Introduction
Johnsongrass [Sorghum halepense (L.) Pers.], a tussock grass from northern Africa and central Asia, actively inhabits grassland ecosystems of North America (Klein and Smith Reference Klein and Smith2021; Paterson et al. Reference Paterson, Kong, Johnston, Nabukalu, Wu, Poehlman, Goff, Isaacs, Lee and Guo2020; Figure 1). Feltus et al. (Reference Feltus, Wan, Schulze, Estill, Jiang and Paterson2004) suggested that S. halepense is a naturally occurring hybrid between two sorghum species: Sorghum bicolor (L.) Moench, an annual, polytypic African species, which includes cultivated sorghum, and Sorghum propinquum (Kunth) Hitchc., a perennial southeast Asian native of moist habitats (Celarier Reference Celarier1958; Doggett Reference Doggett and Simmonds1976; Paterson et al. Reference Paterson, Schertz, Lin, Liu and Chang1995). Currently, S. halepense can be found throughout much of Asia, Africa, Europe, North and South America, and Australia (McWhorter Reference McWhorter1971). In the United States, S. halepense was initially planted as a forage hay crop (Bennett Reference Bennett, Heath, Metcalfe and Barnes1973). As on other continents, however, S. halepense escaped cultivation and spread throughout every U.S. state except for Alaska, Maine, and Minnesota; as far north as Canada; and as far south as Argentina (Hickman et al. Reference Hickman, Goodman, Elmore, Buthod, Duell and Craun2018; USDA-NRCS 2023; Warwick et al. Reference Warwick, Phillips and Andrews1986). Clements and DiTommaso (Reference Clements and DiTommaso2012) suggested that the ability of S. halepense to advance longitudinally and establish in regions that were once considered uninhabitable for this species is derived from wide climatic and environmental tolerance, a relatively short generation time, effective forms of reproduction and dispersal, and competitive ability that allows for colonization in numerous environments (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977; Warwick and Black Reference Warwick and Black1983). Consequently, S. halepense has, for more than a century, been a common weed in cultivated agricultural systems (Heard Reference Heard1917; Monaghan Reference Monaghan1979; Schwinning et al. Reference Schwinning, Meckel, Reichmann, Polley and Fay2017; Squires and Walsh Reference Squires and Walsh2021; Vinall Reference Vinall1921).

Figure 1. Johnsongrass [Sorghum halepense (L.) Pers.] diagram and associated distribution map. Illustration by Chris J. P. Grisham and map from USDA-NRCS PLANTS database(USDANRCS2023).
The dominance of S. halepense in intact grasslands is a relatively recent occurrence largely due to changes in land management associated with declining livestock grazing, increased energy development (renewable and fossil fuel), and anthropogenic housing development (Klein and Smith Reference Klein and Smith2021; Paterson et al. Reference Paterson, Kong, Johnston, Nabukalu, Wu, Poehlman, Goff, Isaacs, Lee and Guo2020; Rocateli and Manuchehri Reference Rocateli and Manuchehri2017). The Southern Great Plains are particularly susceptible to S. halepense because of a preferential climate coupled with rapid land use disturbances (Lakoba et al. Reference Lakoba, Atwater, Thomas, Strahm and Barney2021; Omernik and Griffith Reference Omernik and Griffith2014). Barney and DiTomaso (Reference Barney and DiTomaso2011) found that S. halepense growth has a 50% to 90% climatic match between all 20 designated ecoregions of the continental United States and was greatest in the plains and prairies. The Great Plains, notably the Southern Great Plains, are also increasingly fragmented due to commercial land development for housing or energy development from windmills, solar farms, or oil and gas production (de Castro and Zenteno Reference de Castro, Zenteno, Schiavon and Fernández de Castro2023; Engle et al. Reference Engle, Coppedge, Fuhlendorf and Van Auken2008; Scholtz et al. Reference Scholtz, Polo, Tanner and Fuhlendorf2018). Land fragmentation creates vulnerability to weed invasions by increasing the number of successful sites for weed seedling establishment (Aicher et al. Reference Aicher, Larios and Suding2011; Duncan