Palmer amaranth is one of the most troublesome and economically damaging agronomic weeds in the southern United States. It is able to adapt to diverse climatic and agricultural conditions. The photosynthetic rate of Palmer amaranth (81 µmolm−2s−1) is three to four times that of corn (Zea mays L.), cotton (Gossypium hirsutum L.), and soybean [Glycine max (L.) Merr.] (Ehleringer Reference Ehleringer1983). This high photosynthetic rate translates into its rapid growth rate of 5 cm day−1 under optimum growing conditions (Horak and Loughin Reference Horak and Loughin2000). Consequently, this rapid growth rate results in a narrow window of opportunity for effective POST herbicide application. Palmer amaranth control becomes difficult when it is >10-cm tall (Riar et al. Reference Riar, Norsworthy, Steckel, Stephenson, Eubank and Scott2013). A female Palmer amaranth can produce up to 1 million seeds; however, its seeds are relatively short-lived in the soil, with about 80% mortality in 3 yr (Sosnoskie et al. Reference Sosnoskie, Webster, Culpepper and Kichler2014). With its fast growth rate, high fecundity, season-long emergence, high photosynthetic rate, and high propensity to evolve herbicide resistance, Palmer amaranth has become a serious weed in row crops and vegetables (Ehleringer et al.Reference Ehleringer, Cerling and Helliker1997; Guo and Al-Khatib Reference Guo and Al-Khatib2003; Jha and Norsworthy Reference Jha and Norsworthy2012; Steckel Reference Steckel2007). Palmer amaranth infestation can reduce corn, cotton, and soybean yield 91%, 77%, and 78%, respectively (Bensch et al. Reference Bensch, Horak and Peterson2003; Fast et al. Reference Fast, Murdoch, Farris, Willis and Murray2009; Massinga et al. Reference Massinga, Currie, Horak and Boyer2001). In the past decade, reports abound of Palmer amaranth evolving resistance to glyphosate and other herbicides (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006; Jha et al. Reference Jha, Norsworthy, Riley, Bielenberg and Bridges2008; Norsworthy et al. Reference Norsworthy, Scott, Smith and Oliver2008). To date, Palmer amaranth has been confirmed resistant to acetolactate synthase (ALS) inhibitors (Burgos et al. Reference Burgos, Kuk and Talbert2001; Horak and Peterson Reference Horak and Peterson1995), dinitroanilines (Gossett et al. Reference Gossett, Murdock and Toler1992), 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors (Jhala et al. Reference Jhala, Sandell, Rana, Kruger and Knezevic2014), glyphosate (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006; Norsworthy et al. Reference Norsworthy, Scott, Smith and Oliver2008), photosystem II herbicides (Vencill et al. Reference Vencill, Grey and Culpepper2011), and most recently, protoporphyrinogen oxidase (PPO) inhibitors (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016).
Several chemical families inhibit PPO activity, including diphenylethers (e.g., acifluorfen, fomesafen, lactofen, oxyfluorfen), N-phenylphthalimide (e.g., flumioxazin, flumiclorac), phenylpyrazoles (e.g., fluazolate, pyraflufen-ethyl), oxadiazole (e.g., oxadiazon), oxazolidinones (e.g., pentoxazone), pyrimidinediones (e.g., saflufenacil), thiadiazole (e.g., fluthiacet-methyl), and triazolinone (e.g., carfentrazone, sulfentrazone) (Heap 2017). Diphenylether herbicides are used PRE or POST. Some triazolinones and N-phenylphthalimide have high soil activity and are phytotoxic to the crop when applied foliar, hence most are commonly used PRE (Dayan and Duke Reference Dayan and Duke2010). There are two known nuclear PPO genes in plants, PPX1 and PPX2, which encode plastid- and mitochondrial-targeted PPO isoforms, respectively; however, some PPX2 isoforms are dual targeted to both organelles (Lermontova and Grimm Reference Lermontova and Grimm2000; Lermontova et al. Reference Lermontova, Kruse, Mock and Grimm1997; Watanabe et al. Reference Watanabe, Che, Iwano, Takayama, Yoshida and Isogai2001). The inhibition of PPO causes accumulation of protophorphyrinogen IX, which leaks from the plastid to the cytoplasm, where it is oxidized rapidly into photosensitive protoporphyrin IX (Becerril and Duke Reference Becerril and Duke1989; Jacobs et al. Reference Jacobs, Jacobs, Sherman and Duke1991; Lee et al. Reference Lee, Duke and Duke1993). Upon exposure to light, protoporphyrin IX generates singlet oxygen molecules that cause lipid peroxidation, membrane destruction, and ultimately cell death (Becerril and Duke Reference Becerril and Duke1989; Jacobs et al. Reference Jacobs, Jacobs, Sherman and Duke1991).
The first weed species to evolve resistance to PPO-inhibiting herbicides was common waterhemp in 2001 (Shoup et al. Reference Shoup, Al-Khatib and Peterson2003). Resistance to PPO-inhibiting herbicides in Palmer amaranth was first reported in Arkansas and is confirmed also in Tennessee and Illinois (Heap 2017; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; L Steckel and K Gage, personal communication). When this research was conducted, resistance to PPO herbicides in Amaranthus spp. was attributed to a target-site mutation in the PPX2 gene only (Patzoldt et al. Reference Patzoldt, Hager, McCormick and Tranel2006; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). The Gly-210 deletion mutation was present in all PPO-resistant waterhemp populations in Illinois, Kansas, and Missouri (Thinglum et al. Reference Thinglum, Riggins, Davis, Bradley, Al-Khatib and Tranel2011). However, a novel PPO mutation, Arg-98-Leu, was detected in PPO-resistant common ragweed (Ambrosia artemisiifolia L.) (Rousonelos et al. Reference Rousonelos, Lee, Moreira, VanGessel and Tranel2012).
This study was conducted to investigate the response of Palmer amaranth from Arkansas to various foliar-applied PPO-inhibiting herbicides (acifluorfen, carfentrazone, flumioxazin, fluthiacet-methyl, fomesafen, pyraflufen-ethyl, saflufenacil) and to foliar-applied herbicides with different modes of action (dicamba, glyphosate, glufosinate, trifloxysulfuron). The study also aimed to determine the frequency of Gly-210 deletion among the PPO-resistant biotypes, identify other PPO target-site mutations that endow resistance to PPO-inhibiting herbicides, and compare the resistance level to fomesafen in accessions that contained or lacked the Gly-210 deletion.
