Introduction
Auxin herbicide use has spatially and temporally intensified since the release of 2,4-D– and dicamba-resistant cultivars of soybean [Glycine max (L.) Merr.] and cotton (Gossypium hirsutum L.) (Culpepper et al. Reference Culpepper, Sosnoskie, Shugart, Leifheit, Curry and Gray2018). As previously seen with glyphosate- and glufosinate-resistant crops (Johnson et al. Reference Johnson, Fisher, Jordan, Edminsten, Stewart and York2012), the threat of off-target movement to adjacent crops and natural vegetation that lack engineered resistance has also increased. The most commonly cited processes contributing to off-target exposures include physical spray drift, volatilization, and spray-tank contamination (Egan et al. Reference Egan, Barlow and Mortensen2014a). Wet and dry atmospheric deposition are also potential exposure pathways (Hill et al. Reference Hill, Harker, Hasselback, Moyer, Inaba and Byers2002; Majewski et al. Reference Majewski, Foreman and Goolsby2000; Van Dijk and Guicherit Reference Van Dijk, Guicherit, Dijk, Pul and Voogt1999). An overlooked exposure route is irrigation with water containing residual levels of dissolved herbicide. Monitoring studies have reported concentrations of herbicide residues in tailwater ditches and reservoirs (Kadoum and Mock Reference Kadoum and Mock1978; Moore et al. Reference Moore, Bennett, Cooper, Smith, Shields, Milam and Farris2001), but potential crop damage implications remain largely unknown (Bruns Reference Bruns1954; Scifres et al. Reference Scifres, Allen, Leinweber and Pearson1973).
Investigation of this question has increased in importance since intensification of auxin herbicide use, especially in Mississippi Delta regions affected by the ongoing decline of agriculturally important alluvial aquifers that makes groundwater extraction for irrigation more difficult and costly. In these regions, producers have adopted tailwater recovery systems as conservation practices to reduce reliance on groundwater (Evett et al. Reference Evett, Carman and Bucks2003; Vories and Evett Reference Vories and Evett2010). These networks of ditches or canals, pumps, and surface reservoirs capture precipitation, runoff from fields, and released tailwater for reuse on other fields (Evett et al. Reference Evett, Carman and Bucks2003). The water-saving benefits of on-farm reservoirs have been explored, potentially replacing 25% to 50% of groundwater irrigation (Sullivan and Delp Reference Sullivan and Delp2012). Tailwater reuse may pose a threat of off-target exposure by recirculating herbicide residues within the system to nonresistant crops. This concern is magnified for auxin herbicides, as nonresistant soybean and cotton varieties exhibit extreme sensitivity to dicamba and 2,4-D, respectively (Al-Khatib and Peterson Reference Al-Khatib and Peterson1999; Everitt and Keeling Reference Everitt and Keeling2009; Johnson et al. Reference Johnson, Fisher, Jordan, Edminsten, Stewart and York2012; Kelley et al. Reference Kelley, Wax, Hager and Riechers2005). Alfalfa (Medicago sativa L.), tomato (Solanum lycopersicum L.), grape (Vitis spp.), dry beans (Phaseolus vulgaris L.), watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai], peanut (Arachis hypogaea L.), and other crops, as well as noncultivated vegetation, can also be negatively affected by off-target movement of these herbicides (Bruns Reference Bruns1954; Culpepper et al. Reference Culpepper, Sosnoskie, Shugart, Leifheit, Curry and Gray2018; Egan et al. Reference Egan, Bohnenblust, Goslee, Mortensen and Tooker2014b; Hill et al. Reference Hill, Harker, Hasselback, Moyer, Inaba and Byers2002; Johnson et al. Reference Johnson, Fisher, Jordan, Edminsten, Stewart and York2012). Drift mechanisms have received great focus in assessing crop sensitivities to off-target auxin exposure (Egan et al. Reference Egan, Barlow and Mortensen2014a), but a limited selection of studies suggest irrigation exposure could be a concern for producers implementing tailwater recovery systems. Adverse response to dicamba in cotton, cucumber (Cucumis sativus L.), and sorghum [Sorghum bicolor (L.) Moench] (reduced seedling fresh weights; Scifres et al. Reference Scifres, Allen, Leinweber and Pearson1973) and to 2,4-D in field beans (Phaseolus vulgaris L., ‘Red Mexican’; Bruns Reference Bruns1954) exposed via irrigation have been documented. Further investigation is needed to understand agronomic implications and to systematically assess dose–response relationships at multiple growth stages, as has been done for drift exposure.
