one plant, and all plants were grown in a greenhouse maintained at 30 C/25 C day/night with artificial lighting from metal halide lamps programmed for a 16-hr photoperiod. To quantify the difference in resistance and sensitivity levels at different growth stages, each population was sprayed at a height of 8 to 10 cm (early postemergence [EPOST]) or 13 to 15 cm (late postemergence [LPOST]). Lactofen (Cobra, 240 g ai L−1, Valent USA, Walnut Creek, CA, USA) was applied at rates ranging from 0.219 to 6,920 g ha−1 (1× field rate was 219 g ha-1) with a selection of seven rates evenly spaced along a log10 scale. Similarly, fomesafen (Flexstar, 225 g ai L−1, Syngenta US, Wilmington, DE, USA) was applied at rates ranging from 0.329 to 10,400 g ha−1 (1× field rate was 329 g ha−1). The specific rate applied to each plant depended on whether it was sensitive or resistant to PPO inhibitors and whether the herbicide application timing was EPOST or LPOST. Sensitive plants treated EPOST were sprayed at 0.001× to 1× field rates, sensitive plants treated LPOST were sprayed at 0.00316× to 3.16× field rates, resistant plants treated EPOST were sprayed at 0.01× to 10× field rates, and resistant plants treated LPOST were sprayed at 0.0316× to 31.6× field rates. During the second repetition of the experiment, the range of rates used was shifted up by one rate for all populations and application timings except for the sensitive populations treated EPOST. This occurred to achieve higher levels of control. All herbicide treatments were applied with a compressed-air laboratory spray chamber (DeVries Manufacturing, Hollandale, MN, USA) equipped with an 80° even flat-fan spray nozzle (TeeJet Technologies, Wheaton, IL, USA) calibrated to deliver 187 L ha−1. All lactofen spray mixtures contained 1% v/v crop oil concentrate (COC), and all fomesafen spray mixtures contained 1% v/v COC and 1% v/v 32% urea ammonium nitrate (UAN). Adjuvant-only controls were included. Two weeks after treatment (WAT), survivorship ratings were taken on a scale from 0 to 10 where 0 represented plant mortality, and aboveground biomass of each plant was harvested, dried at 37 C for 5 days, and weighed. The dry weights were multiplied by the survivorship ratings to obtain an adjusted dry weight (Guo et al. Reference Guo, Riggins, Hausman, Hager, Riechers, Davis and Tranel2015). To account for differences in plant growth between each population, these values were converted to a percentage of the untreated control using the adjuvant-only control plants from each respective population. Each treatment was replicated five or six times, and the experiment was conducted twice.
Preemergence Dose Responses
Preemergence (PRE) dose responses were also carried out on these populations to test the differences in resistance and sensitivity to PPO inhibitors at seedling emergence. Fifty-seed weights of each population were planted in 6 cm by 6 cm greenhouse flat inserts filled with a mixture of soil, peat, and torpedo sand (1:1:1 by weight) plus 13-13-13 Osmocote. The same soil mixture was used to cover the seeds at a thickness of 1 to 2 mm, and herbicides were applied immediately after planting. Fomesafen was applied at rates ranging from 0.329 to 3,290 g ha-1 with a selection of seven rates evenly spaced along a log10 scale. Flumioxazin (Valor, 0.51 g ai g−1, Valent USA, Walnut Creek, CA, USA) was applied at rates ranging from 0.107 to 338 g ha−1 (1× field rate was 107 g ha−1). The sensitive populations were sprayed from 0.001× to 1× field rates of both herbicides, and the resistant populations were sprayed from 0.01× to 10× field rates of fomesafen and 0.00316× to 3.16× field rates of flumioxazin. Water-only controls were included. Following herbicide application, overhead water was gently applied to leach the herbicide through the soil profile (simulating approximately 50 mm rainfall), and soil moisture was maintained with subirrigation for the remainder of the experiment. Ten days after treatment (DAT), emergence counts were taken. Seedlings were considered fully emerged when the adaxial leaf surfaces of the cotyledons were no longer touching each other (Wuerffel et al. Reference Wuerffel, Young, Matthews and Young2015). Emergence counts were converted to a percentage of the untreated control using the water-only control counts from each respective population. Each treatment was replicated six times, and the experiment was conducted twice.
