The number of cases of herbicide-resistant weeds has increased substantially worldwide, mainly in row crops. To date, out of 42 cases of weed resistance to herbicides reported in Brazil, eight are glyphosate resistance cases (Heap Reference Heap2016), including sourgrass. This species is a perennial rhizomatous grass native of the American tropics that grows in clumps. It is considered a troublesome weed, especially in the Brazilian and Paraguayan soybean production areas.
Sourgrass seeds have low dormancy potential and high dispersal ability (Clayton et al. Reference Clayton, Vorontsova, Harman and Williamson2006; Mendonça et al. Reference Mendonça, Martins, Martins and Costa2014; Mondo et al. Reference Mondo, Carvalho, Dias and Marcos Filho2010). According to Carvalho et al. (Reference Carvalho, Scherer, Lucio and Alves2009), sourgrass has the greatest competition potential with crops when compared with 18 other weed species, mainly due to its rapid growth. Sourgrass can reduce corn (Zea mays L.) and soybean productivity by up to 32 and 44%, respectively (Gazziero et al. Reference Gazziero, Voll, Fornarolli, Vargas and Adegas2012; Gemelli et al. Reference Gemelli, Oliveira, Constantin, Braz, Jumes, Gheno, Rios and Franchini2013). In 2014 glyphosate-resistant (GR) soybean was planted as 93% of the total Brazilian crop, which is equivalent to 30 million ha (Brookes and Barfoot Reference Brookes and Barfoot2016).
The first case of GR sourgrass was reported in 2005 in Caaguazu County, Alto Parana Province, Paraguay (25.41°S, 56.45°W) (Heap Reference Heap2016). Two years later, the first Brazilian case of GR sourgrass was reported in Guaira County, Parana State (24.06°S, 54.15°W), approximately 350 km east of the first documented resistance case (Heap Reference Heap2016). In 2009 GR populations of sourgrass were identified in soybean fields and citrus orchards (Adegas et al. Reference Adegas, Gazziero, Voll and Osipe2010; Carvalho et al. Reference Carvalho, Hipolito, Torralva, Alves, Christoffoleti and De Prado2011). However, there is limited information about the frequency and distribution of GR sourgrass populations throughout agricultural regions of Brazil.
Resistance monitoring studies designed to understand the frequency and dispersal of resistant weeds in agricultural areas are important tools to raise awareness and contribute to the implementation of integrated management strategies to mitigate such issues at regional levels (Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015). For integrated management strategies to be effective, appropriate monitoring methods are essential to measure and understand the scope, scale, relative frequency, and intensity of resistance cases. Resistance monitoring studies may also be useful to correlate herbicide resistance with weed management practices and ultimately guide the development of specific practices to solve regional weed resistance issues (Soteres and Peterson Reference Soteres and Peterson2015).
Such studies have been conducted for key weed species in countries with relevant agriculture production such as Brazil, Australia, and the United States (Owen et al. Reference Owen, Martinez and Powles2015; Rosenbaum and Bradley Reference Rosenbaum and Bradley2013; Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015; Vidal et al. Reference Vidal, Winkler, Hernandes, Fleck, Merotto and Trezzi2004). Based on the relevance of this information to implementation of an effective resistance management program, the objective of this study was to monitor the frequency and dispersal rate of GR sourgrass populations in agricultural areas of Brazil between 2012 and 2015.
Materials and Methods
Seed Sampling and Sites
Herbicide resistance monitoring (Soteres and Peterson Reference Soteres and Peterson2015) was conducted by collecting seeds from sites where sourgrass plants survived after POST glyphosate applications on GR soybean. Seeds from surviving plants were collected every year between January and May from 2012 to 2015.
