Introduction
Waterhemp [Amaranthus tuberculatus (Moq.) Sauer] is a herbaceous, obligate outcrossing weed species native to the midwestern United States (Nordby et al. Reference Nordby, Hartzler and Bradley2007; Steckel Reference Steckel2007). Surveys conducted by the Weed Science Society of America (WSSA) reported that A. tuberculatus is the second most troublesome weed after Palmer amaranth (Amaranthus palmeri S. Watson) in corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] in the United States and Canada (Van Wychen Reference Van Wychen2020, Reference Van Wychen2022). Season-long A. tuberculatus interference at a density of 8 plants m−1 row reduced soybean yield by 56% (Bensch et al. Reference Bensch, Horak and Peterson2003). It has high fecundity; a single female A. tuberculatus plant can produce more than 400,000 seeds in the absence of any competition from other plants under field conditions (Uscanga-Mortera et al. Reference Uscanga-Mortera, Clay, Forcella and Gunsolus2007), and the majority of seeds germinate within a 2- to 3-yr period (Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996; Steckel et al. Reference Steckel, Sprague, Stoller and Wax2007). Amaranthus tuberculatus exhibits an extended period of emergence in the Midwest, achieving 10% and 90% cumulative emergence with 240 and 937 cumulative growing degree days, respectively (Hartzler et al. Reference Hartzler, Buhler and Stoltenberg1999; Werle et al. Reference Werle, Sandell, Buhler, Hartzler and Lindquist2014).
The evolution of herbicide resistance in A. tuberculatus populations in the United States has resulted in the loss of effective herbicide sites of action (SOAs) for controlling this weed species. The obligate outcrossing behavior of A. tuberculatus leads to high genetic diversity and rapid transfer of herbicide-resistance alleles among populations in the landscape (Liu et al. Reference Liu, Davis and Tranel2012; Sarangi et al. Reference Sarangi, Tyre, Patterson, Gaines, Irmak, Knezevic, Lindquist and Jhala2017). In the United States, A. tuberculatus populations collected from 20 states were confirmed to be resistant to one or more herbicide SOAs, including resistance to 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors, 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS) inhibitors, acetolactate synthase (ALS) inhibitors, photosystem II (PSII) inhibitors, protoporphyrinogen oxidase (PPO) inhibitors, auxin mimics, and very-long-chain fatty-acid inhibitors (Heap Reference Heap2023). Several A. tuberculatus populations were reported resistant to ALS- and PSII-inhibiting herbicides in the 1990s (Heap Reference Heap2023). During the early 2000s, A. tuberculatus was reported as the first weed species to develop resistance to a PPO-inhibiting herbicide (Shoup et al. Reference Shoup, Al-Khatib and Peterson2003). Glyphosate-resistant A. tuberculatus was first confirmed in Missouri by Legleiter and Bradley (Reference Legleiter and Bradley2008), while the initial indication of glyphosate resistance development was reported by Zelaya and Owen (Reference Zelaya and Owen2002) from Iowa. Concerns have arisen recently regarding the resistance to auxin mimics in A. tuberculatus populations in Nebraska (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012) and Illinois (Evans et al. Reference Evans, Strom, Riechers, Davis, Tranel and Hager2019).
The resistance classification criteria used in previous research varied considerably. These differences likely arise from the WSSA definition of herbicide resistance (WSSA 1998), which focuses on individual plants rather than the entire population. For example, a study from Missouri classified A. tuberculatus populations resistant when ≥60% survival was observed at 2× the labeled glyphosate dose (Rosenbaum and Bradley Reference Rosenbaum and Bradley2013). Another study, which used percent plant injury for classification, reported that the A. tuberculatus populations with 0% to 49% injury at a labeled dose (1×) herbicide application were classified as resistant, whereas the populations with 50% to 89% injury were classified as less sensitive (Singh et al. Reference Singh, Garetson, McGinty, Dotray, Morgan, Nolte and Bagavathiannan2020). Two recent studies from Iowa and Wisconsin classified A. tuberculatus populations resistant when more than 50% plants survived the labeled dose (1×) application of herbicides (Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022; Hamberg et al. Reference Hamberg, Yadav, Dixon, Licht and Owen2023a). The classification criteria used in the literature often ignored populations with 10% to 50% survival at 1× the labeled dose, but these populations are important from a herbicide-resistance management perspective at the field level. Therefore, in this paper, we created resistance classification criteria that align with most of the previous research and provide early indications of resistance spread in specific populations.