Sensitive Spray Study
An additional spray study was carried out to determine whether the sensitive populations used in the dose-response experiments were representative of sensitive populations of each species. Six wild-type populations each of Palmer amaranth and waterhemp from various states were used. The wild-type waterhemp populations tested were from Illinois, Iowa, Kansas, Nebraska, Indiana, and Ohio. The Illinois population was the same as that used in the dose-response experiments, the Ohio population was collected during the 2016 growing season as described in Murphy (Reference Murphy2018), and the remaining populations were obtained from the Germplasm Resources Information Network (GRIN). The sensitive Palmer amaranth populations tested were from Arizona, Arkansas, Illinois, Kentucky, Missouri, and Oklahoma. The population from Kentucky was the same population used in the dose-response experiments, the Arizona and Oklahoma populations were from an in-house collection, and the remaining populations were collected in 2009 from the margins of agricultural fields as explained in Davis et al. (Reference Davis, Schutte, Hager and Young2015).
Experimental procedures (e.g., growing plants, herbicide treatments, and plant evaluation) were identical to those described above for EPOST treatments, with the exception of the herbicide rates used. For this particular study, lactofen was applied at 0, 2.19, 11.0, or 110.0 g ha−1 for Palmer amaranth and 0, 0.175, 2.19, or 11.0 g ha−1 for waterhemp, whereas fomesafen was applied at 0, 1.04, 10.4, or 104 g ha-1 for Palmer amaranth and 0, 0.330, 10.4, or 104 g ha−1 for waterhemp. Each treatment was replicated five or six times, and the experiment was conducted twice. The study was conducted as a randomized complete block design (RCBD) in which the blocking factor was experimental run.
Statistical Analysis
Adjusted dry weights (POST dose response) and emergence counts (PRE dose response) were analyzed in R software v. 1.0.143 using the dose response curve (drc) package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). The four-parameter log-logistic function used to generate dose-response curves is expressed as:
where y is the adjusted dry weight or emergence count, b is the slope of the curve, c is the lower asymptote, d is the upper asymptote, x is the herbicide rate, and ED50 is the herbicide rate required to reduce the response halfway between d and c. Homogeneity of variance was achieved through a square root transformation of the data. To determine if the fitted curves were different from one another, ED50 values were calculated and compared. The ED50 value and its standard error associated with each curve were obtained from the fit of the log-logistic model, and these values were used to calculate the 95% confidence intervals of the ED50 values from each fitted curve.
For the sensitive spray study, the data were separated into four groups and analyzed separately: waterhemp treated with lactofen, waterhemp treated with fomesafen, Palmer amaranth treated with lactofen, and Palmer amaranth treated with fomesafen. Adjusted dry weights were calculated as described above. For each set of data, an ANOVA was carried out using the following model:
where yijkl is the adjusted dry weight of the plant under observation, µ is the grand population mean, Ri is the random effect of the ith experimental run, Dj is the random effect of the jth herbicide dose, Pk is the random effect of the kth population, DPjk is the random interaction of the jth herbicide dose and the kth population, and ϵijkl is the random error term, NID (0, σe 2). Data were analyzed using the PROC MIXED procedure using the TYPE 3 model in SAS 9.4 (Statistical Analysis Software, Inc., Cary, NC, USA). The assumption of normality was analyzed by conducting a Shapiro-Wilks normality test on the residuals using PROC UNIVARIATE, and the assumption of homogeneous variances was analyzed using the Browne and Forsythe test in the MEANS option of PROC GLM. Because the residuals were not normally distributed, the Palmer amaranth data were transformed via the cube root, and the waterhemp data were transformed via the square root. Due to a lack of homogeneity of variance after transformation, separate variance groups were set for each data set.