Materials and Methods
Plant Materials
A total of 124 Palmer amaranth accessions were collected in the summer between 2008 and 2015 from 23 counties in Arkansas, primarily from the eastern half of the state. Sampling sites were identified by county extension agents and crop consultants. Samples in 2008 and 2009 were collected from 18 counties (17 from eastern Arkansas and 1 from central Arkansas) to survey resistance to glyphosate. Seven counties were sampled in 2011 from fields that were planted with LibertyLink® crops to verify differential response to glufosinate. Fifty-two fields from 16 counties were sampled in 2014. Samples in 2015 (23 accessions) were collected specifically to survey fields with remnant Palmer amaranth after having been sprayed with PPO herbicides. Inflorescences from at least 10 mature female plants−1 field were collected, dried, and threshed. Equal amounts of seeds from each plant were mixed to make a composite seed sample to represent the field. A sensitive standard population (SS) was included in each experiment for comparison. Plants were grown in a greenhouse maintained at 32/25±3 C day/night temperature with a 16:8-h day:night regime and photon flux density of 0.0005molm−2s−1. Plants were planted using commercial potting soil (Sunshine® Premix No. 1; Sun Gro Horticulture, Bellevue, WA), watered daily, and fertilized with Miracle-Gro® (Scotts Miracle-Gro, Marysville, OH) every 2 wk.
Palmer Amaranth Response to Foliar-applied Fomesafen
One hundred-twenty four Palmer amaranth accessions (Table 1) were tested in the greenhouse for resistance to fomesafen. Composite seed samples were planted in 24 by 54 cm cellular trays. Seedlings were thinned to 1 plant cell−1 and sprayed with 263 g ai ha−1 fomesafen (Flexstar®, Syngenta Crop Protection, Greensboro, NC 27419) when seedlings were 7- to 8-cm tall. The herbicide was applied with 0.5% by volume nonionic surfactant (NIS) (Induce®, Helena Chemical, Collierville, TN 38017) using a laboratory sprayer equipped with a flat-fan spray nozzle delivering 187 L ha−1 at 221 kPa. The experiment was conducted in a randomized complete block design with two replications and repeated. Each replication consisted of 50 seedlings grown in a cellular tray at 1 seedling cell−1. The plants were assessed visually relative to nontreated plants at 21 d after treatment (DAT) using an injury scale of 0 to 100, where 0%=no visible injury and 100%=complete desiccation. Survivors with 0% to 10%, 11% to 30%, 31% to 60%, and 61% to 89% injury were classified as highly resistant, resistant, moderately resistant, and slightly resistant, respectively. Individuals with 90% injury or higher were considered sensitive. In this experiment, an accession with >10% survivors that were at least slightly resistant was considered to be PPO resistant. Data were analyzed using hierarchal clustering in JMP Pro v. 13 (SAS Institute, Cary, NC.).
Table 1 Number of Palmer amaranth accessions in Arkansas tested with foliar-applied fomesafen at 263gha−1.

a Palmer amaranth samples were collected from fields with a history of glyphosate, glufosinate, or protoporphyrinogen oxidase (PPO)-inhibiting herbicide use. Sampling in 2015 was done specifically to survey fields with remnant Palmer amaranth population after having been sprayed with PPO herbicides.
Response to Other Foliar-applied PPO Herbicides
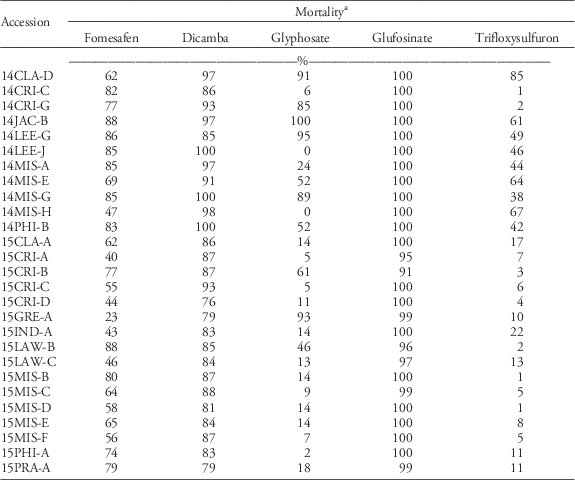
Twelve accessions that had low mortality (<83%) from foliar-applied fomesafen were bioassayed in the greenhouse for their response to other foliar-applied PPO herbicides. A sensitive standard accession was also included. Seedlings (100 per accession, 10-cm tall) were treated with the recommended doses of acifluorfen, carfentrazone, flumioxazin, fluthiacet-methyl, lactofen, pyraflufen-ethyl, or saflufenacil (Table 2). Carfentrazone, pyraflufen-ethyl, flumioxazin, and fluthiacet-methyl treatments included 0.25% NIS (v/v), whereas acifluorfen included 0.125% NIS (v/v). Lactofen was sprayed with 0.5% crop oil concentrate (v/v). Saflufenacil was sprayed with 1% methylated seed oil and 1% ammonium sulfate (w/v). Following herbicide applications, the plants were placed in the greenhouse, grouped by herbicide, and the accessions were randomized within each herbicide group. Mortality and injury of survivors were evaluated at 21 DAT. In the second run of the experiment, herbicides were sprayed when seedlings were 5- to 8-cm tall. Data were analyzed, by herbicide, using JMP Pro v. 13 (SAS Institute, Cary, NC).
Table 2 Common name, trade name, and manufacturer of herbicides used in the study.
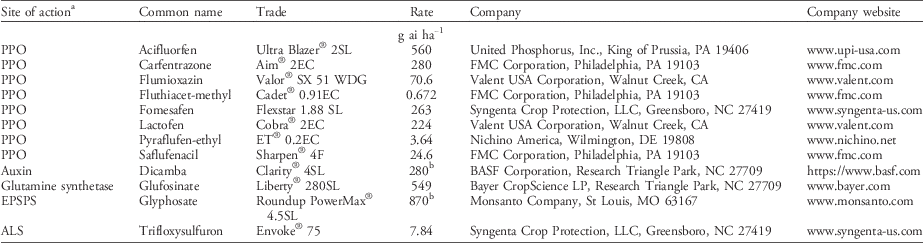
a Abbreviations: ALS, acetolactate synthase; EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase; PPO, protoporphyrinogen oxidase.
b Rate in g ae ha−1.
Response to Foliar-applied Non-PPO Herbicides
Seventy-three accessions collected in 2014 and 2015 were tested in the greenhouse for response to dicamba, glufosinate, glyphosate, and trifloxysulfuron (Table 2). The SS accession was also included for comparison. The experiment was conducted in a completely randomized design with two replications and two runs. Each replication consisted of 50 plants grown in a cellular tray. Composite seed samples were planted as described earlier. The foliar herbicides were applied to uniform-sized plants (7.6-cm tall). Glufosinate and trifloxysulfuron were sprayed with 3,366 g AMS ha−1 and 0.25% NIS (v/v), respectively. Herbicide applications were made as described earlier. Plants were evaluated for injury and mortality at 21 DAT. Data were analyzed by herbicide using JMP Pro v. 13 (SAS Institute, Cary, NC). Response to glyphosate was analyzed using hierarchal clustering, collectively considering mortality and injury of survivors.