Seeds were collected in agricultural areas located in 329 municipalities from 14 Brazilian states: Bahia (BA), Ceará (CE), Goiás (GO), Maranhão (MA), Minas Gerais (MG), Mato Grosso do Sul (MS), Mato Grosso (MT), Pará (PA), Paraná (PR), Piauí (PI), Rio Grande do Sul (RS), Santa Catarina (SC), São Paulo (SP), and Tocantins (TO). Seed sampling did not occur in exactly the same sites over the years, but rather it was influenced by glyphosate control failures observed in the field. Thus, sampling areas were not chosen randomly but focused on sites with possible resistance cases. Seed sampling followed the methodology proposed by Burgos et al. (Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013) and Heap (Reference Heap1994). Briefly, after a crop site with herbicide failures was located, seeds were randomly collected from at least 50 mature plants (at least 1,000 viable seeds per sample), which were combined into a single composite sample, packaged in individual paper bags, and identified appropriately. No samples were collected from roadsides or areas that had not received at least one glyphosate application.
Greenhouse Trials for Resistance Monitoring
Greenhouse trials were conducted in different research institutions: Escola Superior de Agricultura Luiz de Queiroz (Luiz de Queiroz School of Agriculture, Piracicaba, SP, 22.70°S, 47.63°W), which tested seeds from the states of SP, BA, CE, MA, MG, PA, PI, and TO; Universidade Estadual de Maringá (State University of Maringá, Maringá, PR, 23.40°S, 51.94°W), which tested seeds from states of PR, SC, RS, and MS; and Centro Universitário Várzea Grande (University Center of Várzea Grande, Várzea Grande, MT, 15.64°S, 56.10°W), which tested seeds from GO and MT. All three institutions followed the same protocol. Samples were initially cleaned to eliminate impurities. Then seeds were packed in paper bags, identified, and stored at room temperature. During seed sampling, each sample was identified with site information (geographic coordinates, state, and municipality). In total, 2,593 samples were collected throughout the 4 yr: 702 (2012), 976 (2013), 389 (2014), and 526 (2015). Each sample corresponded to a collection site.
The experimental units consisted of a pot (1 dm3) filled with a commercial substrate (Mec Plant®, Mecpret, PR160 Road, 15, Telemaco Borba, Parana, Brazil). Thirty seeds were sowed per pot, and after emergence, specimens were thinned to four plants per pot. Four replicates arranged in a completely randomized design were used for each population, and the pots were rearranged weekly in the greenhouse to minimize positioning effects due to changes in light or temperature.
Discriminatory Rate Determination and Resistance Monitoring
A single discriminatory rate was used to characterize the resistance level to glyphosate, an approach that has been extensively described in the literature (Owen et al. Reference Owen, Martinez and Powles2015; Rosenbaum and Bradley Reference Rosenbaum and Bradley2013; Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015). The discriminatory rate is defined as the minimum rate that provides maximum difference between dose–response curves for resistant (R) and susceptible (S) biotypes, resulting in a minimum of 80% control of biotype S (Beckie et al. Reference Beckie, Friesen, Nawolsky and Morrison1990; Bourgeois and Morrison Reference Bourgeois and Morrison1997; Norsworthy et al. Reference Norsworthy, Talbert and Hoagland1998).
In Brazil, there are 64 commercial glyphosate formulations labeled to control sourgrass, with a range of rates from 325 to 2,160 g ae ha−1 (MAPA 2016). Considering only POST applications in GR soybeans to provide efficient control of grasses with 2 to 3 tillers, the highest recommended rate is 960 g ha−1 (Rodrigues and Almeida Reference Rodrigues and Almeida2011). Additionally, preliminary research was conducted to determine the discriminatory rate by evaluating 17 sourgrass populations sampled in sites with no history of glyphosate use (Melo Reference Melo2015). Based on the dose–response curves obtained in this preliminary study, 960 g ha−1 conferred effective control (100%) of the most susceptible populations and partial control (59%) of the most resistant population. In other studies, Carvalho et al. (Reference Carvalho, Hipolito, Torralva, Alves, Christoffoleti and De Prado2011) and Correia et al. (Reference Correia, Acra and Balieiro2015) demonstrated that 960 g ha−1 was adequate to provide ≥90% control of susceptible sourgrass populations. Based on those previous studies, a glyphosate dose of 960 g ha−1 (Roundup Transorb®, 480 g ae L−1, Monsanto Brazil, Nações Unidas Ave., 12901, São Paulo, Brazil, 04578910) was chosen as discriminatory rate to test all 2,593 samples.