The primary difference between herbicide exposure by irrigation compared with drift is a shift in uptake pathways. Furrow irrigation is the most common irrigation practice for soybean in Arkansas (Vories and Evett Reference Vories and Evett2010), and for these systems, root uptake would be the primary pathway for exposure. Flood-irrigated and pivot-irrigated soybean, which represent smaller acreages in Arkansas, would also likely experience significant root uptake, while maintaining foliar exposure as a commonality with drift. Mechanisms of water and dissolved constituent movement across a foliar surface are different from the root–soil interface, and therefore root uptake of auxins could differ substantially in terms of plant response compared with foliar exposure, but preceding studies have almost exclusively focused on the latter, particularly for auxins. A study by Bruns (Reference Bruns1954) noted marked differences in symptomology in field beans exposed to 2,4-D via irrigation water compared with foliar exposure, most distinctively chlorosis and abnormal root development.
A growth chamber experiment was initiated to determine the dose–response relationship for susceptible soybean exposed to dicamba dissolved in irrigation water. The objectives of the study were to (1) determine the response of nonresistant soybean exposed at two growth stages in a controlled growth chamber environment, (2) develop dose–response curves for various critical response factors, and (3) project yield loss potential for root uptake and compare it with drift exposure data sets. Root uptake response data are compared with foliar (drift) response data from the literature to infer plant response differences between foliar and root uptake pathways. The overall aim of the research is to aid in understanding the risks associated with auxin herbicides dissolved in irrigation water and to assist land managers in developing compatible irrigation and weed control strategies. With the need for a varied arsenal of weed control options and for sustainable water supply quantity and quality, the findings of this and related work are important for addressing two fundamentally essential challenges faced by producers today. Assessing the potential for cross-crop impacts is essential for good stewardship in both weed and water management.
Materials and methods
Vegetative- and reproductive-stage soybean populations were assessed for dose response to dissolved herbicide in a growth chamber experiment. An indeterminate, early-maturing Roundup Ready2® soybean variety (‘RS066R2’, Renk Seed, Sun Prairie, WI) was selected for compatibility with the growth chamber environment. Seeds were pretreated with CruiserMax®, an insecticide/fungicide, and Optimize®, a bacterial inoculant for nitrogen fixation. Seeds were pre-germinated between damp paper towels at 21 C for 1 wk before planting. For each experimental unit, one seedling was planted with the cotyledons just above the soil surface in the center of a polyethylene pot (20.3-cm diameter, 17.8-cm height, Hummert International, Earth City, MO) filled with 4.6-kg sieved field soil obtained from the Arkansas Agricultural Research and Extension Center, located in Fayetteville, AR (Table 1), and lined with a nylon screen to prevent soil loss. Each pot received 250 ml of Hoagland’s solution without nitrogen amendment, supplied to each pot in a shallow catchment dish that also served to aid in irrigation and to prevent cross-contamination through leaching after herbicide treatment.
Table 1. Properties of soils used in the three experimental blocks, including pH and electrical conductivity (EC) in a 1:2 soil to water ratio, percent mass loss on ignition (LOI), and percent sand, silt, and clay fractions measured by hydrometer

After planting, pots were transferred to the growth chamber. Temperature (24 C), relative humidity (75%), and light conditions (photosynthetically active radiation = 500 to 700 μmol m−2 s−1) were maintained in a Conviron (Controlled Environments, Winnipeg, Canada) growth chamber with a 12/12-h day/night interval. Soil volumetric water content was monitored using a Delta-T® SM150 probe (Cambridge, UK) and was maintained within a range of 10% to 30%.
A randomized complete block design was used with growth stage (V3/V4 or R1) and concentration (0.05, 0.10, 0.50, 1.0, and 5.0 mg ae L−1) as treatment factors. Each experimental block consisted of 33 soybean plants, with plants receiving treatment with dicamba at five concentrations (0.05, 0.10, 0.50, 1.0, and 5.0 mg L−1 dicamba) at one of two growth stages (V3/V4 or R1) and three plants assigned to each treatment. Three control plants received irrigation water containing no added herbicide. The experiment was repeated three times from February 18, 2017, to July 27, 2017, at the Altheimer Laboratory at the University of Arkansas, Fayetteville, AR.