Mechanism of Resistance to PPO Herbicides
A total of 316 survivors from 47 accessions treated with foliar-applied fomesafen were analyzed for the Gly-210 deletion mutation (Patzoldt et al. Reference Patzoldt, Hager, McCormick and Tranel2006; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). Young leaf tissues were collected from up to 10 survivors each of 35 accessions sprayed with the recommended dose of foliar-applied fomesafen in the greenhouse bioassays. In addition, leaf tissues were collected from 82 plants in 14 fields with high population density of Palmer amaranth after having been sprayed with PPO inhibitors, among other herbicides. DNA was extracted using a modified Cetyltrimethylammonium bromide (CTAB) protocol (Sales et al. Reference Sales, Shivrain, Burgos and Kuk2008) and quantified using a NanoDrop spectrophotometer (ND-1000, Thermo Scientific, Wilmington, DE). An allele-specific PCR assay was used to detect the codon Gly-210 following the protocol described for common waterhemp, which also worked for Palmer amaranth (Lee et al. Reference Lee, Hager and Tranel2008; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016).
Some PPO-resistant plants that did not have the Gly-210 deletion mutation were selected for sequencing of the PPX2L gene. Total RNA was extracted using the RNeasy extraction kit (Qiagen 74903, Valencia, CA) and converted to cDNA using the Reverse Transcription kit (Promega A3500, Madison, WI). Initially a partial sequence of the PPX2L gene was amplified using the primer pair ppx2-1F (5′-GTAATTCAATCCATTACCCACCTT-3′) and ppx2-3R (5′-TTACGCGGTCTTCTCATCCAT-3′) and sequenced using the internal primers: ppx2-1R (5′-TTCCATACGTCGGGAAATGT-3′), ppx2-2F (5′-TGTTGGAACCATTTCTCTGG-3′), ppx2-2R (5′-GGGGATAAGAACTCCGAAGC-3′) and ppx2-3F (5′-GATGCTGTGGTTGTCACTGC-3′). Eventually, the full-length sequences of PPX2L in PPO-sensitive and PPO-resistant plants were obtained (GenBank accession nos. MF583744 and MF583746) by designing primers based on the upstream and downstream gene sequence of Prince-of-Wales feather (Amaranthus hypochondriacus L.) (GenBank accession no. EU024569.1). These additional primers were: ppx2-5′UTR (5′-TGGCAGATTGAGACAAAATTGG-3′) and ppx2-3′UTR (5′-GGCAGAAAAGTCACTGCACA-3′).
Fomesafen Dose–Response Bioassay
Seven PPO-resistant accessions, including the SS, were used in whole-plant bioassays to determine the resistance level to fomesafen. Three accessions that contained the Gly-210 deletion (14MIS-H, 15MIS-C, 15MIS-E) and four accessions that lacked the Gly-210 deletion mutation (14CRI-C, 15CRI-B, 15PHI-A) were selected. Seedlings were grown in 15-cm-diameter pots and thinned to 5 plants pot−1. Seedlings, 5- to 7-cm tall, were sprayed with 10 doses of fomesafen from 0 to 2,107 g ai ha−1. The SS was sprayed with 9 doses from 2 to 263 gha−1, corresponding to 1/128 to 1X the recommended dose. A nontreated check was included. The herbicide was applied with 0.5% NIS. The experiment was conducted in a randomized complete block design with four replications. At 21 DAT, plants were cut at the soil surface, shoots were dried for 48 h at 60°C, and the dry weights were recorded.
Data were analyzed using SAS JMP Pro v.13 (SAS Institute, Cary, NC) in conjunction with SigmaPlot v.13 (Systat Software, San Jose, CA) for regression analysis. The percentage biomass reduction was fit to a nonlinear, sigmoid, four-parameter logistic regression model defined by
where y is the biomass reduction expressed as percentage relative to the untreated control, a is the growth rate, b is the inflection point, c is the lower asymptote, d is the upper asymptote, and x is the fomesafen dose. Estimates of herbicide dose that cause 50% growth reduction (GR50) were determined using the fitted regression equation.
Results and Discussion
Palmer Amaranth Response to Foliar-applied Fomesafen
The frequency distribution of mortality from fomesafen treatment was highly skewed, with a greater proportion of high mortality; therefore, the median values were used to describe the data, except for the 2015 accessions (Figure 1). The 2008 accessions generally incurred 97% mortality with the field dose of fomesafen, except for three accessions with <90% mortality. One rare accession from Phillips County had only 74% mortality; however, the survivors incurred >75% injury and did not produce seeds. Thus, the early samples had individuals that were relatively more tolerant to fomesafen than others. The accessions in 2009 and 2011 were all susceptible, but one of two accessions with 95% mortality, 11LAW-B, contained rare individuals carrying the Gly-210 deletion mutation (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). Eleven accessions (22%) collected in 2014 and 16 accessions (70%) in 2015 were classified resistant. The 2015 samples were from fields with suspected resistance to PPO herbicides, and the results confirmed most growers’ suspicions. The mortality data with fomesafen treatment was normally distributed from 23% to 100%, with an average of 71%. Accessions classified as PPO resistant in 2015 consisted of individuals with variable response to fomesafen (Figure 2). For example, 15CLA-A had 50% survivors and almost all showed ≤10% injury. On the other hand, 15GRE-A had 75% survivors, and only about one-half showed ≤10% injury. Overall in 2015, 16 accessions from 10 counties showed mostly <90% mortality with fomesafen and were classified resistant based on further evaluations discussed in succeeding sections. This shows that resistance to PPO herbicides among Palmer amaranth has evolved quasi-simultaneously across counties in Arkansas.
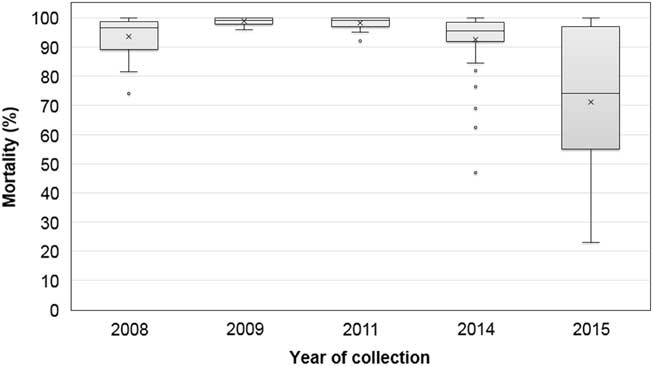
Figure 1 Variability in response to foliar-applied fomesafen (263 gha−1) among Palmer amaranth accessions collected between 2008 and 2015. Box plot shows median values (horizontal line inside the box), mean values (marked X), first and third quartile values (box outlines), minimum and maximum values (whiskers), and outlier values (open circles).