Results and Discussion
Postemergence Dose Responses
Not surprisingly, the populations of both species sprayed at LPOST had significantly higher ED50 values than their respective populations sprayed at EPOST for both lactofen (Figure 1 and Table 1) and fomesafen (Figure 2 and Table 2). The sensitive waterhemp sprayed at EPOST had the lowest ED50 value for both lactofen (Table 1) and fomesafen (Table 2) dose responses. These results aligned with those reported by Hager et al. (Reference Hager, Wax, Bollero and Stoller2003), which found control of common waterhemp was greater after an EPOST timing than after a later POST timing. Results also coincided with those of the study performed by Wuerffel et al. (Reference Wuerffel, Young, Matthews and Young2015), which demonstrated that the response of resistant biotypes of waterhemp to PPO-inhibiting herbicides depends on the target plant growth stage.
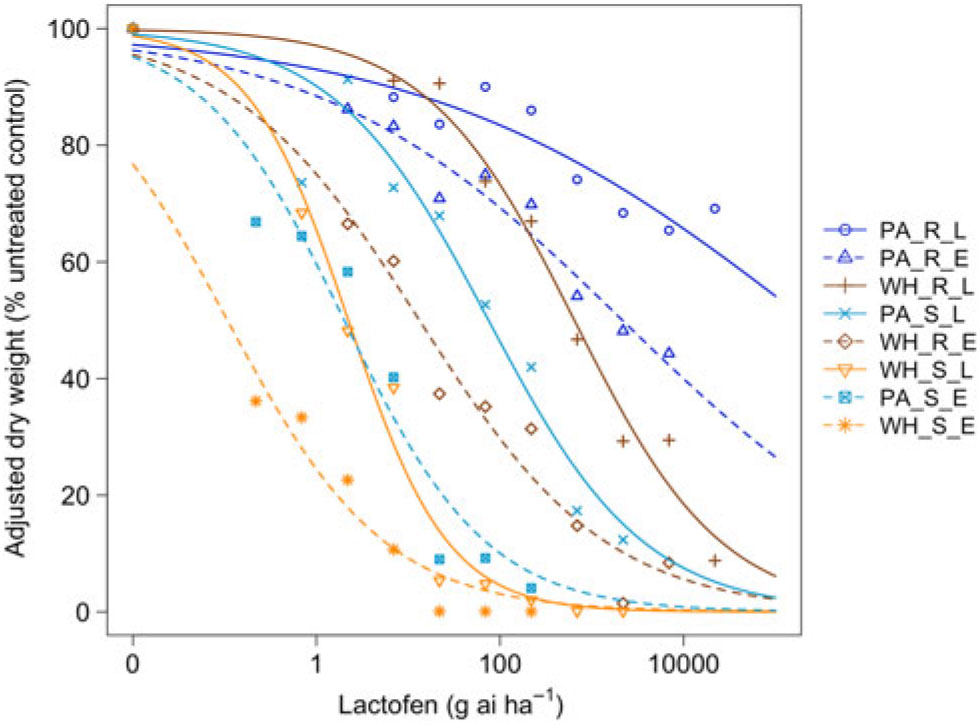
Figure 1. Dose responses of waterhemp (WH_X_X) and Palmer amaranth (PA_X_X) populations homozygous for (XX_R_X) or lacking (XX_S_X) the Gly210 PPO deletion treated with lactofen at early (XX_X_E) and late (XX_X_L) application stages. Dose-response curves were fitted using a four-parameter, log-logistic function in R software.
Table 1. Lactofen ED50 values and relative sensitivities of each biotype sprayed early POST and late POST.

a Abbreviations: PA_X_X, Palmer amaranth; WH_X_X, waterhemp; XX_R_X, homozygous for the Gly210 PPO deletion; XX_S_X, lacking the Gly210 PPO deletion; XX_X_E, early POST application; XX_X_L, late POST application.
b Difference in ED50 values determined using 95% confidence intervals.
c Relative sensitivity values calculated relative to WH_S_E.