Extensive genetic variability and long-distance pollen and seed movement coupled with the selection pressure from herbicides favored A. tuberculatus populations becoming resistant to multiple herbicides. An A. tuberculatus population from Illinois has been documented to show resistance to ALS-, HPPD-, PSII-, and PPO-inhibiting and auxin mimic herbicides, demonstrating a five-way resistance (Evans et al. Reference Evans, Strom, Riechers, Davis, Tranel and Hager2019). Another population from Missouri has been identified as six-way resistant, exhibiting 3-fold or higher resistance to ALS-, EPSPS-, HPPD-, PSII-, PPO-inhibiting and auxin mimic herbicides (Shergill et al. Reference Shergill, Barlow, Bish and Bradley2018). The evolution of multiple herbicide–resistant populations limits the alternate herbicide SOAs available for controlling this weed species. In Minnesota, A. tuberculatus populations resistant to ALS- inhibiting herbicides like imazethapyr and thifensulfuron-methyl were first reported in 1994, with a subsequent report of resistance to an EPSPS inhibitor (glyphosate) in 2007 (Cockerton et al. Reference Cockerton, Kaundun, Nguyen, Hutchings, Dale, Howell and Neve2021; Heap Reference Heap2023). Amaranthus tuberculatus populations resistant to PPO inhibitors were confirmed in Minnesota in 2015 (Heap Reference Heap2023). The instances of A. tuberculatus control failures following postemergence applications have increased over time, but except for these initial reports, the extent of resistance in Minnesota remains unclear.
The objectives of this research were to evaluate the profile and extent of herbicide-resistant A. tuberculatus populations in Minnesota. The goal of this research was not to estimate the proportion of fields in Minnesota infested with herbicide-resistant A. tuberculatus, but rather to assess the spectrum and distribution patterns of those resistant populations within the state. The results of this experiment could establish a baseline for future research for mitigating herbicide-resistance evolution in Minnesota and implementing regional management strategies.
Materials and Methods
Plant Materials
Amaranthus tuberculatus seed samples were collected in the fall of 2020 and 2021 as a part of a row-crop production area survey in Minnesota. The samples were collected by growers, crop consultants, and University of Minnesota Extension Educators, or by members of the University of Minnesota Integrated Cropping Systems Weed Science Lab. Amaranthus tuberculatus plants that survived the postemergence herbicide applications in corn, soybean, and sugar beet (Beta vulgaris L.) fields were selected for this experiment. At each location, seed heads from 10 to 15 female plants were harvested and pooled to establish a population. The GPS coordinates of collection sites were recorded. This experiment involved the evaluation of 90 putative herbicide-resistant A. tuberculatus populations collected from 47 counties across Minnesota. The distribution of the collection sites and the demarcation of different regions are illustrated in Figure 1.

Figure 1. Geographic distribution of Amaranthus tuberculatus populations collected from corn, soybean, and sugar beet fields in Minnesota in 2020 and 2021. Background colors indicate regions of the state of Minnesota.
Seed heads were oven-dried at 35 C for 96 h and threshed using an electric motor-operated Agriculex SPT-1A thresher (Agriculex, Guelph, ONT, Canada). Seeds were cleaned using a seed blower (South Dakota Seed Blower, Seedburo Equipment, Des Plaines, IL, USA), and each population was cold stratified by storing seeds separately in dark in airtight polyethylene bags at −20 C for 3 mo. Following the removal of seeds from the freezer, they were stored at 4 C for a period of 15 d to overcome the seed dormancy.
Seeds from each population were planted in 55 by 28 cm plastic trays filled with germination mix (Jolly Gardener®, Oldcastle Lawn & Garden, Atlanta, GA, USA). The trays were placed in a greenhouse at a day/night temperature of 30/24 C and a 16-h photoperiod. One seedling was transplanted at the 2-leaf stage into a Cone-tainer™ (4-cm top diameter and 21-cm deep) (Stuewe and Sons, Tangent, OR, USA) filled with 3:1 ratio of potting mix (Sun Gro® Professional Growing Mix, Sun Gro Horticulture, Agawam, MA, USA) to sand. Plants were watered daily and fertilized once a week using water-soluble 20-3-19 FeED Plus Mg fertilizer (Jack’s Professional®, JR Peters, Allentown, PA, USA).
Herbicide Assays
Whole-plant bioassays were conducted from 2021 to 2023 in a greenhouse at the Plant Growth Facilities at the University of Minnesota’s St Paul campus to determine resistance to eight postemergence herbicides representing seven different SOAs. The plants from each A. tuberculatus population were divided into three groups, and each group was sprayed either with a labeled dose (1×), three times the labeled dose (3×) of each herbicide, or no herbicide (nontreated control). An individual plant represented an experimental unit. The experimental units within a population and herbicide dose were arranged in a randomized complete block design and replicated seven times. Two runs were completed under the same greenhouse conditions. A population collected from a soybean field in McLeod County, MN, was included in the experiment for comparison and was confirmed to be susceptible to all the herbicides, except for the ALS inhibitors.