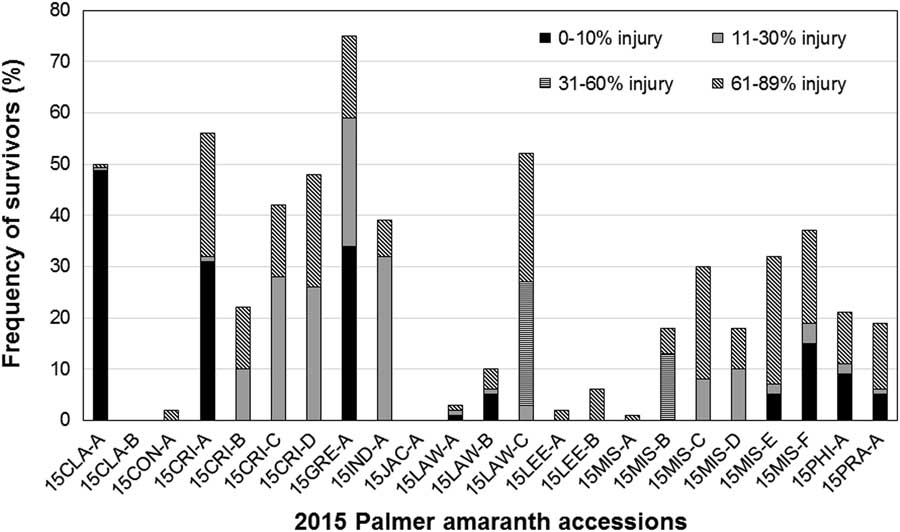
Figure 2 Frequency of fomesafen-resistant plants in Palmer amaranth accessions collected in 2015 from Arkansas. Fomesafen at 263 gha−1 was applied with 0.5% nonionic surfactant to 7.6-cm-tall seedlings. Survivors were categorized based on visible injury.
Prior to 2015, there were fields with a few Palmer amaranth remaining after PPO herbicide application, but such low numbers did not catch the growers’ attention because of the expected variability in plant response under various environmental conditions. In some cases, any escapes would have been blamed on various factors. Continued selection with different herbicides, but with the same PPO-inhibitor mode of action, has increased the frequency of surviving individuals to a field-scale, observable level. PPO-inhibiting herbicides are used extensively to control glyphosate- and ALS-resistant Palmer amaranth in soybean and cotton (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016). Prior to the widespread glyphosate-resistance problem in Amaranthus, fomesafen had been used primarily as PRE or POST herbicide for broadleaf weed control in soybean. Since fomesafen commercialization in the 1960s, several other herbicides targeting the PPO enzyme have been commercialized. With the expansion of PPO-herbicide use pattern to cotton and PPO-herbicide use in preplant applications, the selection pressure from this mode-of-action group has intensified greatly. Considering that rare PPO-resistant Palmer amaranth individuals were detected retroactively in a population collected in 2011, it took 4 yr before several reports arose of field-level escapes from PPO herbicide application in 2015.
Fields sampled in 2015 were those with remnant infestations of Palmer amaranth after a weed management program that included multiple applications of a PPO herbicide. Most of those fields had plants resistant to 263 gha−1 fomesafen. The frequency of resistance reported here pertained mostly to fields with putative PPO herbicide–resistance problem. A random survey of fields infested with Palmer amaranth across the state, irrespective of cropping history, is expected to produce a lower frequency of resistance to PPO herbicides. Of the 23 fields verified for resistance in 2015, only two had 100% mortality with a field dose of fomesafen. The rest had resistant individuals showing different levels of injury of survivors (Figure 2). The majority of survivors from three accessions (15CLA-A and 15CRI-A) incurred minimal injury (0% to 10%). High frequency of PPO-resistant individuals among the 2015 accessions threatens the continued use of PPO herbicides.
Multivariate cluster analysis was conducted to group the 2014 and 2015 accessions based on their response to the field use rate of fomesafen, taking into account both mortality data and injury of survivors. The 2014 and 2015 accessions differentiated into four clusters (Figure 3). The 50 accessions belonging to the first cluster were the most sensitive, the majority of which were from the quasi-random collection in 2014. The second cluster is composed of 19 resistant accessions with the majority of survivors showing >60% injury, from the 2014 and 2015 collection. The third cluster comprises two resistant accessions in which most survivors incurred low (11% to 30%) injury. The fourth cluster contains the three resistant accessions in which a large number of survivors incurred the lowest (<11%) injury. This indicates that a large number of PPO-resistant plants incurred low to moderate levels of injury from field use rate of fomesafen, allowing them to mature and reproduce. The evolution of observable, population-level resistance to PPO herbicides in multiple fields in 2015 demonstrated the quasi-simultaneous evolution of resistance to PPO herbicides in Palmer amaranth. This occurred after several decades of use primarily in soybean, then more recently in cotton. The proportion of resistant populations that evolved via gene flow (pollen or seed) or via independent selection is yet to be determined.

Figure 3 Hierarchal clustering of 2014 and 2015 Palmer amaranth accessions treated with foliar-applied fomesafen at 263 g ha−1. The herbicide was applied with 0.5% nonionic surfactant to 7.6-cm-tall seedlings. Cluster 1, most sensitive to fomesafen (50 accessions); Cluster 2, resistant to fomesafen, the majority of survivors incurred >60% injury (19 accessions); Cluster 3, resistant to fomesafen, majority of survivors sustained 11% to 30% injury (2 accessions); Cluster 4, resistant to fomesafen, the majority of survivors incurred 0% to 10% injury (3 accessions).
Response to Other Foliar-applied PPO-inhibiting Herbicides
The response of Palmer amaranth to other foliar-applied PPO herbicides and to other non-PPO foliar-applied herbicides was evaluated, because PPO herbicides are used heavily to control ALS- and glyphosate-resistant Palmer amaranth. Twelve of the most fomesafen-resistant accessions were tested with other PPO-inhibiting herbicides (Table 3). Mortality with pyraflufen-ethyl and fluthiacet-methyl was similar across runs; data were therefore combined. These herbicides controlled the SS 100%. The mortality of all accessions, excluding the SS, was <72% with fluthiacet-methyl. Palmer amaranth generally has variable sensitivity to this PPO herbicide and is therefore not listed on the label; whereas this herbicide is expected to provide only partial control of common waterhemp (Anonymous 2011). The mortality rating of all PPO-resistant accessions was at least 17% lower than the SS when treated with pyraflufen-ethyl, with the exception of 14CRI-C (>90% mortality). Pyraflufen-ethyl also is naturally weak on Palmer amaranth, although it killed the SS in these experiments, as did the other herbicides.