et al. Reference Duncan, Diez, Sullivan, Wangen and Miller2009) and supporting seed transport along these recent disturbances, like along roadways (Grman et al. Reference Grman, Bassett, Zirbel and Brudvig2015; McConkey et al. Reference McConkey, Prasad, Corlett, Campos-Arceiz, Brodie, Rogers and Santamaria2012); once these species are in the seedbank, established seedlings of invasive species commonly outcompete native species by growing earlier and at higher densities than native plant species (Reid and Holl Reference Reid and Holl2013; Yelenik and D’Antonio Reference Yelenik and D’Antonio2013). In addition, livestock grazing has been decreasing in the Southern Great Plains, as moving livestock among smaller and fragmented paddocks is difficult for producers, while neighboring subdivisions can prove to be problematic neighbors for livestock operations (Brunson et al. Reference Brunson, Huntsinger, Kreuter and Ritten2016; BurnSilver and Mwangi Reference BurnSilver and Mwangi2007). Some might argue that S. halepense would be a preferential weed to eliminate from these systems, as it can outcompete many physiologically similar native tall grasses (Schwinning et al. Reference Schwinning, Meckel, Reichmann, Polley and Fay2017); others see benefits in S. halepense, especially for grazing livestock, as this species can provide high-quality forage throughout the grazing season (Rocateli and Manuchehri Reference Rocateli and Manuchehri2017; Watson et al. Reference Watson, Coats and Kimbrough1980). The objective of this review is, therefore, to provide a background of S. halepense invasion and discuss the benefits and drawbacks of this grassland invader in the Southern Great Plains.
Sorghum halepense Growth and Plant Community Characteristics
Sorghum halepense spreads through the rapid development of rhizomes and prolific seed production (McWhorter Reference McWhorter1961; Ryder et al. Reference Ryder, Dorn, Huitsing, Adams, Ploegstra, DeHaan, Larson and Tintle2018; Tóth and Lehoczky Reference Tóth and Lehoczky2006). It has been shown to produce approximately 100 m of rhizomes per plant each year that are able to withstand subzero winter temperatures with a survival rate of up to 71% (Anderson et al. Reference Anderson, Appleby and Weseloh1960; Johnson et al. Reference Johnson, Li and Wait2003). These rhizome networks can also account for up to 70% of the entire plant dry weight (Paterson et al. Reference Paterson, Kong, Johnston, Nabukalu, Wu, Poehlman, Goff, Isaacs, Lee and Guo2020). As a self-pollinating plant, S. halepense produces up to 80,000 seeds per plant in a single season that can remain viable for up to 10 yr in the soil (Dweikat Reference Dweikat2005; McWhorter Reference McWhorter1961). Sorghum halepense also has a broad seed depth germination rate ranging from 64% at 1 cm-depth to 30% at 20-cm depth, and up to 6% of its seeds can germinate from depths as great as 25 cm (Tóth and Lehoczky Reference Tóth and Lehoczky2006).
Once established, S. halepense creates a feedback cycle whereby it can outcompete many native perennial grass species by growing earlier and faster and having higher biomass than functionally similar native perennial grasses (Kelly et al. Reference Kelly, Fletcher and Barney2020; Schwinning et al. Reference Schwinning, Meckel, Reichmann, Polley and Fay2017). Reichmann et al. (Reference Reichmann, Schwinning, Polley and Fay2016), for example, reported that during early development, S. halepense plants gained up to 4-fold more biomass than the North American prairie grasses switchgrass (Panicum virgatum L.), little bluestem [Schizachyrium scoparium (Michx.) Nash], and big bluestem (Andropogon gerardii Vitman) within the first 17 d of growth, largely due to increased leaf area, higher atmospheric carbon uptake, and photosynthetic nitrogen-use efficiency. Schwinning et al. (Reference Schwinning, Meckel, Reichmann, Polley and Fay2017) also found that when S. halepense was grown with these same warm-season (C4) tallgrass species in a greenhouse experiment, native perennial grasses had 95% less biomass compared with when they were grown alone, while S. halepense only lost 11% of non-root biomass.