Details of herbicide treatments, doses, and adjuvants used in this experiment are included in Table 1. Herbicides were applied using a spray chamber equipped with TeeJet® 8001 EVS nozzle (TeeJet® Technologies, Spraying Systems, Wheaton, IL, USA) calibrated to deliver 140 L ha−1 at 207 kPa pressure. The plants were 10- to 12-cm tall at the time of herbicide application. Each plant was visually assessed at 28 d after treatment (DAT) for percent injury, utilizing a scale ranging from 0% to 100% based on the extent of injury, height, and regrowth, where 0% indicated no injury compared with the nontreated control and 100% denoted plant death. In this experiment, only plants exhibiting <90% injury at 28 DAT were able to survive and produce flowers when transplanted into larger pots and provided regular watering. Therefore, plants with ≥90% injury at 28 DAT were classified as susceptible, and the survival frequency for each population was then estimated as:
Table 1. Dose, site of action, manufacturer, and adjuvant information for herbicides used in greenhouse experiments at University of Minnesota, St Paul, MN

a Abbreviations: ALS, acetolactate synthase; AMS, ammonium sulfate; COC, crop oil concentrate; EPSPS, 5-enolpyruvylshikimate-3-phosphtae synthase; HPPD, 4-hydroxyphenylpyruvate dioxygenase; PPO, protoporphyrinogen oxidase; PS II, photosystem II.
where
![]() $S$
is the number of plants that survived (<90% injury at 28 DAT) following a herbicide treatment, and
$S$
is the number of plants that survived (<90% injury at 28 DAT) following a herbicide treatment, and
![]() $T$
is the total number of plants screened.
$T$
is the total number of plants screened.
Individual plants were cut at the base at 28 DAT and bagged separately. The samples were oven-dried at 60 C for 4 d to estimate aboveground biomass. Biomass data were converted to percent biomass reduction compared with nontreated control using Equation 2:
where,
![]() ${\rm{B}}{{\rm{M}}_{{\rm{NTC\;}}}}$
represents biomass of nontreated control and
${\rm{B}}{{\rm{M}}_{{\rm{NTC\;}}}}$
represents biomass of nontreated control and
![]() ${\rm{BM}}$
represents biomass of an individual plant.
${\rm{BM}}$
represents biomass of an individual plant.
Resistance Level Classification
Populations exhibiting ≥40 % survival at 3× the labeled dose of a herbicide were classified as “highly resistant,” and populations with <40% survival at 3× the labeled dose but ≥40% survival at a labeled dose (1×) were classified as “moderately resistant.” Populations showing 10% to 39% survival at the labeled dose were categorized as “less sensitive,” while those exhibiting <10% survival were classified as “susceptible.” In instances in which a single population showed resistance (moderate to high resistance) to more than one SOA, it was designated as multiple-herbicide resistant.
Statistical Analyses
Data from the two experimental runs were combined and analyzed. Geographic distribution maps depicting percent survival at 1× the labeled dose were developed using ArcGIS Pro (v. 3.1.0, Redlands, CA, USA), adopting the procedure described by Singh et al. (Reference Singh, Garetson, McGinty, Dotray, Morgan, Nolte and Bagavathiannan2020). Percent survival data from the 90 populations were interpolated using the inverse distance weighing method in ArcGIS Pro to include surveyed and adjacent counties (60 counties in total). Map layers were stretched using maximum/minimum stretch; the maximum and minimum values were set at 100% and 0%, respectively. The percentage of populations showing resistance to different herbicides are presented with pie charts and UpSet plots using R (R Core Team 2023).
Results and Discussion
Auxin Mimics (2,4-D and Dicamba)
Out of 90 A. tuberculatus populations, 5 (6%) were classified as highly resistant and 4 (4%) as moderately resistant to 2,4-D (Figure 2). Averaged over the populations, the application of 1× and 3× the labeled doses of 2,4-D reduced A. tuberculatus biomass by 84% and 88%, respectively (Figure 3). Earlier reports indicated the presence of 2,4-D–resistant A. tuberculatus populations in Illinois, Missouri, Nebraska, and Wisconsin (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012; Evans et al. Reference Evans, Strom, Riechers, Davis, Tranel and Hager2019; Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022; Shergill et al. Reference Shergill, Barlow, Bish and Bradley2018). Surveys conducted in Iowa and Wisconsin reported that labeled dose application of 2,4-D resulted in ≥50% plant survival for 7% and 17% of A. tuberculatus populations, respectively (Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022; Hamberg et al. Reference Hamberg, Yadav, Owen and Licht2023b).