Glyphosate-resistant (R) and glyphosate-susceptible (S) sourgrass populations characterized in previous study were included as standards in all three testing locations, as recommended by James et al. (Reference James, Kemp and Moss1995). In this study, resistance was determined by dose–response trials of 17 populations of sourgrass (Melo Reference Melo2015) using methodology described by Burgos et al. (Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013). The susceptible standard population was effectively controlled with 326 g ha−1 of glyphosate. On the other hand, the resistant population was not controlled with the label rate (960 g ha−1). The resistance factor (R/S from the GR50) was 3.6-fold.
POST applications of glyphosate were carried out when plants reached 2 to 3 tillers and 10 to 12 cm, the most appropriate stage for differentiating susceptible from resistant populations (Melo Reference Melo2015). All applications were carried out during the morning, when weather conditions such as temperature, relative humidity, and wind speed were appropriate. A pressurized backpack sprayer was used, equipped with a 1.5-m-long bar containing three AI 110-02 spraying nozzles (0.5 m between nozzles, TeeJet Technologies®, João Paulo Ablas Ave, 287, Cotia, Brazil, 6711250), with a pressure of 245 kPa, providing a spray volume equivalent to 200 L ha−1. Plant mortality was evaluated at 28 d after application (DAA) using the scale described in Table 1. A color scale based on control rates was used for expressing the final control results in this study, as shown in Table 1.
Table 1 Color-coded glyphosate resistance classification of sourgrass populations at 28 d after application (DAA).

Data Analysis
After each population was classified based on resistance criteria, data were grouped by class, year, and state, and the relative frequencies were calculated by state. Maps of the dispersion of GR populations of sourgrass were elaborated for each sampling year using GPS TrackMaker® (Geo Studio Technology®, Corcovado, 432, Belo Horizonte, MG, Brazil) and AutoCAD (Autodesk®, Duke, 10, Montreal, QC, Canada). For the maps, the color-coded classification was used to identify each sample in the respective geographic coordinates related to the sampling site.
Results and Discussion
Overall, 68% of the populations sampled in the first year of monitoring (2012) were considered susceptible to glyphosate (Figure 1). Populations considered as resistant accounted for 9% of all samples, while 23% of populations were classified as intermediates. In the second year (2013), even though the number of resistant populations did not significantly increase (11.4%), the frequency of intermediate populations rose to 31.7%. In the third year of monitoring (2014), half (50%) of the populations were considered resistant, while the incidence of susceptible populations decreased to 36%. In 2015, 57% of the populations were resistant, while only 26% of populations tested were susceptible. These results clearly indicated a significant increase in the frequency of resistant populations from 2012 to 2015 across sampled states. In 4 yr of monitoring, out of 2,593 populations sampled, 671 (26%) were considered resistant to glyphosate.
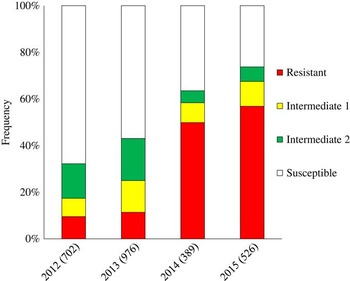
Figure 1 Frequency (%) and total number (in parenthesis) of tested populations of sourgrass during 2012, 2013, 2014, and 2015 in Brazil.
The incidence of resistance cases during the first 2 yr (Figures 2 and 3) were mainly found in western Parana (PR) and southern Mato Grosso do Sul (MS) and can probably be attributed to proximity to the first sites where cases of GR sourgrass in Paraguay and Brazil were reported (Caaguazu, Alto Paraná, Paraguay, and Guaira, PR, Brazil) (Heap Reference Heap2016).

Figure 2 Distribution of sourgrass populations based on glyphosate sensitivity in Brazil in 2012. Detail: Western Paraná and Southern Mato Grosso do Sul, where glyphosate-resistant sourgrass populations were originally identified in Brazil. AL, Alagoas; AM, Amazonas; AC, Acre; AP, Amapá; DF, Distrito Federal; ES, Espírito Santo; PB, Paraíba; PE, Pernambuco; PY, Caaguazú, Paraguay; RN, Rio Grande do Norte; RJ, Rio de Janeiro; RR, Roraima.