To establish the treatment gradient, five dicamba concentrations plus a no-herbicide control (0 mg L−1) were selected to encompass three orders of magnitude, a necessary range for robust statistical analysis of dose response. The maximum dicamba concentration (5.0 mg L−1) was selected based on literature estimates of edge-of-field concentrations where a low-volume runoff event immediately follows herbicide application (Wauchope Reference Wauchope1978). This worst-case scenario sets an upper limit of concentrations that would be expected in a real-world tailwater system and assumes the high-concentration flow would be directly diverted to a susceptible crop for irrigation, without dilution or other dissipation. These simplifying assumptions were not meant to be representative of typical tailwater herbicide concentrations.
Dicamba solutions at specified concentrations were prepared from formulated Engenia®, (BASF, Research Triangle Park, NC) using deionized water as the diluent. For plants assigned treatment at V3/V4 (n = 15), 200 ml of the herbicide solution corresponding to the assigned concentration was applied to the soil surface around the base of each plant, taking care to avoid foliar exposure and minimize stem exposure. Simultaneously, the three control plants and 15 plants assigned to treatment at R1 received 200 ml deionized water. Scaled from the pot area up to field-relevant units, the 200-ml irrigation represented a 6.2 mm ha−1 irrigation event. Herbicide treatment procedures were repeated for the R1 treatment group once flowering occurred.
Plants were assessed for symptomology associated with auxin exposure in soybean (i.e., cupping in newly emerged leaves, epinasty, stacking of nodes, stunted growth; Andersen et al. Reference Andersen, Clay, Wrage and Matthees2004) 14 d after treatment (DAT) for both the V3/V4 and R1 treatment groups and the control. Visible assessments for injury were made using a 0% to 100% scale, with 0% being no injury and 100% being complete death, at 14 DAT for both V3/V4 and R1 treatment groups. Plants were terminated at 14 DAT for the R1 treatment group and at 21 DAT for the V3/V4 treatment group, and plant height to the newest node, number of pod-bearing nodes, and number of pods were recorded for all plants. Plants were bagged, dried for 9 d at 60 C in a drying oven, and weighed to measure aboveground dry mass. Using the following equation, dry mass, height, and node and pod number were scaled relative to control plants and expressed as a percent reduction from the control to minimize variability between experimental blocks.
Thus, positive values indicate smaller plants and fewer nodes or pods than control plants, that is, damaged plants, and negative values indicate larger plants and more nodes or pods than control plants. Plant injury assessments were not adjusted in this way, as these metrics were scaled to controls at the time of recording, and injury to control plants was always 0%.
The metrics percent plant injury at 14 DAT and percent dry mass reduction, percent height reduction, percent pod reduction, and percent node reduction measured at harvest were examined for differences in response related to experimental block, growth stage at application, and concentration of herbicide using the Kruskal-Wallis one-way ANOVA test with post hoc pairwise comparison for identified treatment effects. To examine effective dose, logistic regressions were fit to the nonlinear relationship between the probability of a response metric exhibiting symptoms of herbicide exposure, with concentration as a predictor. The model is described in Equation 2:
where π is the probability of being affected (damaged) more than the threshold in response, and b 0 and b 1 are regression coefficients, with b 0 being the intercept and b 1 indicating a positive or negative impact of log dose on log odds, respectively. Logistic regression analysis was performed only for the metrics that indicated significant (P < 0.05) effects of concentration in Kruskal-Wallis tests. For percent plant injury at 14 DAT, critical response levels of 20% and 40% injury were established to assess probability of damage. For the end of experiment metrics (plant height to the newest node, number of pod-bearing nodes, and number of pods), a binary threshold was constructed based on whether more than 30% reduction occurred or not. These thresholds were selected based on data distribution. Note that dose concentration in Equation 2 was treated as a continuous variable (0 to 5 mg L−1), while the concentrations in the Kruskal-Wallis test were the ordinal categories. Logistic regression models were used to estimate threshold response dose predicted to result in 50% of plants being effected (TRD):
Logistic regression model parameters were assessed for statistical significance (P < 0.05) using the likelihood ratio test, and model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test, with P > 0.05 indicating adequate fit.