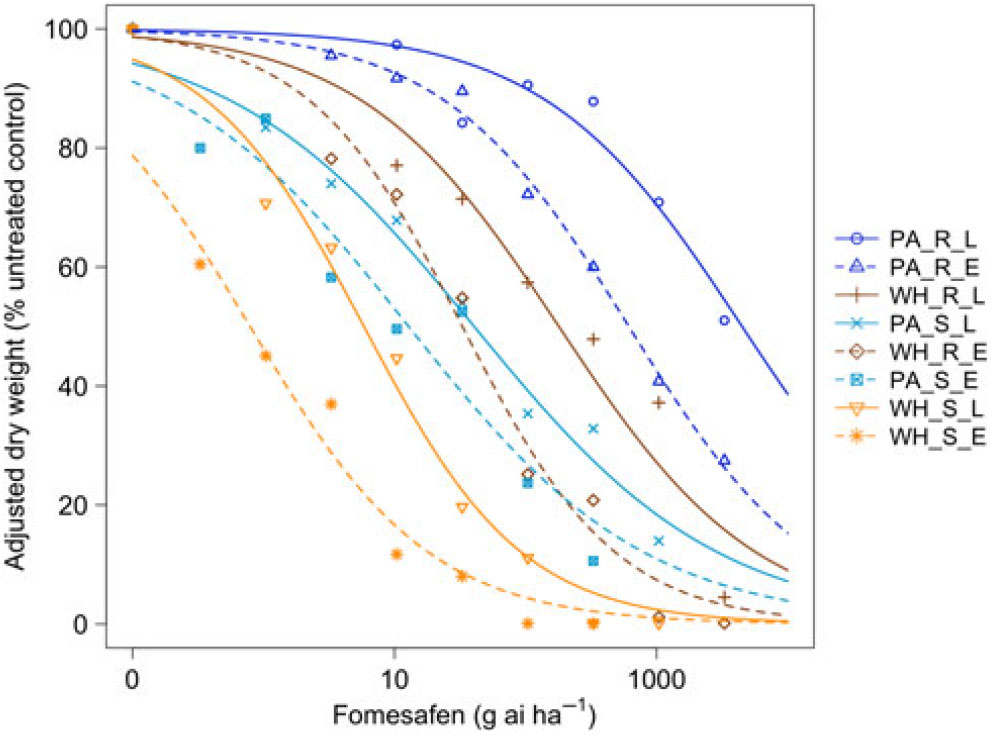
Figure 2. Dose responses of waterhemp (WH_X_X) and Palmer amaranth (PA_X_X) populations homozygous for (XX_R_X) or lacking (XX_S_X) the Gly210 PPO deletion treated with fomesafen at early (XX_X_E) and late (XX_X_L) application stages. Dose-response curves were fitted using a four-parameter, log-logistic function in R software.
Table 2. Fomesafen ED50 values and relative sensitivities of each biotype sprayed early POST and late POST.
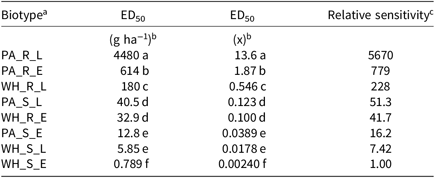
a Abbreviations: PA_X_X, Palmer amaranth; WH_X_X, waterhemp; XX_R_X, homozygous for the Gly210 PPO deletion; XX_S_X, lacking the Gly210 PPO deletion; XX_X_E, early POST application; XX_X_L, late POST application
b Difference in ED50 values determined using 95% confidence intervals.
c Relative sensitivity values calculated relative to WH_S_E.