Response to Climate Disturbances
Extreme climate disturbances, like drought and freezing conditions, are increasing in frequency across the Southern Great Plains (Ojima et al. Reference Ojima, Aicher, Archer, Bailey, Casby-Horton, Cavallaro, Reyes, Tanaka and Washington-Allen2020). Current climate change projections suggest that these climatic extremes will likely become a regular occurrence in the future (Knapp et al. Reference Knapp, Chen, Griffin-Nolan, Baur, Carroll, Gray, Hoffman, Li, Post and Slette2020; Lakoba et al. Reference Lakoba, Atwater, Thomas, Strahm and Barney2021). Clements et al. (Reference Clements, DiTommaso, Upadhyaya, Clements and Shrestha2022) further suggest that S. halepense will likely expand longitudinally (north and south) due to higher global temperatures at northern and southern latitudes.
Long-term and large-scale research sites are likely the best place to evaluate historical climate effects, as other site-specific data, like plant production and management strategy, typically have an associated recorded history. For this effort, the previous 32 yr of precipitation data at six long-term rangeland research sites in the Southern Great Plains were acquired to gain a better understanding of the dynamic precipitation in these areas. Historical climate data, including precipitation and temperature, were acquired from the gridMET database (https://webapps.jornada.nmsu.edu/weather; Abatzoglou Reference Abatzoglou2013) for the years 1990 to 2022. The six long-term research sites included USDA-Agricultural Research Service sites in Woodward, OK (36.3745°N, 99.2455°W), El Reno, OK (35.5335°N, 97.9549°W), Riesel, TX (31.4755°N, 96.9247°W), and Temple, TX (31.0982°N, 97.3428°W), and research sites associated with Oklahoma State University in Pawhuska, OK (36.6634°N, 96.3410°W) and Texas A&M University in LaCopeda, TX (27.66661°N, 98.20892°W). An ANOVA on the differences in average daily precipitation (mm) at these six sites across the years of 1990 to 2005 and again from 2005 to 2022 was then run using JMP (SAS Statistical Software © 2022, SAS Institute, Cary, NC). Results on precipitation trends from 1990 to 2005 indicate that only 2004 had significantly higher precipitation compared with all other years, and this was only at the Riesel and Temple, TX, sites; all other sites and years