Table 3 Response of fomesafen-resistant Palmer amaranth accessions to the recommended rate of various foliar-applied protoporphyrinogen oxidase herbicides.
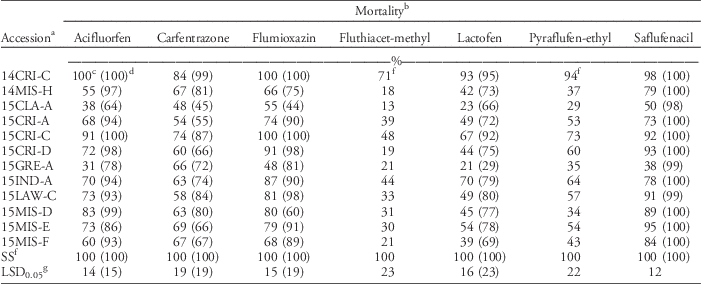
a Accessions were collected between 2014 and 2015 in Arkansas. Accessions were confirmed resistant to foliar-applied fomesafen (263 g ha−1), except for the susceptible standard (SS).
b Mortality ratings from acifluorfen, carfentrazone, flumioxazin, lactofen, and saflufenacil treatments were different across runs; data were therefore analyzed separately. Carfentrazone (280 g ha−1), pyraflufen-ethyl (3.64 g ha−1), and flumioxazin (70.6 g ha−1) treatments included 0.25% v/v nonionic surfactant (NIS). Acifluorfen at 560 g ha−1 included 0.125% v/v NIS. Lactofen (224 g ha−1) was sprayed with 0.5% v/v crop oil concentrate. Saflufenacil (24.6 g ha−1) was applied with 1% methylated seed oil and 1% ammonium sulfate.
c Values obtained from the first run of experiment. Herbicides were applied to 10-cm-tall seedlings.
d Numbers in parentheses were data from the second run of the experiment. Herbicides were applied to 5- to 8-cm-tall seedlings.
e Average mortality across two runs. Mortality ratings across runs were similar in pyraflufen-ethyl and fluthiacet-methyl treatments; data were therefore combined.
f SS, susceptible standard population.
g Fisher’s protected LSD was used to compare accessions within each herbicide.
Mortality was different between runs for acifluorfen, carfentrazone, flumioxazin, lactofen, and saflufencil; data were therefore analyzed separately. In general, mortality was higher in the second run than in the first run due to smaller plant size at the time of herbicide treatment. With acifluorfen, only 2 of 12 fomesafen-resistant accessions tested (14CRI-C and 15CRI-C) were effectively controlled (>90% mortality) in the first run; however, 75% of fomesafen-R accessions were susceptible to acifluorfen when smaller plants were sprayed in the second run. With carfentrazone, all 12 fomesafen-resistant accessions showed only 48% to 83% mortality in the first run. Similar results were observed in the second run. With flumioxazin, eight accessions that had ≥74% mortality in the first run were verified to be sensitive in the second run. About 42% of fomesafen-resistant accessions tested were cross-resistant to foliar-applied flumioxazin. The mortality of all accessions was <70% with lactofen in the first run, with the exception of 15CRI-C (93% mortality). In the second run, all accessions were still poorly to moderately controlled, except for 14CRI-C and 15CRI-C (>90% mortality). Saflufenacil was effective only on five accessions (>90% mortality) with larger seedlings. With smaller seedlings, all 12 fomesafen-resistant accessions showed >97% mortality with saflufenacil. Across all chemistries, regardless of herbicidal strength, application on small seedlings is necessary for maximum possible efficacy. Application on bigger seedlings is risky. Previous reports indicated that resistance of common waterhemp to foliar-applied PPO herbicides becomes prevalent at the 4- to 6-leaf stage (Falk et al. Reference Falk, Shoup, Al-Khatib and Peterson2006). Plant size is a critical factor in the efficacy and consistency of performance of most foliar-applied herbicides. When growers miss the application window, selection for resistance is expected to be stronger. If survival in greenhouse bioassays is seen to be consistent across replications and repetitions, then the risk of having survivors in the field populations is high.
Consistent in both runs, fluthiacet-methyl was the least effective and saflufenacil was the most effective of all foliar-applied PPO herbicides. The response to PPO herbicides, in order of decreasing efficacy, was as follows: saflufenacil>acifluorfen=flumioxazin>carfentrazone=lactofen>pyraflufen-ethyl>fluthiacet-methyl. In this series, fomesafen would fall between the last two herbicides. The fomesafen-resistant accessions were generally cross-resistant to other foliar-applied PPO herbicides, with the exception of saflufenacil. However, saflufenacil is not an in-crop option for soybean or cotton. Although saflufenacil showed the greatest efficacy, some accessions already showed reduced sensitivity to the field use rate of saflufenacil when applied to 10-cm-tall seedlings. Previous studies on diphenylether-resistant tall waterhemp [Amaranthus tuberculatus (Moq.) Sauer] also showed cross-resistance to other foliar-applied PPO herbicide families (Patzoldt et al. 2005; Shoup et al. Reference Shoup, Al-Khatib and Peterson2003; Wuerffel et al. Reference Wuerffel, Young, Matthews and Young2015a).
Response to Foliar-applied Non–PPO Inhibiting Herbicides
Palmer amaranth accessions collected between 2014 and 2015 were tested with dicamba, glufosinate, glyphosate, and trifloxysulfuron. The 73 accessions, including the SS accession, differentiated into four clusters in response to glyphosate (Figure 4A). The first cluster was composed of 13 resistant accessions that had 0% to 36% mortality with glyphosate, with the majority of survivors incurring 31% to 60% injury. Twenty-three accessions constituted the second cluster, with resistance to glyphosate in which a large number of the survivors incurred <11% injury. The third cluster was composed of 24 accessions with ≥65% mortality, 17 of which were classified as sensitive (at least 90% mortality). The fourth cluster included 14 resistant accessions (36% to 68% mortality), in which most of the survivors sustained >60% injury from glyphosate treatment. Overall, about 50% of accessions (Clusters 1 and 2) were resistant to glyphosate, with mortality ranging from 0% to 42%. In addition, all accessions were also resistant to trifloxysulfuron, showing <86% mortality (Figure 4B). The widespread occurrence of ALS- and glyphosate-resistant Palmer amaranth in Arkansas was reported previously (Burgos et al. Reference Burgos, Alcober, Lawton-Rauh, Estorninos, Tseng and Smith2009). The ALS-inhibiting herbicides and glyphosate had been used extensively in the past. Overall, the 27 PPO-resistant accessions were also resistant to either ALS inhibitor or glyphosate, or both (Figure 5; Table 4). The majority of PPO-resistant accessions (85%) exhibited resistance to both glyphosate and trifloxysulfuron.