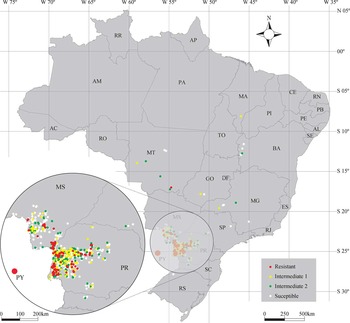
Figure 3 Distribution of sourgrass populations based on glyphosate sensitivity in Brazil in 2013. See Figure 2 caption for detail information and abbreviation(s).
Many Brazilian growers from PR and MS states also have properties in Paraguay, with frequent movement of equipment and people that may have contributed to the dispersal between these contiguous areas. In South America there is evidence that combines used for harvesting may have played an important role on the spread of other important GR-weeds such as johnsongrass [Sorghum halepense (L.) Pers.] in Argentina (Pengue et al. Reference Pengue, Monterroso and Binimelis2009). The presence of hairlike appendages on sourgrass seeds facilitates adhesion to other crop seeds (Kissmann and Groth Reference Kissmann and Groth1997) and could also contribute to its dispersion. Wind dispersal may have been a contributing factor to short-distance spread between neighboring properties. In 2014 the incidence of plants with some level of resistance (resistant and intermediates) in PR and MS states significantly increased to more than 80% of the tested populations, indicating a resistance consolidation and lack of proper mitigation and quarantine programs implementation in these areas (Figures 4 and 5).

Figure 4 Distribution of sourgrass populations based on glyphosate sensitivity in Brazil in 2014. See Figure 2 caption for detail information and abbreviation(s).
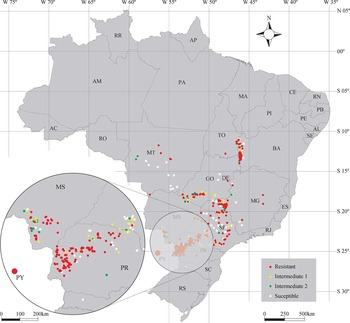
Figure 5 Distribution of sourgrass populations based on glyphosate sensitivity in Brazil in 2015. See Figure 2 caption for detail information and abbreviation(s).
In 2014 an increase in the incidence of resistant and intermediate populations occurred in other Brazilian agricultural states, such as SP, MG, MT, GO, and BA (Table 2). In 2015 the incidence of these populations was 45% in SP, 53% in MG, 24% in MT, 34% in GO, and 54% in BA, which demonstrated a significant increase over the years of monitoring (Figures 2 to 5).
Table 2 Number of sourgrass populations grouped by glyphosate sensitivity from 2012 to 2015 in several Brazilian states.

a BA, Bahia; GO, Goiás; MG, Minas Gerais; MS, Mato Grosso do Sul; MT, Mato Grosso; PR, Paraná; SP, São Paulo.
The frequency of resistant sourgrass populations increased considerably in 2014 and 2015 (Figures 4 and 5) in regions other than PR and MS, suggesting a rapid dispersion of GR populations toward the midwest and northeast regions. Resistant populations were detected in Teresina (PI), Balsas (MA), and Redencao (PA), which are respectively 3,135, 2,749, and 2,534 km from the outbreak point (Guaira, PR). This occurrence may be related to two important aspects of sourgrass biology. Studies on the physiology of the sourgrass seeds show that this species depends on light to germinate and that germination rate is optimum at a temperature of 35 C (Mondo et al. Reference Mondo, Carvalho, Dias and Marcos Filho2010). Thus, both germination and growth are favored by higher temperatures, which could explain the adaptation and dissemination of this species to low latitudes (Machado et al. Reference Machado, Ferreira, Ferreira, Fialho, Tuffi Santos and Machado2006). The second aspect is related to the high potential of dispersion of these populations by harvesting equipment, such as combines (Pengue et al. Reference Pengue, Monterroso and Binimelis2009). Soybean-producing states such as MS, MT, TO, BA, and PI normally have larger production areas than the properties in southern states. Since soybean harvest season traditionally initiates earlier in PR, the combines are usually transported to provide rental services in the midwest region (MS and MT) and later in the north and northeast regions (TO, PA, PI, MA, and BA).