were similar. Drought and flooding had become more frequent for the years 2005 to 2022, where four of the six sites had significantly different precipitation across years, and one site was moderately significant (Pawhuska, OK; P = 0.0596). Across all significant sites, from 2005 to 2022, 2011 was significantly lower and 2015 had significantly higher precipitation (P < 0.05; Figure 2). Collectively, these results indicate that precipitation is becoming more dynamic in recent years, and dynamic precipitation fluctuations will likely facilitate plant species that can withstand these perturbations, which often are plant species with rapid adaptation mechanisms.
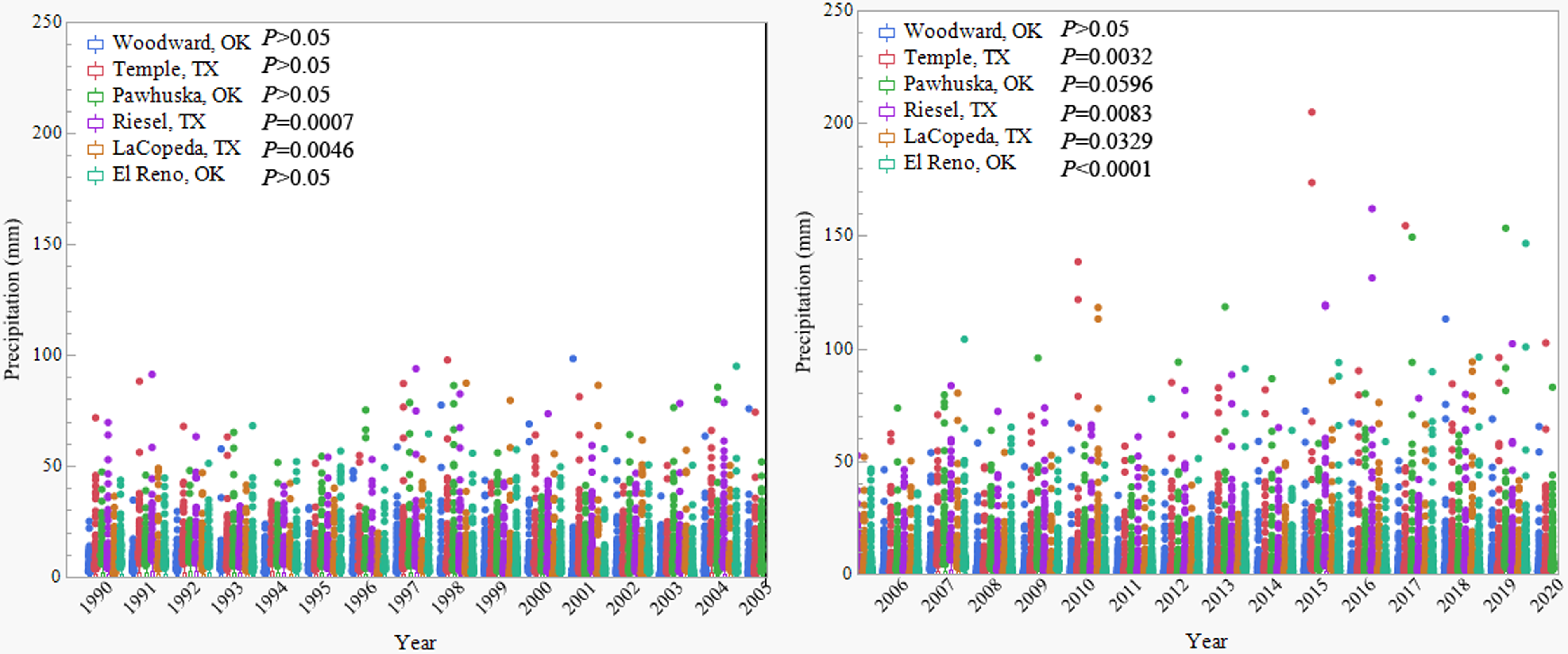
Figure 2. Historical average daily precipitation (mm) ± SE for the years of 1990–2020 across six long-term Southern Great Plains grassland research sites to demonstrate the variation in precipitation from 1990 to 2005 and from 2005 to 2020. Sites are all colocated at long-term plant production monitoring regions in the Southern Great Plains. P-values refer to one-way ANOVA models of year by average daily precipitation, where P < 0.05 refers to a significantly different relationship and P > 0.05 indicates no significant differences in precipitation across years.
Sorghum halepense appears to be well suited to adapt to these changing climate conditions. This is partially because S. halepense seeds quickly adapt to modified temperature and water environments (Fletcher et al. Reference Fletcher, Varnon and Barney2020). Its physiologically adaptive climate traits, as described earlier, especially in comparison to native species growing in these ecosystems, may result in S. halepense becoming a widespread species of concern (Schwinning et al. Reference Schwinning, Meckel, Reichmann, Polley and Fay2017). Currently, however, there are limited and/or hypothetical expectations on how species and plant communities within ecosystems respond to dynamic climatic cycles like these (Briske et al. Reference Briske, Joyce, Polley, Brown, Wolter, Morgan, McCarl and Bailey2015; Knapp et al. Reference Knapp, Chen, Griffin-Nolan, Baur, Carroll, Gray, Hoffman, Li, Post and Slette2020; Polley et al. Reference Polley, Jin and Fay2012, Reference Polley, Aspinwall, Collins, Gibson, Gill, Jackson, Jin, Khasanova, Reichmann and Fay2019).