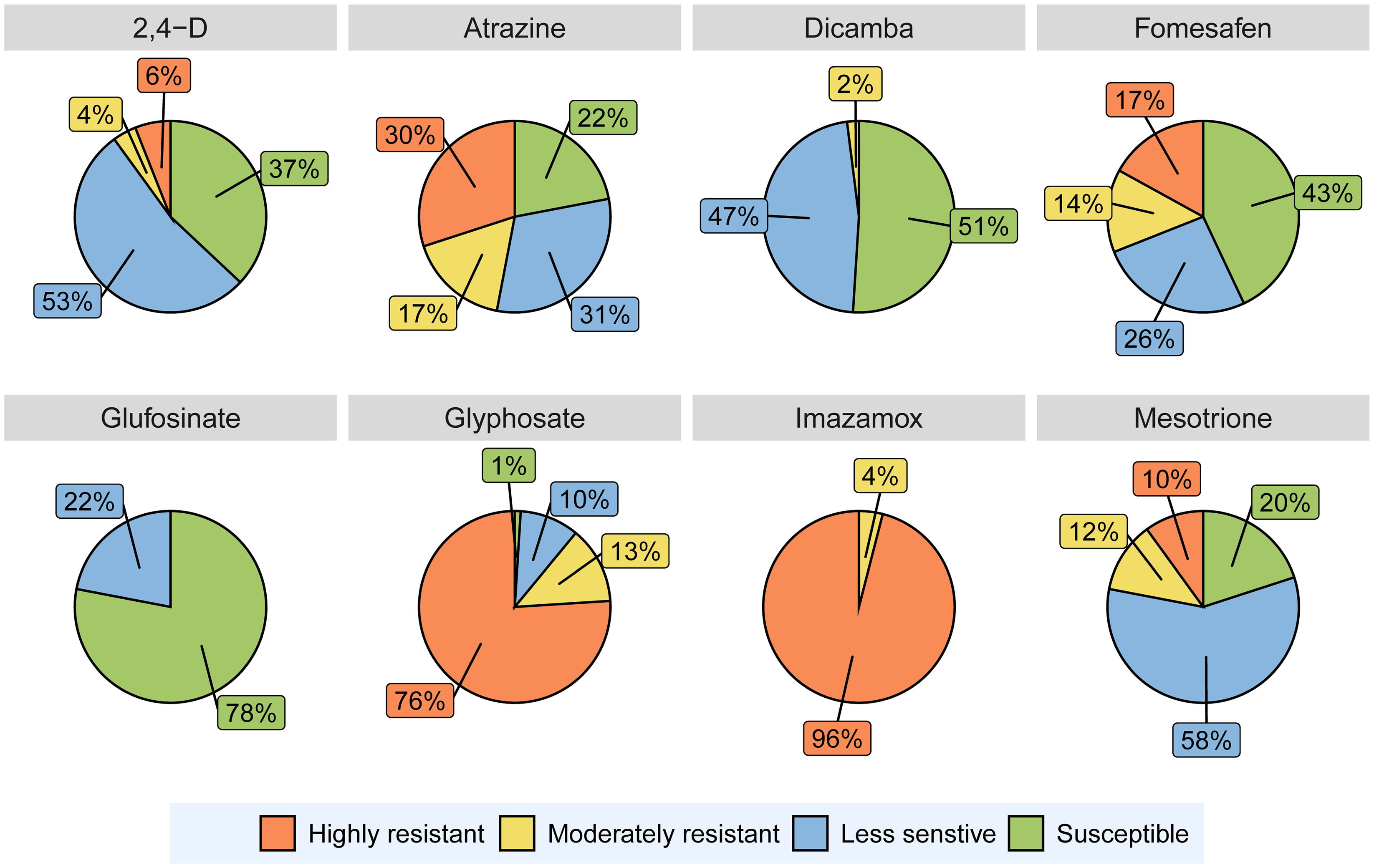
Figure 2. The percentage of 90 Amaranthus tuberculatus populations classified as highly resistant, moderately resistant, less sensitive, and susceptible to different herbicides used in the experiments conducted in a greenhouse at the University of Minnesota. Populations with ≥40 % plant survival at 3× the labeled dose of a herbicide were categorized as “highly resistant,” and populations with <40% survival at 3× the labeled dose but ≥40% survival at the labeled dose (1×) were categorized as “moderately resistant.” Populations exhibiting 20% to 39% and <20% plant survival at labeled dose (1×) were classified as “less sensitive” and “susceptible,” respectively.