Yield loss, expressed as yield proportion of control plant yield due to varying levels of exposure to dicamba in irrigation water, was projected based on models available in the literature using 14 DAT for plant injury assessments (Egan et al. Reference Egan, Barlow and Mortensen2014a) and pod counts (Sitompul et al. Reference Sitompul, Sari, Krisnawati, Mulia and Taufiq2015). These estimates were then related to estimates of dicamba dose (mg cm−2) delivered at each dicamba concentration to the surface area of the potted field soil, which was subsequently scaled up to approximate field rates (g ha−1). The relationship between yield proportion (y) and dicamba dose was modeled by a two-parameter log-logistic relationship (Knezevic et al. Reference Knezevic, Streibig and Ritz2007):
where b is the relative slope around e = ED50. The upper and lower limits of y were 0 and 1. All analyses were performed using R v. 3.3.2 statistical software (R Core Team, Vienna, Austria). R packages PGIRMESS and DRC were used for multiple comparisons of the Kruskal-Wallis test and fitting the log-logistic model, respectively.
Results and discussion
Dicamba concentration was the main significant factor for all growth response metrics (Table 2). Growth stage was a significant explanatory variable only for the height response metric. Previous findings from spray-drift exposure studies indicate growth stage at time of exposure is a significant factor in determining response to dicamba in soybean (Andersen et al. Reference Andersen, Clay, Wrage and Matthees2004; Auch and Arnold Reference Auch and Arnold1978; Egan et al. Reference Egan, Barlow and Mortensen2014a; Kelley et al. Reference Kelley, Wax, Hager and Riechers2005; Wax et al. Reference Wax, Knuth and Slife1969), with flowering plants more sensitive to exposure than plants in vegetative stages. Conversely, Bruns (Reference Bruns1954) reported that field beans exposed at early vegetative stage had greater yield reduction than those treated at early bloom stage, but noted the differences were not significant at the α = 0.05 level. While data from the present study suggest no growth stage effects, follow-up studies performed in the field are needed before definitive conclusions can be drawn.
Table 2. Kruskal-Wallis tests for plant growth response to experimental block, growth stage at exposure, and concentration of dicamba

a Plant injury ratings were collected 14 DAT. Percent dry weight, height, pod, and node reduction relative to control plants were recorded at completion of the experiment.
b A P-value < 0.05 was considered to indicate a significant effect.
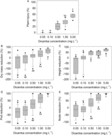
In Figure 1, Kruskal-Wallis pairwise comparisons of five dicamba concentrations revealed that injury tends to increase as concentration in irrigation increases for all responses (Figure 1A−E for each response). Dicamba concentrations could generally be grouped into three effect levels, with 0.05 to 0.50 mg L−1 (3.1 to 31 g ha−1) associated with no measurable effects. Plants exposed to these concentrations performed similarly to control plants. Low and high effect levels were 0.50 to 1.0 (31 to 62 g ha−1) and 1.0 to 5.0 mg L−1 (62 to 310 g ha−1), respectively. Differentiation between no effect and low effect levels was most defined for percent height reduction, the only metric for which 0.50 mg L−1 (31 g ha−1) was statistically different from 0.05 and 0.10 mg L−1 (3.1 and 6.2 g ha−1). Plants exposed to 1.0 to 5.0 mg L−1 (62 to 310 g ha−1) exhibited dicamba symptomology and severe growth and reproductive restrictions. Though responses to dicamba exposure at 1.0 and 5.0 mg L−1 (62 and 310 g ha−1) were statistically the same for percent pod and percent node reduction, notably, plants exposed to 5.0 mg L−1 (310 g ha−1) consistently had no pod-bearing nodes (100% pod and node reduction; Figure 1D and E).

Figure 1. Growth response metrics (A) percent plant injury, (B) percent dry mass reduction, (C) percent height reduction, (D) percent pod reduction, and (E) percent node reduction at five dicamba concentrations in irrigation water. Lowercase letters indicate the results of post hoc pairwise comparisons of differences in growth responses by dicamba concentration in irrigation following identification of a significant difference for at least one concentration in a Kruskal-Wallis analysis.