Several factors influence the infestation capacity of a weed in a particular area, such as ecological adaptability, prolific seed production, seed longevity and dormancy, herbicide use frequency, herbicide efficacy, and additional methods used in weed control (Gressel and Segel Reference Gressel and Segel1990). In the case of sourgrass, several of these factors may be associated. For example, 65% of sourgrass seeds remain viable for 270 d while scattered on soil surface (Reinhert Reference Reinhert2013). Regarding its adaptability, in addition to being a perennial species that produces large amounts of seeds, seedling emergence may be greater than 90% (Mondo et al. Reference Mondo, Carvalho, Dias and Marcos Filho2010). Although major emergence flushes occur in the summer, smaller flushes may occur during other periods of the year (Gemelli et al. Reference Gemelli, Oliveira, Constantin, Braz, Jumes, Oliveira Neto, Dan and Biffe2012; Lacerda Reference Lacerda2003), since water stress does not impair seed viability. Thus, the dissemination of resistant sourgrass populations in Brazil might be the result of a combination of biological characteristics of the species and agricultural practices, especially the use of glyphosate as the main herbicide in soybean-growing areas.
In 2015 the presence of resistant populations increased in other locations outside the route of soybean-harvesting equipment, especially in SP and in the western and central regions of MG, where semiperennial crops such as sugarcane and perennials such as orange and coffee are predominant crops. Previous studies conducted by Carvalho et al. (Reference Carvalho, Hipolito, Torralva, Alves, Christoffoleti and De Prado2011) and Melo (Reference Melo2015) demonstrated the presence of GR sourgrass in 2009 in Itapira (22.433°S, 46.821°W) and Matao (21.603°S, 48.365°W), both in São Paulo State, in orange orchards with at least a 15-yr history of glyphosate use. Since these areas do not grow soybeans, these populations might have been independently selected in SP and MG.
It is evident that the incidence of resistant populations is higher in the more traditional soybean-producing areas, such as southern (PR) and midwestern (GO, MS, and MT) Brazil. However, in areas where soybean cultivation is more recent, such as the northeast (MA, PI, TO, and western BA) the resistant populations’ frequency and spread are lower.
The adaptation of the perennial species sourgrass to new areas cultivated with soybean in Brazil is a serious concern for Brazilian agriculture. In addition to glyphosate, sourgrass is primarily controlled by POST applications of acetyl-CoA carboxylase (ACCase)-inhibiting herbicides. Limited herbicide options for POST control of sourgrass have raised concerns for a short-term multiple-resistance evolution to both 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) and ACCase inhibitors. Multiple resistance to EPSPS and ACCase inhibitors has been identified in goosegrass [Eleusine indica (L.) Gaertn.] and Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] and rigid ryegrass (Lolium rigidum Gaudin) (Heap Reference Heap2016), in which the time between the detection of resistant to the first and second mechanism of action was, on average, 9 yr. In Bolivia, the occurrence of two sourgrass populations with low levels of resistance to glyphosate and to clodinafop (ACCase inhibitor) was recently reported (Franco et al. Reference Franco, Condori and Flores2015). Therefore, it is important to introduce a greater diversity of management alternatives for GR sourgrass populations in order to increase the life span of ACCase inhibitors in Brazilian agriculture.
We have documented a rapid increase in the frequency and dispersion of GR sourgrass populations from 2012 to 2015 in Brazil. Resistant populations spread from the initial resistance cases reported in Paraguay toward the north to Brazil’s soybean-growing areas. Our data illustrate the importance of an appropriate herbicide resistance monitoring program that would contribute to the prevention of weed resistance dissemination by focusing efforts toward addressing issues regionally and bringing awareness to stakeholders in each region. Such measures should ultimately lead to implementation of improved weed management practices in Brazilian agriculture.