Figure 4 (A) Cluster analysis of Palmer amaranth accessions collected in 2014 and 2015 treated with 870 gha−1 glyphosate. Cluster 1 (n=13 accessions; resistant to glyphosate, the majority of survivors incurred 31% to 60% injury), Cluster 2 (n=23 accessions; resistant to glyphosate, the majority of survivors incurred <11% injury), Cluster 3 (n=23 accessions; most resistant to glyphosate), and Cluster 4 (n=14 accessions; resistant to glyphosate, the majority of survivors incurred 61% to 89% injury). Glyphosate was applied to 7.6-cm-tall seedlings. (B) Variability in response to dicamba (280 g ae ha−1), glufosinate (549 gha−1), and trifloxysulfuron (7.84 gha−1) among Palmer amaranth accessions collected in 2014 and 2015 from Arkansas. Box plot shows median values (horizontal line inside the box), mean values (marked with X), first and third quartile values (box outlines), minimum and maximum values (whiskers), and outlier values (open circles).
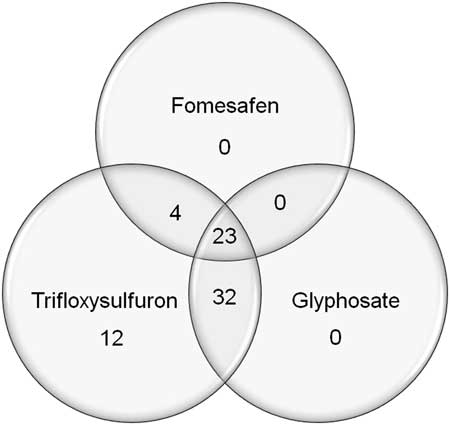
Figure 5 Herbicide resistance profiles of Palmer amaranth populations from Arkansas sampled in 2014 and 2015.
Table 4 Herbicide resistance profile of all protoporphyrinogen oxidase (PPO)-resistant Palmer amaranth accessions to other foliar-applied non-PPO herbicides.
a Fomesafen (263 g ai ha−1), dicamba (280 g ae ha−1), glufosinate (549 g ai ha−1), glyphosate (870 g ae ha−1), and trifloxysulfuron (7.84 g ha−1) were applied to 7.6-cm-tall seedlings.
The 2014 and 2015 accessions were all sensitive to the 549 g ai ha−1 glufosinate (>90% mortality) (Figure 4B). All accessions incurred >95% mortality, except for 15CRI-B (91%). In the same manner, dicamba controlled all accessions (Figure 4B). Dicamba caused >90% mortality in 61% of the accessions. Mortality of the remaining accessions was <89%; however, the survivors incurred >75% injury. Anecdotal reports, however, indicated poor performance of dicamba in fields infested with PPO-resistant Palmer amaranth.
Prevalence of the PPO-Gly-210 Deletion Mutation in Resistant Palmer Amaranth
The Gly-210 deletion in the PPX2L gene confers resistance to PPO-inhibiting herbicides in PPO-resistant common waterhemp and Palmer amaranth (Patzoldt et al. Reference Patzoldt, Hager, McCormick and Tranel2006; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; Wuerffel et al. Reference Wuerffel, Young, Lee, Tranel, Lightfoot and Young2015b). A point mutation in PPX2 of common ragweed also confers resistance to PPO herbicides; however, whereas a plastid-targeting signal was not found to be encoded by PPX2 in common ragweed (Rousonelos et al. Reference Rousonelos, Lee, Moreira, VanGessel and Tranel2012), PPX2L in common waterhemp encodes plastidic and mitochondrial isoforms of PPO (Patzoldt et al. Reference Patzoldt, Hager, McCormick and Tranel2006). This dual-targeting phenomenon has been reported previously for spinach (Spinacia oleracea L.) and corn (Watanabe et al. Reference Watanabe, Che, Iwano, Takayama, Yoshida and Isogai2001). The Gly-210 deletion in PPX2L imparts herbicide resistance to the dual-targeted protein by altering the architecture of the substrate binding domain (Dayan et al. Reference Dayan, Daga, Duke, Lee, Tranel and Doerksen2010).
The molecular survey was carried out on 316 fomesafen-resistant plants from 38 accessions representing 13 counties in Arkansas including Clay, Conway, Crittenden, Greene, Independence, Jackson, Lawrence, Lee, Independence, Mississippi, Phillips, White, and Woodruff (Figure 6). The Gly-210 codon deletion mutation was found in 46% and 60% of the survivors from 2014 and 2015 accessions, respectively (unpublished data). The PPO Gly-210 was prevalent among the PPO-resistant accessions; however, a substantial number of the resistant plants did not carry this mutation (Figure 7, A and B). In most of the PPO-resistant accessions (68%), resistant individuals were mixtures of carriers and noncarriers of the Gly-210 deletion. Only 13% of the PPO-resistant accessions (n=6) contained individuals that were all carriers of the Gly-210 deletion. In nine accessions, none of the resistant individuals carried the Gly-210 deletion. This indicates that either an alternative target-site mutation is present or another resistance mechanism is occurring within and among resistant populations in the field. To verify the occurrence of alternative target-site mutations, we generated a 1,542-bp sequence of PPX2L in selected resistant individuals without the Gly-210 deletion (GenBank accession no. MF583745) from the noncarrier accession 15CRI-B. Resistant plants in this accession contained a different amino acid mutation, Arg-128-Gly. This mutation was reported recently in parallel investigations of PPO-resistant Palmer amaranth, along with an Arg-128-Met mutation (Giacomini et al. Reference Giacomini, Umphres, Nie, Mueller, Steckel, Young, Scott and Tranel2017). A mutation at the homologous site was identified previously in PPO-resistant common ragweed in the form of an Arg-98-Leu substitution (Rousonelos et al. Reference Rousonelos, Lee, Moreira, VanGessel and Tranel2012).