Figure 3. Amaranthus tuberculatus biomass reduction compared with nontreated control from herbicide application (1× and 3× the labeled doses) in greenhouse experiments at the University of Minnesota, St Paul, MN. The horizontal black line represents the mean biomass reduction from each herbicide application. Individual data points depict the average percent biomass reduction for a population.
Only 2% of A. tuberculatus populations tested in this experiment were moderately resistant to dicamba (Figure 2). An A. tuberculatus population (A49; see Supplementary Table 1) from Lincoln County, MN, exhibited 71% and 21% plant survival after the application of 1× and 3× the labeled dose of dicamba, respectively (data not shown). Forty-seven percent of the populations evaluated were categorized as less sensitive to dicamba (Figure 2). Without the implementation of effective management practices, these populations have the potential to evolve into highly resistant populations within a few years. Similarly, a smaller number of A. tuberculatus populations tested in Iowa and Wisconsin showed reduced sensitivity to dicamba compared with 2,4-D (Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022; Hamberg et al. Reference Hamberg, Yadav, Owen and Licht2023b). An A. tuberculatus population from Illinois was reported to exhibit 5.6- to 10.6-fold resistance to dicamba (Bobadilla et al. Reference Bobadilla, Giacomini, Hager and Tranel2022).
In this research, A. tuberculatus populations exhibiting resistance to 2,4-D and dicamba were found in southwestern and west central Minnesota (Lincoln, Lyon, Pipestone, Rock, and Yellow Medicine counties; Figure 4). The corn–soybean rotation is the prevalent cropping sequence in this region, with both crops representing 89% to 95% of the total harvested acreage in these counties (USDA-NASS 2017a, 2017b). In addition to application in corn, 2,4-D and dicamba are extensively used in soybean after the commercialization of the Enlist® and Xtend® traits, respectively (Kruger et al. Reference Kruger, Alves, Schroeder, Golus, Reynolds, Dodds, Brown, Fritz and Hoffmann2022; Werle et al. Reference Werle, Mobli, Striegel, Arneson, Dewerff, Brown and Oliveira2022). Furthermore, the southwestern part of Minnesota often experiences limited weed control with preemergence herbicides due to insufficient rainfall, intensifying selection pressure from postemergence treatments (J Gunsolus, personal communication). Two A. tuberculatus populations from Lincoln County and Lyon County, MN, were moderately resistant to dicamba and highly resistant to 2,4-D. Cross-resistance between 2,4-D and dicamba is possible in A. tuberculatus, and this phenomenon has been observed in a population from Illinois (Bobadilla et al. Reference Bobadilla, Giacomini, Hager and Tranel2022).

Figure 4. Interpolated geographic distribution of survival percentage of Amaranthus tuberculatus populations following the labeled dose (1×) application of (A) 2,4-D, (B) atrazine, (C) dicamba, (D) fomesafen, (E) glufosinate, (F) glyphosate, (G) imazamox, (H) mesotrione in greenhouse experiments conducted at University of Minnesota, St. Paul, MN.
ALS-inhibiting Herbicide (Imazamox)
All A. tuberculatus populations evaluated in this experiment were moderately to highly resistant to imazamox (Figure 2). Irrespective of dose, 65% of all the populations exhibited 70% or more plant survival (data not shown). Resistance to ALS-inhibiting herbicides in A. tuberculatus is widespread in the midwestern United States (Heap Reference Heap2023). An experiment from Missouri reported that 186 out of 187 A. tuberculatus populations were resistant to an ALS-inhibiting herbicide, chlorimuron (Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015). Another recent study from Wisconsin reported ≥50% plant survival at 3× the labeled dose of imazethapyr in 98% of the A. tuberculatus populations evaluated (Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022). The ALS-inhibiting herbicides were first commercialized in 1982 and were used intensively for weed control in agricultural crops (Tranel and Wright Reference Tranel and Wright2002). Amaranthus tuberculatus populations resistant to ALS-inhibiting herbicides became widespread within 5 yr of the initial report of ALS inhibitor resistance (Tranel Reference Tranel2021). Amaranthus tuberculatus resistance to imazamox is widespread across the sampled area in Minnesota (Figure 4). The resistance could be linked to the historical extensive use of chemicals from this SOA and the existence of a high initial frequency of genes conferring resistance (Tranel et al. Reference Tranel, Riggins, Bell and Hager2011).