For each plant response metric, logistic regression analysis showed probability of damage at critical response thresholds (20% and 40% for plant injury; 30% for growth response metrics) increased nonlinearly with increasing dicamba concentration in irrigation water (Table 3; Figure 2A–F). The greatest increases in plant injury probability were associated with lower concentrations (0.05 to 0.50 mg L−1) when the critical response level was low and with higher concentrations (1.0 to 5.0 mg L−1) when the critical response level was high. Thus, the projected TRD for the 20% plant injury assessment level (i.e., concentration at which 50% of plants will express 20% injury or greater) was 0.60 mg L−1 (95% confidence interval = 0.45 to 0.75 mg L−1), but the projected TRD for 40% plant injury was more than three times higher (2.0 mg L−1 ± 1.37–3.33 mg L−1). The TRD estimates for 30% reduction in dry mass, height, and number of pods and pod-bearing nodes ranged from 0.36 to 0.96 mg L−1. Plant height was the most sensitive indicator of dicamba damage, with number of pods and nodes also projected to decline in the population at exposure levels that were lower than the TRD for 20% plant injury (TRD = 0.36 for plant height and 0.47 mg L−1 for number of pods and nodes vs. 0.60 mg L−1 for 20% plant injury). Reductions in pods and nodes in response to dicamba exposure in irrigation water were clearly related, resulting in the same TRD = 0.47 mg L−1. Dry mass, in contrast, was less sensitive than the other plant response metrics, with TRD = 0.96 mg L−1 falling between TRD for 20% and 40% plant injury.
Table 3. Parameters and summary statistics for logistic regression describing the relationship between the probability of plant response thresholds and log-dose dicamba as a predictora

a Model parameters (b 0 and b 1, see Equation 2) were assessed using the likelihood ratio test where a smaller P-value (<0.05) for the χ 2 statistic indicates a significant effect. The Hosmer-Lemeshow test was used to show the goodness of fit where a high P-value (>0.05) for the χ 2 statistic indicates no evidence of lack of fit.

Figure 2. Probability of damage at assessment levels for growth response metrics (A) 20% plant injury, (B) 40% plant injury, (C) 30% dry mass reduction, (D) 30% height reduction, (E) 30% pod reduction, and (F) 30% node reduction at five dicamba concentrations in irrigation water. Shaded areas around the threshold response dose (TRD) estimates indicate the 95% confidence intervals. Logistic regression curve parameters are reported in Table 3.
Differences in TRD between plant injury assessment levels indicates that three times more dicamba is required to reach the threshold of 40% injury than the threshold of 20% injury. This finding may reassure producers that transfer of low-level residual herbicides to nonresistant crops in tailwater recovery systems is unlikely to cause extensive crop damage. Edge-of-field measures show that concentrations in the part per million range (mg L−1) occur infrequently in runoff and under worst-case scenarios, such as a small runoff volume immediately following herbicide application (Wauchope Reference Wauchope1978). High runoff concentrations are further remediated by dilution in ditches and surface reservoirs and through other biological and chemical herbicide dissipation mechanisms (Moore et al. Reference Moore, Bennett, Cooper, Smith, Shields, Milam and Farris2001). Conversely, even low-concentration irrigation water applied at large volumes, such as a typical 30.5 mm ha−1 event (Tacker and Vories Reference Tacker and Vories2000), could result in effect-level exposures, as total dose delivered increases with irrigation volume. In fact, an equivalent phenomenon has been observed in simulated spray-drift experiments, with the ratio of herbicide to carrier volume affecting plant injury (Banks and Schroeder Reference Banks and Schroeder2002). In this study, the herbicides were applied in a small volume of water compared with a typical field irrigation event (6.2 vs. 30.5 mm ha−1). The estimated TRD values reported as concentrations are dependent on the volume of irrigation water applied, and may be different if herbicide is delivered at more dilute concentrations but in larger irrigation volumes or vice versa. Further irrigation exposure studies are needed to elucidate the influence concentration has on crop response compared with total mass delivered. Given that root uptake is driven by diffusion, a significant interaction between mass and volume is possible.
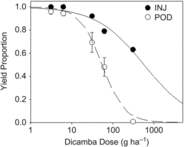
Projected yield proportions estimated from percent plant injury (Egan et al. Reference Egan, Barlow and Mortensen2014a) and from pod counts (Sitompul et al. Reference Sitompul, Sari, Krisnawati, Mulia and Taufiq2015) ranged from 1 to 0.63 and 0.95 to 0, respectively (Figure 3). For both sets of projections, yield proportion decreased with increasing dicamba dose above 6.2 g ha−1 (dose equivalent to the 0.1 mg L−1 treatment). The extensive meta-analysis by Egan et al. (Reference Egan, Barlow and Mortensen2014a) demonstrated that yield loss resulting from foliar exposure to dicamba follows a sigmoidal pattern across doses ranging from 0.56 to 560 g ha−1, which encompasses the estimated field doses in this study (3.1 to 310 g ha−1). Here, the relationship between yield loss and dose was well described by a log-logistic model only when pod count was used to estimate yield, with P = 0.605 as evidence of an adequate fit (Table 4). The yield proportion estimated from percent plant injury at the maximum dicamba dose (5 mg L−1, estimated 310 g ha−1) was out of range of the theoretical minimum 0 yield proportion, poorly constraining estimates of the inflection point e and any estimates of yield proportion above 310 g ha−1. Further, the two models differ in the rate at which yield declined between 6.2 and 62 g ha−1, with projections based on percent plant injury apparently underestimating yield loss.