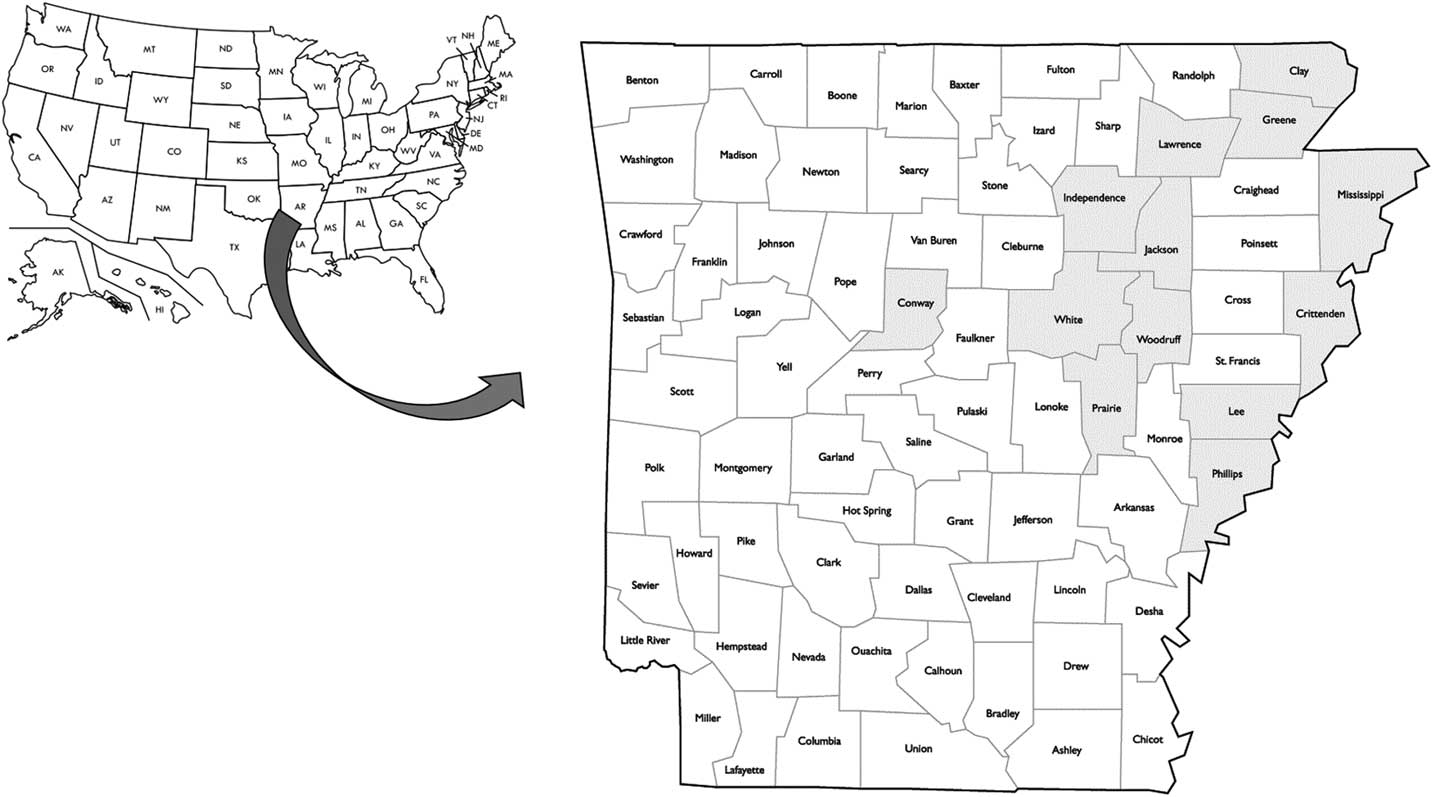
Figure 6 Distribution of fields with confirmed protoporphyrinogen oxidase (PPO)-resistant Palmer amaranth in Arkansas. Counties that are shaded had at least one field with a PPO-resistant Palmer amaranth population carrying the Gly-210 deletion in the PPO gene.
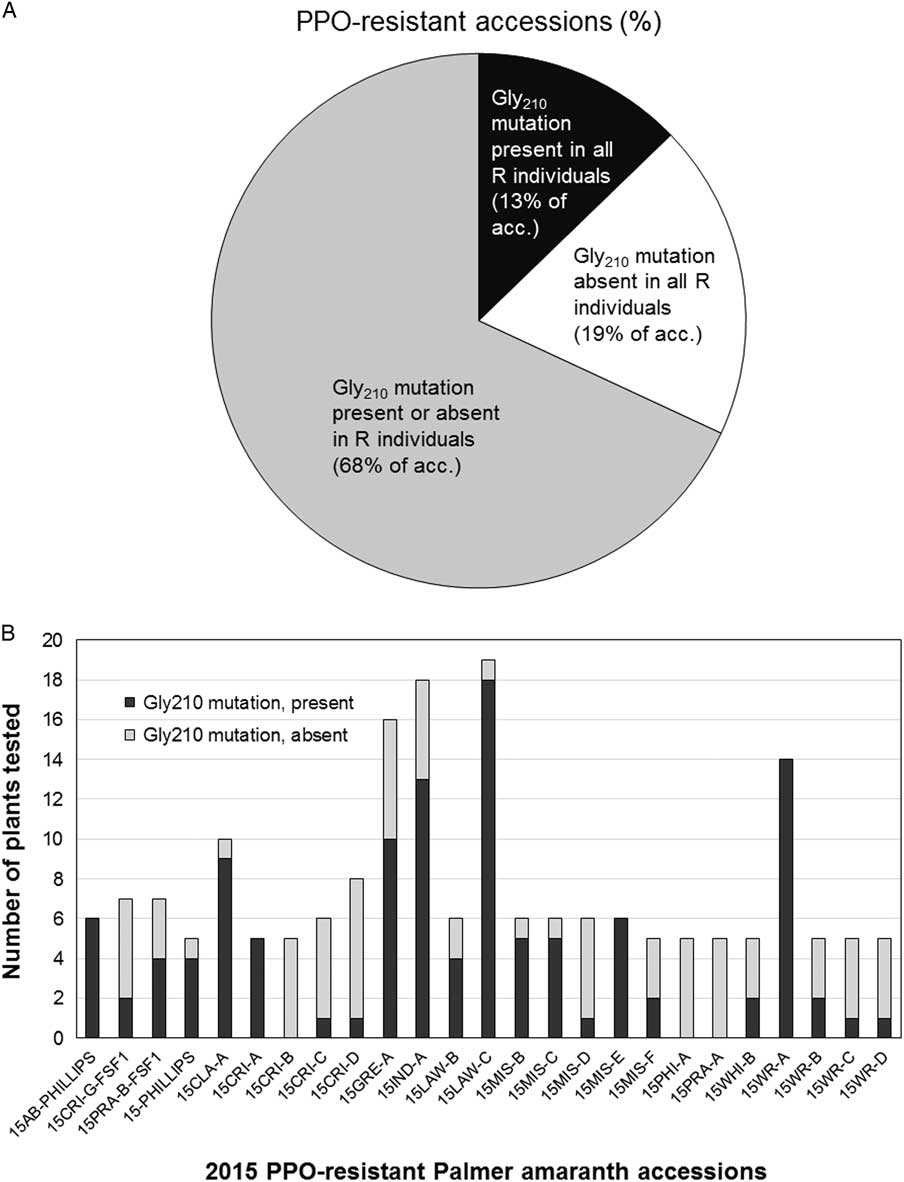
Figure 7 Prevalence of protoporphyrinogen oxidase (PPO) Gly-210 deletion in PPO-resistant Palmer amaranth from Arkansas. (A) The proportion of PPO-resistant accessions that contained, or lacked, the PPO Gly-210 deletion. Black, all fomesafen survivors in these accessions contained the Gly-210 deletion; white, all survivors in these accessions lacked the Gly-210 deletion; gray, survivors in these accessions are a mixture of carriers and noncarriers of the Gly-210 deletion mutation. (B) Proportion of Gly-210 deletion carriers in PPO-resistant accessions from 2015. 15CRI-B, 15PHI-A, and 15PRA-A were noncarriers of the Gly-210 deletion mutation, but had 77%, 74%, and 79% mortality, respectively, and estimated resistance factors of 51- to 125-fold.