EPSPS-inhibiting Herbicide (Glyphosate)
Sixty-eight (76%) of the populations were classified as highly resistant to glyphosate, while 12 (13%) populations were moderately resistant (Figure 2). Glyphosate at 1× and 3× the labeled doses reduced A. tuberculatus biomass by 46% and 62%, respectively, across populations (Figure 3). A study conducted in Wisconsin reported that 88% of putative herbicide-resistant A. tuberculatus populations demonstrated ≥50% survival in response to 3× the labeled dose of glyphosate (Faleco et al. Reference Faleco, Oliveira, Arneson, Renz, Stoltenberg and Werle2022). Another experiment reported that 27% of A. tuberculatus populations from Texas demonstrated <50% injury when exposed to 1× the labeled dose of glyphosate (Singh et al. Reference Singh, Garetson, McGinty, Dotray, Morgan, Nolte and Bagavathiannan2020).
Glyphosate use increased dramatically after the commercialization of Roundup Ready™ crops in the mid-1990s. According to USDA-NASS reports, glyphosate was applied to 75% to 81% of soybean and corn acreages in Minnesota (USDA-NASS 2020, 2021). The widespread adoption of glyphosate-resistant crops led to a significant decline in the utilization of herbicides from other SOAs in agronomic crops, particularly from the mid-1990s to the early 2000s (Kniss Reference Kniss2017). The effectiveness of glyphosate for weed control has diminished over time, and there has been an increase in variability in weed control due to the evolution of glyphosate-resistant weeds, including A. tuberculatus (Landau et al. Reference Landau, Bradley, Burns, Flessner, Gage, Hager, Ikley, Jha, Jhala, Johnson, Johnson, Lancaster, Legleiter, Lingenfelter and Loux2023).
The widespread distribution of glyphosate-resistant A. tuberculatus populations in Minnesota demonstrates the consequences of intense selection pressure exerted by the extensive use of glyphosate (Figure 4). Amaranthus tuberculatus populations collected from northwestern Minnesota, particularly along the Red River Valley, exhibited >90% survival when treated with glyphosate at 1× the labeled dose (Figure 4). High plant survival frequency from this region is likely associated with the production of multiple Roundup Ready™ crops and heavy reliance on glyphosate. Sugar beet is one of the major crops in rotation in the Red River Valley, but sugar beet is sensitive to carryover residue of numerous herbicides commonly used in corn and soybean; therefore, growers have limited herbicide choices when rotating such crops with sugar beet (Renner and Powell Reference Renner and Powell1991; Robinson and McNaughton Reference Robinson and McNaughton2012). In a survey conducted between 2009 and 2014, sugar beet growers in Minnesota and North Dakota reported two to three in-season glyphosate applications in the sugar beet crop (Peters and Carlson Reference Peters and Carlson2014). Additionally, burndown application of glyphosate for grass and broadleaf weed control following small grain harvest is common in this region
Glutamine Synthetase–Inhibiting Herbicide (Glufosinate)
Although no populations tested in this experiment showed resistance to glufosinate, 22% of all populations contained a few plants that survived the labeled dose of glufosinate and were classified as less sensitive (Figure 2). Recent studies from Arkansas, Missouri, and North Carolina have documented the occurrence of glufosinate-resistant A. palmeri, a species closely related to A. tuberculatus (Geist Reference Geist2022; Jones Reference Jones2022; Priess et al. Reference Priess, Norsworthy, Godara, Mauromoustakos, Butts, Roberts and Barber2022). With the rising prevalence of multiple herbicide–resistant weeds in the United States, the use of glufosinate in glufosinate-resistant crops will likely increase (Takano and Dayan Reference Takano and Dayan2020). Therefore, the increased reliance on glufosinate for weed management could impose severe selection pressure on A. tuberculatus to promote the evolution and spread of glufosinate-resistant populations (Tranel Reference Tranel2021).
HPPD-inhibiting Herbicide (Mesotrione)
Among the A. tuberculatus populations tested, 10% and 12% were highly and moderately resistant to mesotrione, respectively (Figure 2). Averaged across the populations, mesotrione reduced A. tuberculatus biomass by 78% and 84% at 1× and 3× the labeled dose, respectively (Figure 3). Amaranthus tuberculatus is one of three weed species in the world reported to be resistant to HPPD-inhibiting herbicides (Jhala et al. Reference Jhala, Kumar, Yadav, Jha, Jugulam, Williams, Hausman, Dayan, Burton, Dale and Norsworthy2023).