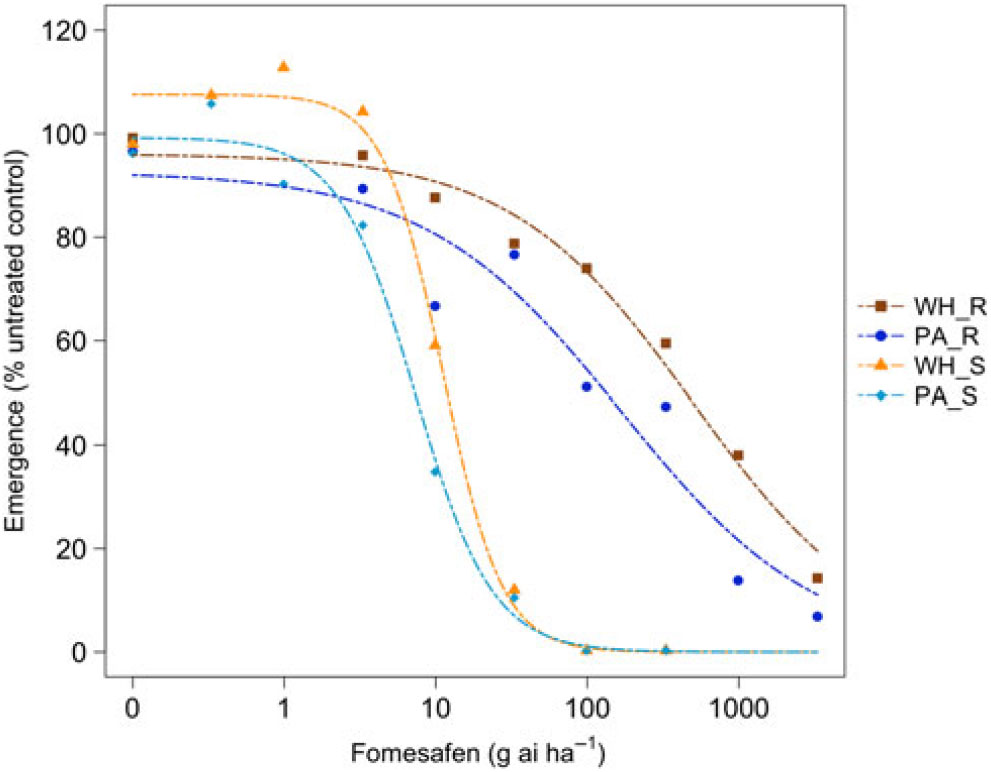
Figure 3. Dose responses of waterhemp (WH_X) and Palmer amaranth (PA_X) populations homozygous for (XX_R) or lacking (XX_S) the Gly210 PPO deletion treated with fomesafen at the PRE application stage. Dose-response curves were fitted using a four-parameter, log-logistic function in R software.
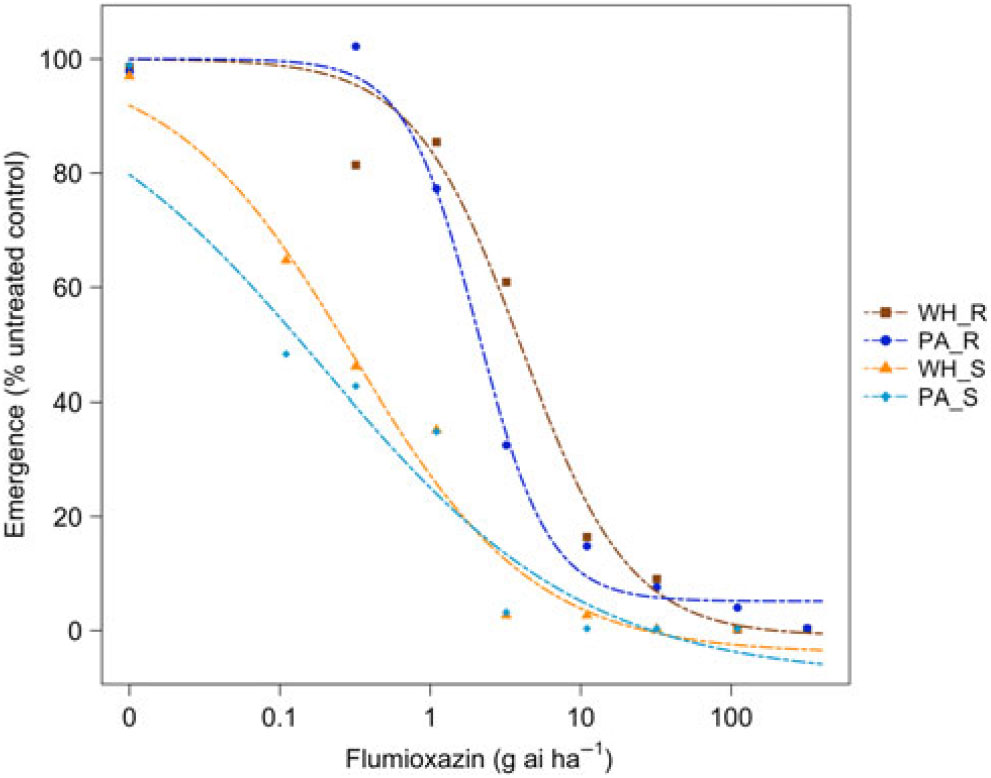
Figure 4. Dose responses of waterhemp (WH_X) and Palmer amaranth (PA_X) populations homozygous for (XX_R) or lacking (XX_S) the Gly210 PPO deletion treated with flumioxazin at the PRE application stage. Dose-response curves were fitted using a four-parameter, log-logistic function in R software.