Chemical Control of Sorghum halepense
Sorghum halepense is extremely resistant to herbicides (Heap Reference Heap2012). This is because herbicide-sprayed S. halepense plants can cross within selfing populations, store genetic variation in seedbanks, and evolve phenotypic plasticity (Clements et al. Reference Clements, DiTommaso, Jordan, Booth, Cardina, Doohan, Mohler, Murphy and Swanton2004). In 2002, for example, a glyphosate-resistant biotype was discovered in Argentina that covered 10,000 ha (Binimelis et al. Reference Binimelis, Pengue and Monterroso2009). Moreover, given its historical link to S. bicolor species and strong ability to cross with S. bicolor, chemical control of S. halepense near S. bicolor crops is extremely difficult, as chemical applications could directly affect S. bicolor production and potentially create more herbicide-resistant strains of S. halepense (Tang and Liang Reference Tang and Liang1988; Warwick and Black Reference Warwick and Black1983). In Texas and Nebraska, for example, Morrell et al. (Reference Morrell, Williams‐Coplin, Lattu, Bowers, Chandler and Paterson2005) reported that up to 32% of unique S. bicolor alleles were identified in S. halepense populations adjacent to long-term S. bicolor production sites. The evidence strongly suggests that engineered genes and herbicide resistance could potentially be transferred into S. halepense and widely disseminated (Morrell et al. Reference Morrell, Williams‐Coplin, Lattu, Bowers, Chandler and Paterson2005). Presently, susceptible S. halepense plants may be able to be controlled using acetolactate synthase–inhibiting herbicides like sulfosulfuron, nicosulfuron, primisulfuron, or imazapic; acetyl-CoA carboxylase–inhibiting herbicides like clethodim or sethoxydim; or 5-enolpyruvylshikimate-3-phosphate synthase inhibitors like glyphosate (McCollough and Shilling Reference McCullough and Shilling2022). Appropriate use of these herbicides has been shown to result in an 88% to 97% efficacy rate (Johnson et al. Reference Johnson, Li and Wait2003). Repeated herbicide use can, however, create herbicide resistance. Hernández et al. (Reference Hernández, León, Fischer, Gebauer, Galdames and Figueroa2015), for example, ascertained that recurrent nicosulfuron application to seedling- and rhizome-emerged S. halepense has created resistant S. halepense biotypes with 33 to 46 times higher herbicide resistance than susceptible control plants. Similarly, S. halepense has also shown glyphosate resistance, likely in part because glyphosate has evolved as a preferred herbicide, with more than 8.6 billion kg sold since 1974, coupled with S. halepense’s adaptive phenology (Baylis Reference Baylis2000; Benbrook Reference Benbrook2016; Fernández et al. Reference Fernández, De Haro, Distefano, Carolina Martínez, Lía, Papa, Olea, Tosto and Esteban Hopp2013; Heap and Duke Reference Heap and Duke2018; Vila-Aiub et al. Reference Vila-Aiub, Balbi, Gundel, Ghersa and Powles2007). Presently, there is an agenda to reduce synthetic herbicide applications, notably glyphosate, as many of these products have recently been identified as carcinogens (IARC 2017; Tarazona et al. Reference Tarazona, Court-Marques, Tiramani, Reich, Pfeil, Istace and Crivellente2017; Williams et al. Reference Williams, Aardema, Acquavella, Berry, Brusick, Burns, de Camargo, Garabrant, Greim, Kier and Kirkland2016).
Biological Control of Sorghum halepense
Biological control can be an ecologically viable way to tackle weed invasions (McFadyen Reference McFadyen1998; Zachariades et al. Reference Zachariades, Paterson, Strathie, Hill and van Wilgen2017). Classical biological control includes introducing host-specific, coevolved natural enemies (biological control agents) from a weed’s native range to the introduced range to keep the invasive species under control (McFadyen Reference McFadyen1998). Historically, however, few invasive grasses have been targeted for biological control (Pemberton and Lee Reference Pemberton and Lee1996; Schwarzländer et al. Reference Schwarzländer, Hinz, Winston and Day2018). This is likely because there are few coevolved enemies of grasses that are host specific (Gill and Blacklow Reference Gill and Blacklow1984; Pemberton Reference Pemberton2002). Witt and McConnachie (Reference Witt and McConnachie2004), for example, noted that in Australia, the biggest obstacle to the biological control of invasive dropseed species (Sporobolus spp.) is that there are 13 native Sporobolus spp., which will largely govern which agents can be selected for biocontrol. Given the high risk of non-target damage posed to economically valuable crops, like S. bicolor, and/or native biodiversity, it is unlikely that biological control will be a practical control mechanism for S. halepense in U.S. grasslands (Sutton et al. Reference Sutton, Canavan, Day, Den Breeyen, Goolsby, Cristofaro, McConnachie and Paterson2019; Wapshere Reference Wapshere1990). Targeted grazing, alternatively, may be a viable control method, as S. halepense used as forage can provide multiple socioeconomic benefits to producers that, managers suggest, compensate for negative ecological effects.