Mesotrione is the most widely used active ingredient among HPPD-inhibiting herbicides. A total of 193,000 kg of mesotrione was applied in the United States in 2018, and approximately 66% of corn acreage in Minnesota was treated with mesotrione in 2021 (USDA-NASS 2021), highlighting the importance of this active ingredient in corn production in the midwestern United States. Southern Minnesota is primarily known for grain corn production, while southeastern and central Minnesota are predominantly dairy production regions where corn is grown for both grain and silage. Extensive application of mesotrione in corn in these regions may potentially be associated with the evolution and distribution of mesotrione-resistant populations (Figure 4).
PPO-inhibiting Herbicide (Fomesafen)
Seventeen and 14% of A. tuberculatus populations were highly and moderately resistant to fomesafen, respectively (Figure 2). Fomesafen applied at 1× and 3× the labeled doses caused ≥80% reduction in aboveground biomass across populations (Figure 3). The PPO-inhibiting herbicides gained popularity for preemergence and postemergence weed control in corn and soybean due to the evolution and spread of glyphosate-resistant weeds (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; Tranel Reference Tranel2021). Amaranthus tuberculatus populations resistant to at least one PPO-inhibiting herbicide have already been identified in Illinois, Indiana, Iowa, Kansas, Minnesota, Missouri, Nebraska, North Carolina, and Wisconsin (Heap Reference Heap2023; Mansfield Reference Mansfield2021; Murphy et al. Reference Murphy, Larran, Ackley, Loux and Tranel2019; Sarangi et al. Reference Sarangi, Stephens, Barker, Patterson, Gaines and Jhala2019; Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015). Amaranthus tuberculatus populations resistant to fomesafen were distributed primarily in the southeastern and southwestern parts of Minnesota (Figure 4). West-central and northwestern Minnesota has significant acreages under sugar beet production. The use of fomesafen is limited in these regions due to the 18-mo rotation interval restriction associated with sugar beet crops (Anonymous 1996). The selection pressure from fomesafen was lower on A. tuberculatus in these regions.
Photosystem II (Atrazine)
Twenty-seven (30%) of 90 A. tuberculatus populations showed at least 40% plant survival when subjected to 3× the labeled dose of atrazine; therefore, these populations were classified as highly resistant (Figure 2). Additionally, 15 populations (17%) were moderately resistant to atrazine. Averaged over the populations, atrazine applied at 3× the labeled dose reduced biomass by 82% (Figure 3). Amaranthus tuberculatus populations resistant to atrazine were confirmed in the midwestern United States and Canada, and it is believed that the intensive use of atrazine for preemergence and postemergence weed control in corn and sorghum [Sorghum bicolor (L.) Moench] contributed to increased atrazine resistance in A. tuberculatus in this region (Anderson et al. Reference Anderson, Roeth and Martin1996). An experiment from Nebraska reported that 73% and 49% of A. tuberculatus populations exhibited at least 50% survival at 1× (1,345 g ai ha−1) and 3× (4,035 g ai ha−1) the labeled dose of atrazine, respectively (Vennapusa et al. Reference Vennapusa, Faleco, Vieira, Samuelson, Kruger, Werle and Jugulam2018). Similarly, another experiment from Ontario, Canada, reported atrazine resistance in 76% of the A. tuberculatus populations evaluated (Schryver et al. Reference Schryver, Soltani, Hooker, Robinson, Tranel and Sikkema2017).
Amaranthus tuberculatus populations from southwestern and west-central Minnesota, including Lincoln, Lyon, Pipestone, Rock, and Yellow Medicine counties, exhibited plant survival ranging from 64% to 100% following the 1× the labeled dose application of atrazine. In contrast, populations from the northwestern Minnesota, including Becker, Clay, Mahnomen, Norman, Pennington, Polk, and Red Lake counties, demonstrated substantially lower plant survival (<35%) at the same atrazine dose (Figure 4). Historically, atrazine use in northwest Minnesota has been lower than in southwest Minnesota (USDA-NASS 2017a, 2017b; USGS-NAWQA 2017). Lower use of atrazine in northwestern Minnesota could be attributed to fewer corn acreages, soil type restrictions, and carryover potential to subsequent sugar beet and small grain crops in this region. Thus, it is likely that the lower overall use of atrazine in northwestern Minnesota contributed to the lower atrazine resistance in A. tuberculatus in this region.