When sprayed with lactofen, the resistant Palmer amaranth population was not sufficiently controlled at the highest dose for either application timing (Figure 1 and Table 1). Additionally, the ED50 value of the sensitive Palmer amaranth sprayed EPOST was not significantly different than the ED50 value of the sensitive waterhemp sprayed LPOST (Table 1). When sprayed LPOST, the sensitive Palmer amaranth population was significantly less sensitive than the resistant waterhemp population when sprayed EPOST (Table 1). Furthermore, when sprayed LPOST with fomesafen, the ED50 value of the sensitive Palmer amaranth was not significantly different than the ED50 value of the resistant waterhemp sprayed EPOST (Table 2). These results suggest that Palmer amaranth does not necessarily need a resistance mechanism against PPO inhibitors to survive a PPO-inhibitor application; rather, it is enough just to be taller and have more biomass.
The resistant to sensitive (R/S) ratios for waterhemp treated with fomesafen POST were similar to the R/S ratio that Wuerffel et al. (Reference Wuerffel, Young, Matthews and Young2015) reported. They calculated an R/S ratio of 38, which lies between the R/S ratios of 30.8 and 41.7 obtained in this study for waterhemp treated with fomesafen LPOST and EPOST, respectively (Table 5). The waterhemp population used in the Wuerffel et al. study was a field population that contained the ΔGly-210 mutation. For waterhemp treated with lactofen, Patzoldt et al. (Reference Patzoldt, Hager, McCormick and Tranel2006) calculated an R/S ratio of 53 in a waterhemp population that was homozygous for ΔGly-210, which is similar to the R/S ratio of 48.9 obtained in this study for the LPOST application timing (Table 5). Both the Wuerffel et al. and Patzold et al. studies followed herbicide rate structures, COC rates, and application heights that were similar to this study. Salas et al. (Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016) obtained R/S ratios ranging between 6 and 21 for Palmer amaranth treated with fomesafen POST. These values were much smaller than the R/S ratios of 48.0 and 111 calculated in the current study for Palmer amaranth treated with fomesafen EPOST and LPOST, respectively, but the study by Salas et al. followed a different herbicide rate structure, and their herbicide mixtures included 0.5% nonionic surfactant, which may account for this difference. Additionally, the Palmer amaranth populations used in that study were a mixture of plants both homozygous and heterozygous for the ΔGly-210 mutation.