Mechanical Control of Sorghum halepense
It has been suggested that S. halepense spread can be well controlled using mechanical inputs like hand weeding, mowing, or tilling (Arle and Everson Reference Arle and Everson1955; Ceseki et al. Reference Ceseki, Al-Khatib and Dahlberg2017; Heard Reference Heard1917; Johnson et al. Reference Johnson, Li and Wait2003). Mechanical control is, however, only a temporary fix in most perennial grassland regions, as hand weeding and tillage are impractical in large-scale perennial grasslands, and frequent mowing can deplete carbohydrate reserves of all species, even favoring the invaders (McCollough and Shilling Reference McCullough and Shilling2022; Simberloff et al. Reference Simberloff, Souza, Nuñez, Barrios-Garcia and Bunn2012). Entsminger et al. (Reference Entsminger, Jones, Guyton, Strickland and Leopold2017), for example, suggested that frequent mowing (four times per year) produced lower native species abundance along native seeded roadways compared with mowing only once per year or onetime mowing events accompanied by additional seedings of desirable species. Consequently, while mechanical control may not be an effective means to eliminate S. halepense, using mechanical control to reduce weed abundance before seeding desirable species that can fill the niches once occupied by weed species may be an effective ecologically based management strategy.
Sorghum halepense in Rangelands and Pasturelands
Land managers hold conflicting views of S. halepense on native rangelands and introduced pasturelands (Bennett et al., Reference Bennett, Heath, Metcalfe and Barnes1973; Hawkins et al. Reference Hawkins, Kelley and Smith1958; Rankins and Darrell Reference Rankins and Darrell1995; Rocateli and Manuchehri Reference Rocateli and Manuchehri2017). S. halepense provides quality forage with approximately 10% to 14% crude protein and 55% to 60% total digestible nutrients and is preferred by large-mouth herbivores, like horses and cattle, across grassland ecosystems (Bennett et al., Reference Bennett, Heath, Metcalfe and Barnes1973; Watson et al. Reference Watson, Coats and Kimbrough1980). Cattle show a strong grazing preference for S. halepense and have been known to kill S. halepense plants by overgrazing this species (Andrae Reference Andrae2009; Sherrill Reference Sherrill1947). However, S. halepense can contain high amounts of nitrate and prussic acid, also known as hydrocyanic acid, during early life-history stages and following distinct climactic events, like first frost or first rain after prolonged drought (Harris and Shearer Reference Harris and Shearer2003; Selk Reference Selk1988; Slade Reference Slade1903; Vinall Reference Vinall1921). Nitrate poisoning occurs when accumulated nitrates in the plant material (primarily plant stems) are converted to nitrite in the rumen (Selk Reference Selk1988). Nitrite is absorbed from the rumen and converts blood hemoglobin to methemoglobin. Because methemoglobin cannot transport oxygen to body tissues, ruminant animals die from oxygen insufficiency (Selk Reference Selk1988). Prussic acid, alternatively, interferes with oxygen use at the cellular level (Vinall Reference Vinall1921), and animals generally die from asphyxiation within a few minutes when a lethal dose of prussic acid is consumed (Harris and Shearer Reference Harris and Shearer2003; Selk Reference Selk1988; Slade Reference Slade1903; Vinall Reference Vinall1921). Recommendations from both researchers and land managers on the best way to manage high nitrate and prussic acid levels are to avoid grazing when the risk of these toxic compounds is high, such as in early spring, after freezing events, or for approximately 10 d following the first rain after prolonged drought (Harris and Shearer Reference Harris and Shearer2003). Timing S. halepense grazing in the Southern Great Plains can be complicated, however, as the climate in the Southern Great Plains is notably dynamic, and weather patterns are becoming more extreme (Harmel et al. Reference Harmel, King, Richardson and Williams2003; Ojima et al. Reference Ojima, Aicher, Archer, Bailey, Casby-Horton, Cavallaro, Reyes, Tanaka and Washington-Allen2020).