Figure 3. Relationship between average projected yield loss across the range of dicamba doses delivered to the soil surface scaled to up to approximate areal field application rates. Yield loss was estimated based on percent plant injury (INJ) and pod counts (POD). Nonlinear regression curve parameters are reported in Table 4.
Table 4. Parameters and summary statistics for nonlinear regression models describing the relationship between projected yield loss based on percent plant injury or pod counts and the dicamba dose delivered, scaled from pot area to approximate areal field rates in hectaresa

a Parameters: b = the relative slope around e = ED50.
b Lack of model fit is indicated by P < 0.05.
Nevertheless, the yield projection model based on percent plant injury is useful for making comparisons of potential differences between soybean exposure to dicamba via root uptake versus foliar uptake. The yield proportion projections based on percent plant injury reflect observations of injury under foliar exposure and assume that injury from root uptake results in yield loss similar to that seen with foliar uptake. Projections based on pod count are linked to observed symptomology following root uptake of dicamba and reflect that plants irrigated with the 5 mg L−1 solution consistently had no pods. Taken together, these findings suggest that soybean may express both magnitude of injury and specific symptomology differently when exposure to dicamba is via root uptake and that drift-based, foliar exposure models may be inappropriate for predicting soybean yield loss when injury results from irrigation. Here, maximum plant injury associated with exposure to 310 g ha−1 in irrigation water was 70%, in contrast with drift studies linking exposure to comparable doses of 280 g ha−1 with up to 100% plant injury (Egan et al. Reference Egan, Barlow and Mortensen2014a) and suggesting plant injury symptomology will be less severe when dicamba exposure is via soil-applied irrigation. However, many plants assessed with injury ranging from 20% to 70% also had no pods, suggesting no yield potential even in plants with low percent plant injury when exposure occurs via irrigation water. Further, soybean exposed at the V3/V4 stage with 20% injury (7 DAT) exhibited yield reduction in the 5% to 10% range in drift studies (Griffin et al. Reference Griffin, Bauerle, Stephenson, Miller and Boudreaux2013). These findings suggest that relying on drift studies to assess damage from a dicamba exposure via irrigation could lead to overestimation of the likelihood of crop recovery. This outcome is potentially even more economically challenging for producers, as the opportunity to replant may no longer be a mitigation option later in the season when pod reduction or absence would become apparent. Alternatively, given that plants were destructively sampled approximately 14 d after flowering, it is possible that pod production was only delayed, but would not have been reduced if given additional time to reach maturity.
This study offers a preliminary comparison of root and foliar uptake as pathways of dicamba exposure. Data exploring soybean growth response to root uptake of dicamba, or herbicides in general, have not previously been reported in the literature. These data are highly relevant to understanding the full suite of dicamba exposure pathways, especially in regions with an intersection of auxin herbicide usage and irrigation sources that are likely to contain residual herbicides. It is difficult to compare the results of irrigation exposure with those of drift exposure on a mass basis. With irrigation exposure, herbicide mass delivered can be calculated from the product of concentration and irrigation volume applied. In simulated drift, actual mass delivered to the plant is not reported and is a function of spray concentration and intercepted spray volume. In neither case, though, is actual mass uptake measured. Further, caution should be taken in extrapolating these findings to the field, as these measurements were collected in a growth chamber study, and yield is indirectly estimated. Field trials are needed to directly relate yield to dicamba dose and to develop robust correlations for predicting yield losses based on metrics such as injury and plant height. Furthermore, monitoring efforts to quantify herbicide-residue levels in tailwater recovery systems and other sources of irrigation water are needed to assess the risk of exposure to dicamba at relevant concentrations under real-world agronomic conditions.
Author ORCIDs
Cammy D. Willett https://orcid.org/0000-0002-5704-5379, Jason K. Norsworthy https://orcid.org/0000-0002-7379-6201.
Acknowledgments
Funding for the project was provided by the Arkansas Soybean Promotion Board. Additional support was provided by the University of Arkansas System Division of Agriculture. No conflicts of interest have been declared.