Preemergence Dose Responses
There were similarities and differences in the patterns of response in both species when sprayed PRE (Figure Reference Ehleringer3 and Figure Reference Ehleringer4) compared with when they were sprayed POST. When sprayed with fomesafen PRE, the resistant waterhemp demonstrated the same level of resistance as the resistant Palmer amaranth (Table 3). The sensitive waterhemp was also just as sensitive as the sensitive Palmer amaranth. The resistant populations of both species were significantly less sensitive than the sensitive populations for both herbicides (Table 3 and Table 4). When sprayed with flumioxazin PRE, the resistant waterhemp was more resistant than the resistant Palmer amaranth, but the sensitive waterhemp exhibited the same level of sensitivity as the sensitive Palmer amaranth (Table 4). These results were similar to those of the POST dose responses in that the resistant populations had significantly higher ED50 values than the sensitive populations. However, the flumioxazin PRE results showed the resistant waterhemp population was more resistant than the resistant Palmer amaranth population, a finding that differed from the POST results. Overall, the PRE dose response study indicated that there were fewer differences in resistance and sensitivity levels between waterhemp and Palmer amaranth when sprayed PRE than when sprayed EPOST or LPOST.
Table 3. Fomesafen ED50 values and relative sensitivities of each biotype sprayed PRE.

a Abbreviations: PA_X, Palmer amaranth; WH_X, waterhemp; XX_R, homozygous for the Gly210 PPO deletion; XX_X, lacking the Gly210 PPO deletion.
b Difference in ED50 values determined using 95% confidence intervals.
c Relative sensitivity values calculated relative to PA_S.
Table 4. Flumioxazin ED50 values and relative sensitivities of each biotype sprayed PRE.

a Abbreviations: PA_X, Palmer amaranth; WH_X, waterhemp; XX_R, homozygous for the Gly210 PPO deletion; XX_X, lacking the Gly210 PPO deletion.
b Difference in ED50 values determined using 95% confidence intervals.
c Relative sensitivity values calculated relative to PA_S.
The R/S ratio of 44.8 for waterhemp treated with fomesafen PRE (Table 5) was similar to that reported by Wuerffel et al. (Reference Wuerffel, Young, Matthews and Young2015), who calculated an R/S ratio of 33 in a field population of waterhemp that was resistant via the ΔGly-210 mutation. Falk et al. (Reference Falk, Shoup, Al-Khatib and Peterson2006) reported an R/S ratio of 2.5 for fomesafen applied PRE to an uncharacterized population of waterhemp, which was much smaller than the R/S ratio of 44.8 obtained in this study. Umphres et al. (Reference Umphres, Steckel and Mueller2018) reported an R/S ratio of 5.2 for a field population of resistant Palmer amaranth sprayed with fomesafen, which was much smaller than the R/S ratio of 22.9 we report in this study. However, the resistance mechanism in population of plants in the Umphres et al. study was not characterized. Both studies followed different herbicide rate structures than the current study did. For flumioxazin, Umphres et al. (Reference Umphres, Steckel and Mueller2018) reported an R/S ratio of 4.7, which is not far from the R/S ratio of 10.6 calculated in this study.
Table 5. Resistant to sensitive ratios of Palmer amaranth and waterhemp sprayed EPOST, LPOST, or PRE with lactofen, fomesafen, or flumioxazin.

a R/S ratios not calculated are represented by (–).
b Abbreviations: EPOST, early postemergence; LPOST, late postemergence; PRE, preemergence; R/S, resistant to sensitive ratio.
A study by Vila-Aiub et al. (Reference Vila-Aiub, Casas and Gundel2018) found that an increase in size from the seedling stage of glyphosate-resistant johnsongrass [Sorghum halepense (L.) Pers.] resulted in increasing R/S ratios. The R/S ratios of the fomesafen LPOST, EPOST, and PRE dose responses (Table 5) reflected this same pattern occurring in Palmer amaranth but not in waterhemp. For waterhemp, the R/S ratios were more likely to decrease rather than increase across these timings. When treated with lactofen, the R/S ratios for waterhemp decreased from EPOST to LPOST. Why the R/S ratios exhibited different trends across application timings for the two species is not clear. Perhaps developmentally regulated differences exist in tolerance mechanisms (e.g., differential expression of a gene encoding an enzyme that contributes to herbicide metabolism) that influence the R/S ratio.