Multiple-Herbicide Resistance
Amaranthus tuberculatus populations were tested for multiple-herbicide resistance by applying herbicides singly to a population, not by treating with herbicides in a mixture or sequentially. Twenty-six A. tuberculatus populations demonstrated resistance to two herbicide SOAs (two-way resistant), whereas, three-way resistance was confirmed in 30 populations (Figure 5). Fifteen, seven, and four populations were confirmed to be four-, five-, and six-way resistant, respectively. Two populations, A49 and A50 (Supplementary Table 1), from Lincoln and Lyon counties, MN, respectively, were resistant to 2,4-D, atrazine, dicamba, fomesafen, glyphosate, imazamox, and mesotrione. Two additional populations, A53 and A75, from Redwood and Martin counties, MN, respectively, were resistant to 2,4-D, atrazine, fomesafen, glyphosate, imazamox, and mesotrione (Figure 5). Previously, an A. tuberculatus population resistant to 2,4-D, atrazine, fomesafen, glyphosate, and mesotrione was reported from Missouri (Shergill et al. Reference Shergill, Barlow, Bish and Bradley2018). All six-way-resistant A. tuberculatus populations in this study were reported from southwestern Minnesota (Figure 6). Growers in southwestern Minnesota relied heavily on postemergence herbicides, and multiple years of selection pressure from the postemergence herbicides have likely contributed to the evolution of six-way-resistant A. tuberculatus populations in some fields in this region. Furthermore, this region has limited crop diversity beyond the corn and soybean rotation, with herbicide-based weed control prevailing in this system (USDA-NASS 2017a, 2017b).

Figure 5. Number of multiple herbicide–resistant Amaranthus tuberculatus populations out of 90 populations evaluated in the experiment. The combination matrix at the bottom identifies interactions between the herbicides, and the bars above intersection specify the size of interaction, that is, the number of populations confirmed to be resistant (moderately and highly) to those herbicides in intersection, where populations with ≥40 % plant survival at 3× the labeled dose of a herbicide were categorized as “highly resistant,” and populations with <40% survival at 3× the labeled dose but ≥40% survival at 1× the labeled dose were categorized as “moderately resistant.”

Figure 6. Geographic distribution of multiple herbicide–resistant Amaranthus tuberculatus populations in Minnesota. The two-, three-, four-, five-, and six-way resistance means the same population were either moderately or highly resistant to herbicides from two-, three-, four-, five-, and six herbicide sites of action, respectively, where populations with ≥40 % plant survival at 3× the labeled dose of a herbicide were categorized as “highly resistant,” and populations with <40% survival at 3× the labeled dose but ≥40% survival at the labeled dose (1×) were categorized as “moderately resistant.” Herbicide treatments were applied separately (not in a tank mix).
Management Considerations
The experiment demonstrated that A. tuberculatus evolved resistance to six herbicide SOAs used in Minnesota row-crop production systems. The presence of populations with plants resistant to 2,4-D and dicamba could jeopardize the utility of recently approved herbicide-resistant soybean traits. Additionally, five- and six-way-resistant populations would limit in-season herbicide choices for A. tuberculatus management. Glufosinate remains the sole choice for in-season control of six-way-resistant populations in glufosinate-resistant corn and soybean; however, the presence of individual plants surviving glufosinate in less sensitive populations requires continuous monitoring and timely implementation of alternative management strategies.
The distribution patterns of herbicide-resistant A. tuberculatus populations emphasize the necessity of regional resistance management through information sharing and coordinated interventions. Minimizing the selection pressure from herbicides is critical to mitigate the evolution of herbicide resistance (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). Implementing agroecological weed management strategies, such as seedbank management (harvest weed seed control or electrocution of weeds), enhancing crop competitiveness (narrow row spacing or intercropping), utilizing tillage, increasing crop diversity, and cover cropping, is essential. These strategies target A. tuberculatus at various stages of its life cycle, thereby reducing the number of plants exposed to herbicide applications. Moreover, adopting sound herbicide use practices, including the use of effective preemergence herbicides, rotating and/or mixing effective herbicide SOAs, and adhering to label-recommended application doses and stages are critical for effective weed management and resistance mitigation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2024.64
Acknowledgments
The authors thank stakeholders and Extension Educators (Angie Peltier, Bruce Potter, Dave Nicolai, Jared Goplen, and Lisa Behnken) for submitting A. tuberculatus seed samples. The assistance of the Integrated Cropping Systems Weed Science Lab members in this project is greatly appreciated. The authors also acknowledge Jeffrey L. Gunsolus, Professor Emeritus, University of Minnesota, and Micheal D. Owen, Professor Emeritus Iowa State University, for their valuable review of the article.
Funding statement
The authors thank the Minnesota Soybean Research and Promotion Council for their financial support to this research and the graduate research assistant involved in this research.
Competing interests
The authors declare no competing interests.