Grazing Management for Sorghum halepense Invasion
The high forage quality of S. halepense and ability to manage this species through grazing has largely limited S. halepense spread in grazing lands (Hawkins et al. Reference Hawkins, Kelley and Smith1958; Watson et al. Reference Watson, Coats and Kimbrough1980). Heard (Reference Heard1917), for example, suggested that the best eradication measure for S. halepense was to irrigate to establish a good stand followed by heavy sheep grazing. In native rangelands, where soils are undisturbed, S. halepense has more species to compete with and less opportunity to dominate (Paterson et al. Reference Paterson, Kong, Johnston, Nabukalu, Wu, Poehlman, Goff, Isaacs, Lee and Guo2020). This is especially true when grazing occurs on native rangelands, as livestock show a strong preference for S. halepense, given its forage quality relative to native grasses, and will often preferentially graze S. halepense out of the plant community (Bennett et al., Reference Bennett, Heath, Metcalfe and Barnes1973; Watson et al. Reference Watson, Coats and Kimbrough1980). Pasturelands, alternatively, differ from native rangelands, as they are periodically plowed every 5 to 20 yr, seeded with productive introduced species, and receive regular fertilization and herbicide management inputs (Sollenberger et al. Reference Sollenberger, Newman, Macoon, Moore, Collins, Nelson and Redfearn2020; USDA-NRCS 2024). Sorghum halepense can, therefore, have a greater ability to dominate pasturelands, as there are fewer physiologically similar species to compete with and reduced competition from broadleaf herbaceous species (Rocateli and Manuchehri Reference Rocateli and Manuchehri2017). While S. halepense is still preferentially grazed in pasture, many pasturelands have an established grazing system where livestock are rotated throughout the year (Badgery et al. Reference Badgery, Millar, Broadfoot, Martin, Pottie, Simmons and Cranney2017; Paine et al. Reference Paine, Undersander and Casler1999; Williams and Hammond Reference Williams and Hammond1999). Livestock, therefore, may only have access to a specific pasture once per year in rotationally grazed systems. When temporal grazing disturbances are limited by rotation, S. halepense should be quite productive (Paterson et al. Reference Paterson, Kong, Johnston, Nabukalu, Wu, Poehlman, Goff, Isaacs, Lee and Guo2020; Rocateli and Manuchehri Reference Rocateli and Manuchehri2017). At a long-term agroecosystem study site in Riesel, TX, for example, areas that are rotationally grazed for more than 10 yr were found to have almost two times the plant production compared with areas that were continuously grazed (unpublished data). It was postulated in this paper that the reason for this high forage availability was the preferential growth of S. halepense in pastures that were not subjected to continuous grazing (unpublished data). While this hypothesis has yet to be tested, as total plant production was not sorted by species, it seems plausible that, especially in pasturelands that have rotational grazing, S. halepense could improve forage availability and forage quality by growing in tandem with seeded introduced species.
Conclusions
Sorghum halepense has been a challenging invader on croplands for decades and has more recently started increasing on intact native range and pasture grassland ecosystems. The spread and dominance of S. halepense is not only due to its morphology of rapid development of rhizomes and prolific seed production but also due to changing land use, like less livestock grazing due to higher anthropogenic development on Southern Great Plains grasslands. Once established, S. halepense can outcompete many native perennial grass species by growing earlier and faster and having higher biomass than functionally similar native perennial grasses. S. Sorghum halepense also appears to be well suited to adapting to extreme weather, like frequent drought and flooding that are actively occurring across the Southern Great Plains. While chemical, biological, and mechanical control can be used to control S. halepense, these options are costly and/or impractical to use across much of the Southern Great Plains grazing lands. Alternatively, there can be multiple socioeconomic benefits of having S. halepense on grazing lands, not least among them the potential for higher and more nutritious forage for grazing livestock. It is, however, likely that higher forage availability will only be possible when grazing can be excluded for a period to allow S. halepense to regrow, as S. halepense is often preferentially grazed out of the plant community in continuously grazed systems. There is still much work to be done to fully comprehend the benefits and drawbacks of S. halepense growing on grazing lands, but as this review has indicated, this species should be monitored to balance its increasing spread with greater forage stability and availability in the dynamic climate conditions facing the Southern Great Plains.
Acknowledgments
Thank you to Chris Grisham and David Rowley who assisted in gathering data and reviewing the article. Thank you also to all reviewers and editors who provided quality feedback on this review.
Funding statement
This work was supported by the USDA-ARS CRIS project (no. 3098-21600-001-000D). This research was a contribution from the Long-Term Agroecosystem Research (LTAR) network. LTAR is supported by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Competing interests
The authors declare no conflicts of interest.