Sensitive Spray Study
The ANOVA results from the sensitive spray study showed that the population main effect and the interaction between population and herbicide dose were insignificant for all experiments, indicating that the means of all populations were not statistically different, both overall and at each dose of herbicide applied (Table 6). This suggests that the populations used as the sensitive controls for each species in the dose responses were representative of wild-type populations for the corresponding species. The block effect, experimental run, was highly insignificant for the analysis of the waterhemp populations applied with lactofen. The block effect was significant in the remainder of analyses, which is likely due to different experimental conditions such as greenhouse temperature, because each run of the experiment was temporally separated. The effect of dose was significant for all analyses because significant differences in plant response existed between a 0× dose and all other doses. Because the sensitive populations used in the dose-response experiments are representative of wild-type populations, it can be assumed that the results of the dose-response experiments are indicative of each species as a whole.
Table 6. One-way analysis of variance tables comparing the effect of dose of lactofen or fomesafen on six different wild-type populations of either Palmer amaranth or waterhemp, blocked by experimental run.

Collectively, the results reported herein indicate that Palmer amaranth is more tolerant to PPO inhibitors than waterhemp when treated POST. This could be why Palmer amaranth took a decade longer to evolve resistance to PPO inhibitors compared with that of waterhemp (i.e., because Palmer amaranth is naturally more tolerant to POST application of these herbicides, there is less of a selective advantage for it to evolve resistance to PPO inhibitors). If a Palmer amaranth plant is sufficiently large at the time of application of a PPO inhibitor, it does not need a resistance mechanism to survive, and therefore, it is not under as strong of a selection pressure for resistance. This could be attributed to its high growth and photosynthetic rates (Ehleringer Reference Ehleringer1983; Horak and Loughin Reference Horak and Loughin2000) or various tolerance mechanisms (e.g., greater ability to metabolize the herbicide). Aside from herbicide-resistance evolution, understanding the mechanism underlying this natural tolerance could give insight into herbicide tolerance mechanisms that could be utilized in crops.
Another factor that could have contributed to waterhemp having evolved resistance to PPO inhibitors before Palmer amaranth did is that initially, these herbicides may have demonstrated a greater selection for waterhemp than there was for Palmer amaranth. In 1994, PPO-inhibitor use was highest primarily in midwestern states (Dayan et al. Reference Dayan, Barker and Tranel2018), wherein waterhemp was likely being targeted, because this species was more prevalent in the Midwest than Palmer amaranth was at that time. The total usage of PPO inhibitors in the United States during the early 1990s was relatively high (Dayan et al. Reference Dayan, Barker and Tranel2018), as this time period was just before the introduction of glyphosate-resistant crops. After these crops were introduced, PPO-inhibitor use declined, and did not rebound until after glyphosate-resistant weeds became common. It was not until this second wave of high PPO-inhibitor use that resistance to these herbicides was observed in Palmer amaranth. A study by Webster and Nichols (Reference Webster and Nichols2012) evaluated how weed species have changed over the 14-year period between 1994 and 2008 in the southern United States. In cotton and soybean systems, in which PPO inhibitors are typically used, Palmer amaranth went from being the 10th and 23rd most troublesome weed species, respectively, to the first and second most troublesome weed species, respectively. Perhaps the slower evolution of PPO-inhibitor resistance in Palmer amaranth relative to waterhemp is due simply to a higher number of individuals of the latter species being exposed to these herbicides before the era of glyphosate-resistant crops.
Regardless of the evolutionary history of resistance to PPO inhibitors in these two species, our results highlight the importance of being able to identify Palmer amaranth and waterhemp growing in the field to inform timely herbicide application. Based on our results, it is more important to make early applications of a PPO inhibitor when Palmer amaranth is present in the field. A PRE application would be the best way to control both species, because both have the same level of resistance or sensitivity to these herbicides at this application stage. Additionally, these results indicate that when making a POST application of a PPO inhibitor, it may be difficult to determine whether Palmer amaranth in the field is resistant or not based only on survival.
Acknowledgments
We thank J.D. Green and James Martin for the original collection of Kentucky seed accessions that were used to generate the homozygous-resistant and -sensitive Palmer amaranth populations used in this project. This research was partially funded by Valent U.S.A. No other conflicts of interest have been declared.