Our survey of more than 300 PPO inhibitor–resistant individuals representing 38 field populations showed that a resistant population may contain a mixture of plants carrying either one of these mutations. Henceforth, testing for these mutations should be done simultaneously on suspected PPO inhibitor–resistant plants using available tools (Giacomini et al. Reference Giacomini, Umphres, Nie, Mueller, Steckel, Young, Scott and Tranel2017). Sequencing of Palmer amaranth with different resistance levels to PPO inhibitors, with or without the Gly-210 mutation, is ongoing.
The occurrence of other resistance-conferring mutations in other loci of the PPO gene is rare, as indicated by previous research. Many single or double point mutations were reported in mutant PPO genes in an attempt to obtain PPO herbicide–resistant Arabidopsis (Li and Nicholl Reference Li and Nicholl2005). Those authors’ data showed that single point mutations either provided low resistance or resulted in substantial fitness penalty.
Resistance Level to Fomesafen
The fomesafen dose that caused 50% growth reduction (GR50) ranged from 116 to 232 g ha−1 in accessions that contained the Gly-210 deletion, whereas GR50 ranged from 51 to 153 g ha−1 in accessions that lacked the Gly-210 deletion (Table 5). Based on these GR50 values, the level of resistance to fomesafen relative to the SS ranged from 8- to 15-fold in accessions that contained the Gly-210 and from 3- to 10-fold in accessions that lacked the Gly-210 deletion.The dose–response bioassay indicated high resistance level in accessions carrying the Gly-210 deletion mutation. However, some accessions (14CRI-G and 15CRI-B) that lacked the Gly-210 deletion had GR50 values comparable to those of accessions that contained the Gly-210 deletion. Accession 15CRI-B, which contained the Arg-128-Gly mutation, had similar GR50 values to those of the resistant accessions carrying the Gly-210 deletion. This indicates that Gly-210 and Arg-128-Gly mutations result in comparable levels of resistance to fomesafen. Accessions 14CRI-C and 15PHI-A, which lacked the Gly-210 deletion, had lower GR50 values than the other PPO-resistant accessions but higher GR50 values than the SS. These accessions have not yet been studied for the occurrence of other PPO mutations or other resistance mechanisms. Although our results indicated that the Arg-128-Gly mutation may confer a resistance level similar to that of the Gly-210 deletion, additional data are needed to quantify the impact of this mutation on resistance to PPO herbicides. In addition, we have not ruled out the possibility that other resistance mechanism(s) exist in some PPO inhibitor–resistant accessions, particularly those exhibiting low-level resistance to PPO inhibitors. It is also possible also the populations showing high-level resistance harbor multiple resistance mechanisms, as in the case of ALS-resistant turnipweed [Rapistrum rugosum (L.) All.] (Hatami et al. Reference Hatami, Gherekhloo, Rojano-Delgado, Osuna, Alcantara, Fernandez, Sadeghipor and de Prado2016).
Table 5 Resistance levels of protoporphyrinogen oxidase–resistant Palmer amaranth accessions in Arkansas.
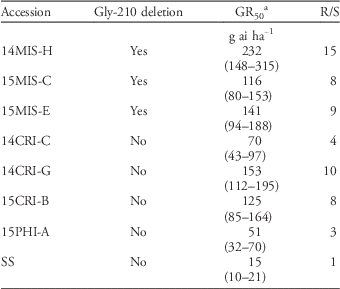
a GR50, dose of herbicide required to cause 50% biomass reduction. Values in parentheses are 95% confidence intervals.
The increasing number of Palmer amaranth populations with resistance to PPO herbicides is a great concern, because this limits herbicide options for cotton and soybean. Most PPO-resistant populations were also resistant to glyphosate and trifloxysulfuron, which leaves almost no herbicides for POST Palmer amaranth control. The remaining POST herbicide options for PPO-resistant Palmer amaranth include glufosinate in LibertyLink® crops, dicamba in Roundup Xtend® or Engenia®, or 2,4-D in Enlist Duo® crops. However, overdependence on glufosinate and phenoxy herbicides must be avoided, as some Palmer amaranth populations show high tolerance to dicamba (unpublished data). If we lose glufosinate and dicamba, there will be zero POST options for weed control in soybean and cotton, unless HPPD and 2,4-D traits are commercialized soon.
Overall, the majority of the PPO-resistant individuals carried the PPO Gly-210 deletion. An alternative target-site mutation, Arg-128-Gly, was identified in resistant plants of one accession analyzed that did not carry the Gly-210 mutation. The occurrence of other resistance-conferring mutations or other resistance mechanisms is being investigated. The combination of these mutations in one plant may be lethal, but the presence of these mutations in different plants in one field, or different mutations in proximal fields, may accelerate the evolution and spread of resistance to PPO inhibitors in Palmer amaranth.
Acknowledgments
The authors thank the Arkansas Soybean Promotion Board, Syngenta Crop Protection, and Cotton Inc. for funding this research. The authors also thank Seth Bernard Abugho, Claudia Oliveira, Josiane Argenta, Leopoldo Estorninos, Vijay Singh, Teal Penka, and Christopher Rouse for assistance in research activities and several Arkansas County Extension Agents for facilitating the plant sampling.














